Biochemical and Hematological Correlates of Elevated Homocysteine in National Surveys and a Longitudinal Study of Urban Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Databases
2.1.1. NHANES III, Phase 2 and 1999–2006
2.1.2. HANDLS 2004–2018
2.2. Study Samples
2.3. Serum Homocysteine
2.4. Biochemical and Hematological Indices
2.5. Covariates
2.6. Data Handling and Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BIC | Bayesian Information Criterion |
| CDC | Centers for Disease Control and Prevention |
| cv | Cross-validation |
| HANDLS | Healthy Aging in Neighborhood of Diversity Across the Life Span |
| Hcy | Homocysteine |
| LASSO | least absolute shrinkage and selection operator |
| LOWESS | Locally weighted regression |
| MCH | Mean cell hemoglobin |
| MEC | mobile examination center |
| MMA | methylmalonic acid |
| MRV | Medical Research Vehicles |
| MTHF | N-5-methyl-tetrahydrofolate |
| NCHS | National Center for Health Statistics |
| NHANES | National Health and Nutrition Examination Surveys |
| RDW | Red cell distribution width |
| ROC | Receiver Operating Characteristic |
| SAH | S-adenosylhomocysteine |
| SAM | S-adenosylemethionine |
| SUA | Serum Uric Acid |
References
- Selhub, J. Public health significance of elevated homocysteine. Food Nutr. Bull. 2008, 29, S116–S125. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Smith, A.D.; Jobst, K.A.; Refsum, H.; Sutton, L.; Ueland, P.M. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch. Neurol. 1998, 55, 1449–1455. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Gamaldo, A.A.; Teel, A.; Zonderman, A.B.; Wang, Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health 2014, 14, 643. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; Rogers, G.; Bowman, B.A.; Gunter, E.W.; Wright, J.D.; Johnson, C.L. Serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey (1991–1994): Population reference ranges and contribution of vitamin status to high serum concentrations. Ann. Intern. Med. 1999, 131, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Booth, G.L.; Wang, E.E. Preventive health care, 2000 update: Screening and management of hyperhomocysteinemia for the prevention of coronary artery disease events. The Canadian Task Force on Preventive Health Care. CMAJ 2000, 163, 21–29. [Google Scholar]
- Marti-Carvajal, A.J.; Sola, I.; Lathyris, D.; Dayer, M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2017, 8, CD006612. [Google Scholar] [CrossRef]
- Selhub, J.; Miller, J.W. The pathogenesis of homocysteinemia: Interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am. J. Clin. Nutr. 1992, 55, 131–138. [Google Scholar] [CrossRef]
- Hatzis, C.M.; Bertsias, G.K.; Linardakis, M.; Scott, J.M.; Kafatos, A.G. Dietary and other lifestyle correlates of serum folate concentrations in a healthy adult population in Crete, Greece: A cross-sectional study. Nutr. J. 2006, 5, 5. [Google Scholar] [CrossRef]
- Manavifar, L.; Nemati Karimooy, H.; Jamali, J.; Talebi Doluee, M.; Shirdel, A.; Nejat Shokohi, A.; Fatemi Nayyeri, M. Homocysteine, Cobalamin and Folate Status and their Relations to Neurocognitive and Psychological Markers in Elderly in Northeasten of Iran. Iran J. Basic Med. Sci. 2013, 16, 772–780. [Google Scholar]
- Song, J.H.; Park, M.H.; Han, C.; Jo, S.A.; Ahn, K. Serum Homocysteine and Folate Levels are Associated With Late-life Dementia in a Korean Population. Osong Public Health Res. Perspect. 2010, 1, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, F.; Liu, Y. Association of homocysteine with immunological-inflammatory and metabolic laboratory markers and factors in relation to hyperhomocysteinaemia in rheumatoid arthritis. Clin. Exp. Rheumatol. 2015, 33, 900–903. [Google Scholar] [PubMed]
- Cheng, C.H.; Huang, Y.C.; Chen, F.P.; Chou, M.C.; Tsai, T.P. B-vitamins, homocysteine and gene polymorphism in adults with fasting or post-methionine loading hyperhomocysteinemia. Eur. J. Nutr. 2008, 47, 491–498. [Google Scholar] [CrossRef]
- Selhub, J.; Jacques, P.F.; Wilson, P.W.; Rush, D.; Rosenberg, I.H. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA 1993, 270, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Rozen, R. Genetic predisposition to hyperhomocysteinemia: Deficiency of methylenetetrahydrofolate reductase (MTHFR). Thromb. Haemost. 1997, 78, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Fujita, T.; Suzuki, D.; Nishida, T.; Asukai, M.; Matsuyama, Y. Serum homocysteine levels are affected by renal function during a 3-year period of minodronate therapy in female osteoporotic patients. J. Bone Miner. Metab. 2019, 37, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Kafai, M.R. Demographic, lifestyle, and health characteristics and serum B vitamin status are determinants of plasma total homocysteine concentration in the post-folic acid fortification period, 1999–2004. J. Nutr. 2009, 139, 345–352. [Google Scholar] [CrossRef][Green Version]
- Francis, M.E.; Eggers, P.W.; Hostetter, T.H.; Briggs, J.P. Association between serum homocysteine and markers of impaired kidney function in adults in the United States. Kidney Int. 2004, 66, 303–312. [Google Scholar] [CrossRef]
- Ganji, V.; Kafai, M.R.; Third National, H.; Nutrition Examination, S. Demographic, health, lifestyle, and blood vitamin determinants of serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Clin. Nutr. 2003, 77, 826–833. [Google Scholar] [CrossRef]
- Fukagawa, N.K.; Martin, J.M.; Wurthmann, A.; Prue, A.H.; Ebenstein, D.; O’Rourke, B. Sex-related differences in methionine metabolism and plasma homocysteine concentrations. Am. J. Clin. Nutr. 2000, 72, 22–29. [Google Scholar] [CrossRef][Green Version]
- Sadre-Marandi, F.; Dahdoul, T.; Reed, M.C.; Nijhout, H.F. Sex differences in hepatic one-carbon metabolism. BMC Syst. Biol. 2018, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.F.; Pan, G.G. Red blood cell distribution width predicts homocysteine levels in adult population without vitamin B12 and folate deficiencies. Int. J. Cardiol. 2017, 227, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Haj Mouhamed, D.; Ezzaher, A.; Neffati, F.; Douki, W.; Najjar, M.F. Effect of cigarette smoking on plasma homocysteine concentrations. Clin. Chem. Lab. Med. 2011, 49, 479–483. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Targher, G.; Montagnana, M.; Guidi, G.C. Plasma gamma-glutamyl transferase activity predicts homocysteine concentration in a large cohort of unselected outpatients. Intern. Med. 2008, 47, 705–707. [Google Scholar] [CrossRef][Green Version]
- Tanaka, T.; Scheet, P.; Giusti, B.; Bandinelli, S.; Piras, M.G.; Usala, G.; Lai, S.; Mulas, A.; Corsi, A.M.; Vestrini, A.; et al. Genome-wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am. J. Hum. Genet. 2009, 84, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes.htm (accessed on 25 October 2019).
- NCHS. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–1994; NCHS: Highlandsville, MD, USA, 1994. [Google Scholar]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention (CDC). Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf (accessed on 25 October 2019).
- Evans, M.K.; Lepkowski, J.M.; Powe, N.R.; LaVeist, T.; Kuczmarski, M.F.; Zonderman, A.B. Healthy aging in neighborhoods of diversity across the life span (HANDLS): Overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn. Dis. 2010, 20, 267–275. [Google Scholar]
- Araki, A.; Sako, Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. 1987, 422, 43–52. [Google Scholar] [CrossRef]
- Abbott Homocysteine (HCY) assay package insert fo IMX Analyzer. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/l06_b_met_homocysteine_IMX.pdf (accessed on 25 October 2019).
- Pernet, P.; Lasnier, E.; Vaubourdolle, M. Evaluation of the AxSYM homocysteine assay and comparison with the IMx homocysteine assay. Clin. Chem. 2000, 46, 1440–1441. [Google Scholar] [CrossRef]
- Laboratories, B.R. Instruction Manual, Bio-Rad Quantaphase Folate Radioassay Kit; Bio-Rad Laboratories: Hercules, CA, USA, 1987. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Surveys (NHANES 2005–06): Description of Laboratory Methodology: Vitamin B-12. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/B12_D.htm (accessed on 15 February 2019).
- Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Surveys (NHANES 2005–06): Description of Laboratory Methodology: Folate. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/FOLATE_D.htm (accessed on 15 February 2019).
- Diagnostics, Q. Vitamin B-12 (Cobalamin) and Folate Panel. Available online: https://testdirectory.questdiagnostics.com/test/test-detail/7065/vitamin-b12-cobalamin-and-folate-panel-serum?cc=MASTER (accessed on 21 October 2019).
- STATA. Statistics/Data Analysis: Release 16.0; Stata Corporation: College Station, TX, USA, 2019. [Google Scholar]
- Zou, H. The adaptive Lasso and it oracle properties. J. Am. Stat. Assoc. 2006, 101, 1418–1428. [Google Scholar] [CrossRef]
- Albeck, M.J.; Borgesen, S.E. ROC-curve analysis. A statistical method for the evaluation of diagnostic tests. Ugeskr Laeger 1990, 152, 1650–1653. [Google Scholar] [PubMed]
- Soreide, K. Receiver-operating characteristic (ROC) curve analysis in diagnostic, prognostic and predictive biomarker research. J. Clin. Pathol. 2008. [Google Scholar] [CrossRef]
- Jacques, P.F.; Bostom, A.G.; Wilson, P.W.; Rich, S.; Rosenberg, I.H.; Selhub, J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am. J. Clin. Nutr. 2001, 73, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Levi, A.; Vecht-Lifshitz, S.E.; Goldberg, E.; Garty, M.; Krause, I. Assessment of a possible link between hyperhomocysteinemia and hyperuricemia. J. Investig. Med. 2015, 63, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Canas, J.A.; Fanelli-Kuczmarski, M.T.; Tajuddin, S.M.; Evans, M.K.; Zonderman, A.B. Genetic risk scores, sex and dietary factors interact to alter serum uric acid trajectory among African-American urban adults. Br. J. Nutr. 2017, 117, 686–697. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Fanelli-Kuczmarski, M.T.; Canas, J.A.; Beydoun, H.A.; Evans, M.K.; Zonderman, A.B. Dietary factors are associated with serum uric acid trajectory differentially by race among urban adults. Br. J. Nutr. 2018, 120, 935–945. [Google Scholar] [CrossRef]
- Aslinia, F.; Mazza, J.J.; Yale, S.H. Megaloblastic anemia and other causes of macrocytosis. Clin. Med. Res. 2006, 4, 236–241. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H.; Tang, Q. Red Blood Cell Distribution Width: A Novel Predictive Indicator for Cardiovascular and Cerebrovascular Diseases. Dis. Markers 2017, 2017, 7089493. [Google Scholar] [CrossRef]
- Tajuddin, S.M.; Nalls, M.A.; Zonderman, A.B.; Evans, M.K. Association of red cell distribution width with all-cause and cardiovascular-specific mortality in African American and white adults: A prospective cohort study. J. Transl. Med. 2017, 15, 208. [Google Scholar] [CrossRef]
- Hoffmann, J.J. Red cell distribution width and mortality risk. Clin. Chim. Acta 2012, 413, 824–825. [Google Scholar] [CrossRef]
- Perlstein, T.S.; Weuve, J.; Pfeffer, M.A.; Beckman, J.A. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch. Intern. Med. 2009, 169, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.V.; Semba, R.D.; Ferrucci, L.; Newman, A.B.; Fried, L.P.; Wallace, R.B.; Bandinelli, S.; Phillips, C.S.; Yu, B.; Connelly, S.; et al. Red cell distribution width and mortality in older adults: A meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Margalit, I.; Cohen, E.; Goldberg, E.; Krause, I. Reconsidering the relation between serum homocysteine and red blood cell distribution width: A cross-sectional study of a large cohort. Biomarkers 2018, 23, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Poupon, R. Liver alkaline phosphatase: A missing link between choleresis and biliary inflammation. Hepatology 2015, 61, 2080–2090. [Google Scholar] [CrossRef]
- Loohuis, L.M.; Albersen, M.; de Jong, S.; Wu, T.; Luykx, J.J.; Jans, J.J.M.; Verhoeven-Duif, N.M.; Ophoff, R.A. The Alkaline Phosphatase (ALPL) Locus Is Associated with B6 Vitamer Levels in CSF and Plasma. Genes (Basel) 2018, 10, 8. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G. Cigarette smoking and serum liver enzymes: The role of alcohol and inflammation. Ann. Clin. Biochem. 2010, 47, 321–326. [Google Scholar] [CrossRef]
- Bailey, R.L.; Looker, A.C.; Lu, Z.; Fan, R.; Eicher-Miller, H.A.; Fakhouri, T.H.; Gahche, J.J.; Weaver, C.M.; Mills, J.L. B-vitamin status and bone mineral density and risk of lumbar osteoporosis in older females in the United States. Am. J. Clin. Nutr. 2015, 102, 687–694. [Google Scholar] [CrossRef]
- Nakamura, Y.; Suzuki, T.; Kato, H. Serum bone alkaline phosphatase is a useful marker to evaluate lumbar bone mineral density in Japanese postmenopausal osteoporotic women during denosumab treatment. Ther. Clin. Risk Manag. 2017, 13, 1343–1348. [Google Scholar] [CrossRef]
- Kim, D.B.; Oh, Y.S.; Yoo, K.D.; Lee, J.M.; Park, C.S.; Ihm, S.H.; Jang, S.W.; Shim, B.J.; Kim, H.Y.; Seung, K.B.; et al. Passive smoking in never-smokers is associated with increased plasma homocysteine levels. Int. Heart J. 2010, 51, 183–187. [Google Scholar] [CrossRef][Green Version]
- Lee, W.; Lee, S.; Roh, J.; Won, J.U.; Yoon, J.H. The Association between Involuntary Smoking Exposure with Urine Cotinine Level and Blood Cadmium Level in General Non-Smoking Populations. J. Korean Med. Sci. 2017, 32, 568–575. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, J.E.; Bartolome, M.; Canas, A.I.; Huetos, O.; Navarro, C.; Rodriguez, A.C.; Arribas, M.; Esteban, M.; Lopez, A.; Castano, A. Anti-smoking legislation and its effects on urinary cotinine and cadmium levels. Environ. Res. 2015, 136, 227–233. [Google Scholar] [CrossRef] [PubMed]
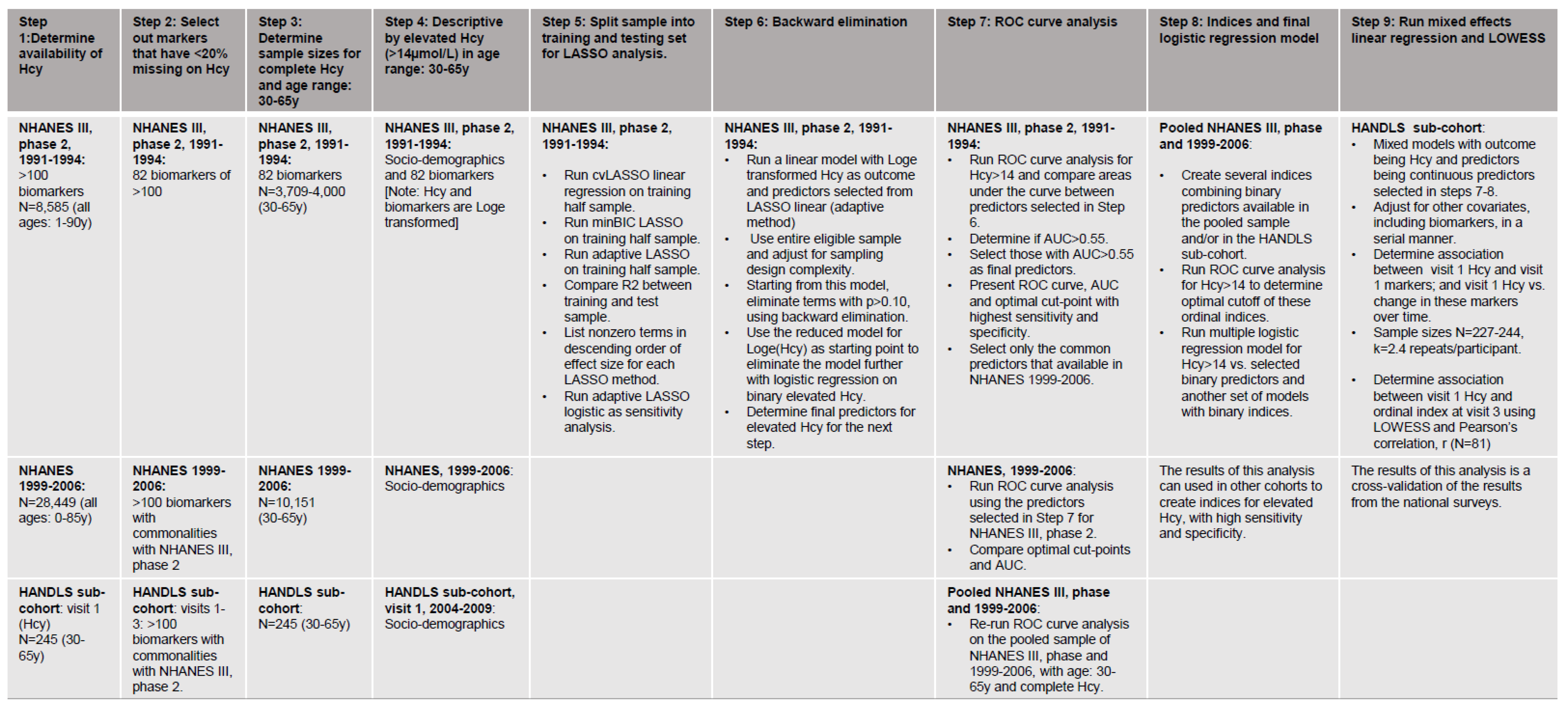
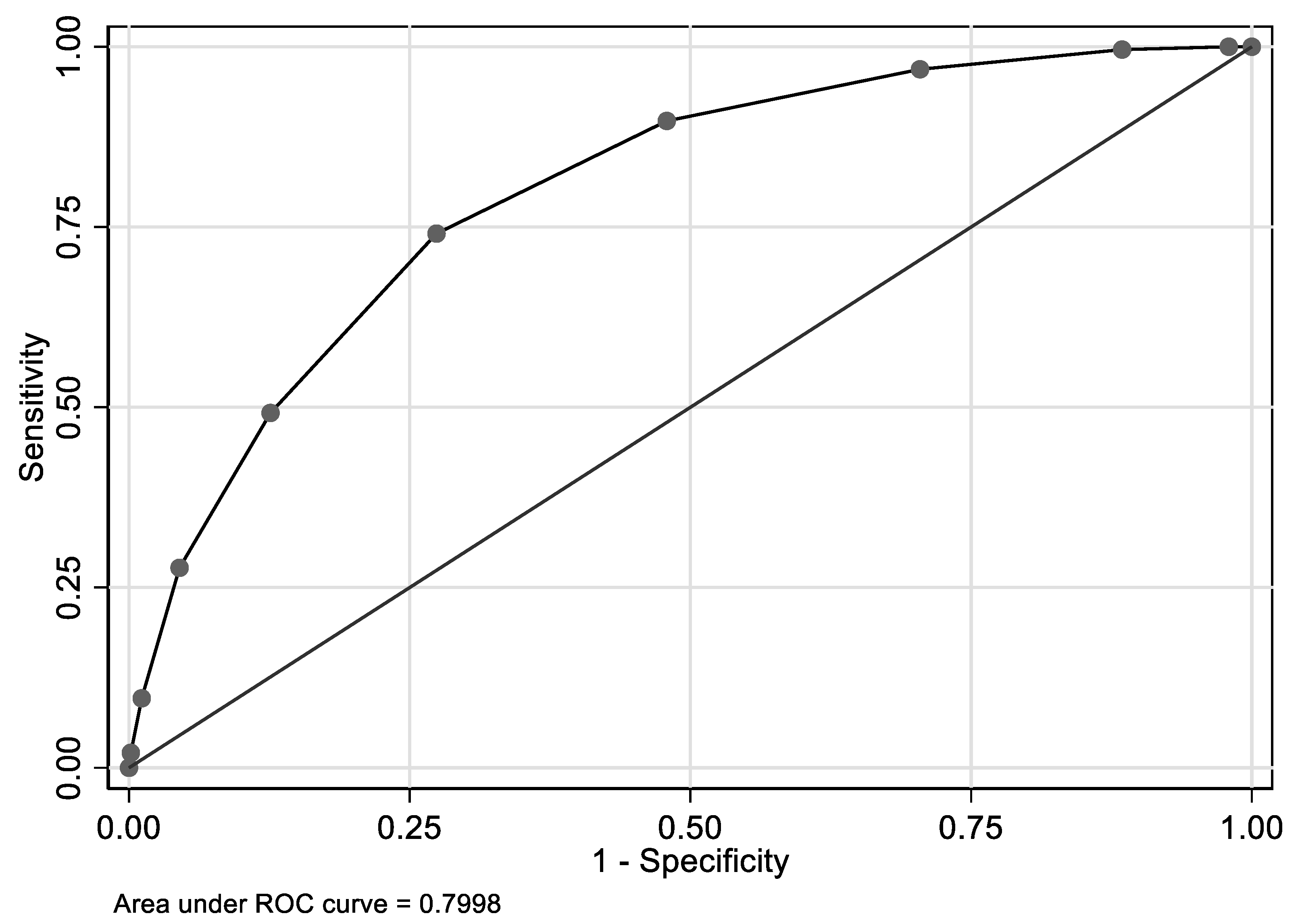
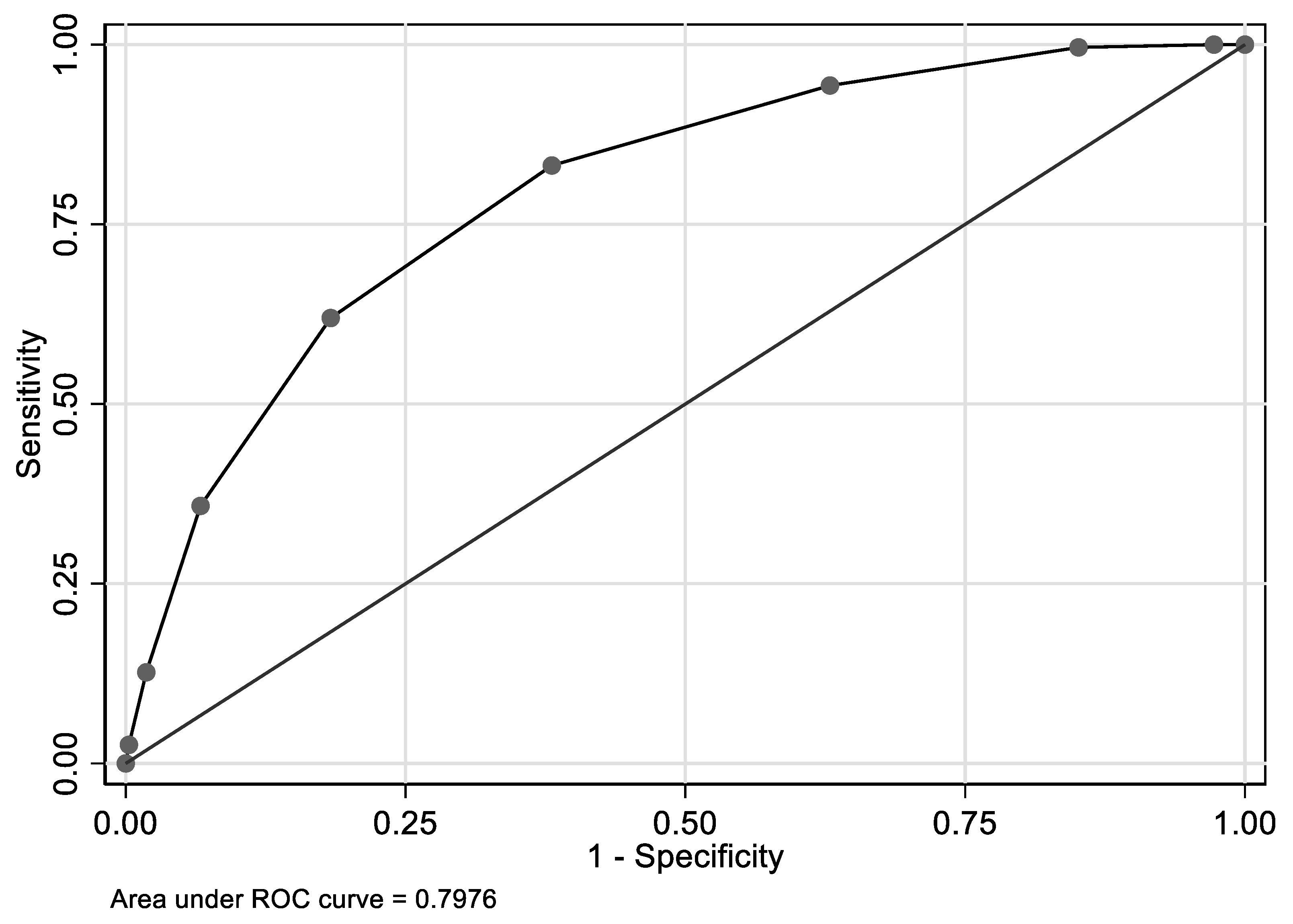
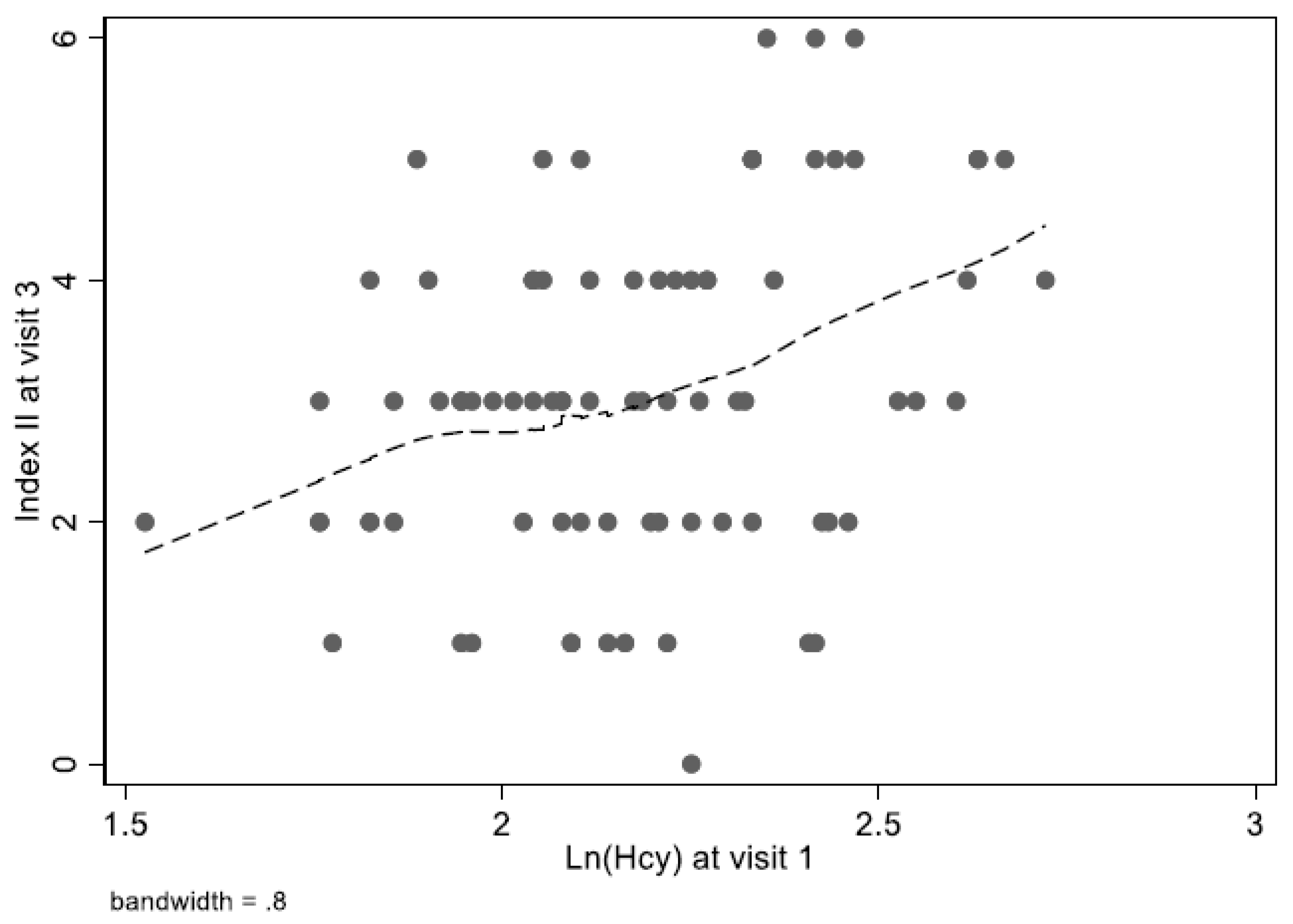
| Overall | Hcy ≤ 14 µmol/L | Hcy > 14 µmol/L | PHcy | ||||||||||
| n | Mean | % | SE | n | Mean | % | SE | n | Mean | % | SE | ||
| NHANES III, phase 2: 1991–94 | 4000 | 100.0 | 0.0 | 3663 | 91.5 | 0.7 | 337 | 8.4 | 0.7 | ||||
| Age (y) | 4000 | 44.3 | 0.42 | 3663 | 44.0 | 0.45 | 337 | 47.1 | 0.76 | 0.001 | |||
| Sex, % men | 4000 | 49.0 | 1.2 | 3663 | 47.8 | 1.2 | 62.8 | 4.9 | 0.008 | ||||
| Race/ethnicity | 4000 | 3663 | 337 | 0.51 | |||||||||
| NH white | 74.8 | 2.1 | 74.6 | 2.2 | 75.8 | 3.7 | |||||||
| NH black | 11.1 | 1.0 | 10.9 | 1.1 | 13.0 | 1.9 | |||||||
| MA | 5.2 | 0.7 | 5.4 | 0.7 | 3.7 | 0.7 | |||||||
| Other | 8.8 | 1.6 | 9.0 | 1.6 | 7.6 | 2.6 | |||||||
| Poverty status | |||||||||||||
| PIR ≥ 125% | 3714 | 14.0 | 1.9 | 3388 | 14.0 | 1.9 | 326 | 14.6 | 2.3 | 0.79 | |||
| PIR < 125% | |||||||||||||
| Region | 4000 | 18.9 | 2.2 | 3663 | 18.4 | 2.5 | 337 | 23.8 | 4.8 | 0.30 | |||
| Northeast | 22.6 | 4.4 | 22.8 | 4.5 | 20.7 | 4.4 | |||||||
| Midwest | 36.0 | 7.3 | 35.8 | 7.4 | 36.9 | 7.6 | |||||||
| South | 22.6 | 7.5 | 22.9 | 7.6 | 18.3 | 6.9 | |||||||
| West | |||||||||||||
| Urban/Rural | 4000 | 3663 | 337 | 0.59 | |||||||||
| Urban | 50.5 | 7.5 | 50.7 | 7.6 | 48.2 | 8.2 | |||||||
| Rural | 49.6 | 7.5 | 49.3 | 7.6 | 51.8 | 8.2 | |||||||
| Hcy, Loge | 4000 | +2.17 | 0.01 | 3663 | +2.10 | 0.01 | 337 | +2.93 | 0.04 | <0.001 | |||
| Selected biochemical and hematological indices, Loge | Mean, Loge | Mean, exp | SE, Loge | Mean, Loge | Mean, exp | SE, Loge | Mean, Loge | Mean, exp | SE, Loge | ||||
| Serum cotinine, ng/mL | 3966 | +0.33 | 1.39 | 0.12 | 3630 | +0.17 | 1.190 | 0.11 | 336 | +2.08 | 8.0 | 0.27 | <0.001 |
| Serum vitamin D, nmol/L | 3997 | +4.21 | 67.4 | 0.02 | 3660 | +4.21 | 67.40 | 0.02 | 337 | +4.11 | 60.9 | 0.04 | 0.015 |
| Serum thyroxine, nmol/L | 3997 | +4.7 | 109.9 | 0.01 | 3660 | +4.70 | 109.9 | 0.01 | 337 | +4.63 | 102.5 | 0.03 | 0.12 |
| Serum TSH, mU/L | 3925 | +0.42 | 1.52 | 0.03 | 3594 | +0.42 | 1.522 | 0.03 | 331 | +0.41 | 1.506 | 0.06 | 0.94 |
| Serum antimicrosomal Ab, U/mL | 3927 | −0.61 | 0.54 | 0.06 | 3596 | −0.61 | 0.543 | 0.06 | 331 | −0.55 | 0.576 | 0.18 | 0.73 |
| Serum anti-thyroglobulin Ab, U/mL | 3927 | −0.06 | 0.94 | 0.04 | 3596 | −0.06 | 0.942 | 0.04 | 331 | −0.03 | 0.970 | 0.09 | 0.76 |
| White blood cell count | 3998 | +1.94 | 6.96 | 0.01 | 3661 | +1.93 | 6.890 | 0.01 | 337 | +2.01 | 7.463 | 0.03 | 0.015 |
| Lymphocyte percent | 3998 | +3.46 | 31.81 | 0.01 | 3661 | +3.47 | 32.14 | 0.01 | 337 | +3.40 | 29.96 | 0.03 | 0.026 |
| Mononuclear percent | 3920 | +1.67 | 5.310 | 0.04 | 3584 | +1.67 | 5.312 | 0.04 | 336 | +1.69 | 5.419 | 0.05 | 0.77 |
| Granulocyte percent | 3920 | +4.10 | 60.34 | 0.01 | 3584 | +4.10 | 60.34 | 0.01 | 336 | +4.13 | 62.17 | 0.02 | 0.12 |
| Lymphocyte number | 3998 | +0.80 | 2.23 | 0.01 | 3661 | +0.79 | 2.20 | 0.01 | 337 | +0.81 | 2.247 | 0.03 | 0.67 |
| Mononuclear number | 3905 | −0.99 | 0.370 | 0.03 | 3572 | −1.00 | 0.368 | 0.03 | 333 | −0.91 | 0.402 | 0.05 | 0.039 |
| Granulocyte number | 3920 | +1.43 | 4.18 | 0.02 | 3584 | +1.43 | 4.18 | 0.02 | 336 | +1.53 | 4.618 | 0.03 | 0.017 |
| Red blood cell count, SI | 3997 | +1.55 | 4.71 | 0.00 | 3660 | +1.55 | 4.71 | 0.00 | 337 | +1.55 | 4.711 | 0.00 | 0.74 |
| Hemoglobin, g/L | 3998 | +4.96 | 142.5 | 0.00 | 3661 | +4.95 | 141.2 | 0.00 | 337 | +4.97 | 144.0 | 0.01 | 0.001 |
| Hematocrit, L/L = 1 | 3997 | −0.87 | 0.420 | 0.00 | 3660 | −0.87 | 0.419 | 0.00 | 337 | −0.85 | 0.427 | 0.01 | 0.001 |
| Mean cell volume, fL | 3998 | +4.49 | 89.12 | 0.00 | 3661 | +4.49 | 89.12 | 0.00 | 337 | +4.51 | 90.92 | 0.00 | <0.001 |
| Mean cell hemoglobin, pg | 3997 | +3.41 | 30.3 | 0.00 | 3660 | +3.41 | 30.27 | 0.00 | 337 | +3.43 | 30.87 | 0.00 | <0.001 |
| Mean cell hemoglobin conc., SI | 3997 | +5.82 | 337.0 | 0.00 | 3660 | +5.82 | 337.0 | 0.00 | 337 | +5.83 | 340.3 | 0.00 | 0.30 |
| Red cell distribution width, % | 3998 | −2.05 | 0.130 | 0.00 | 3661 | −2.05 | 0.129 | 0.00 | 337 | −2.02 | 0.132 | 0.00 | <0.001 |
| Platelet count: SI | 3998 | +5.54 | 254.7 | 0.01 | 3661 | +5.53 | 252.1 | 0.01 | 337 | +5.54 | 254.7 | 0.02 | 0.87 |
| Platelet distribution width, % | 3973 | +2.81 | 16.61 | 0.00 | 3640 | +2.80 | 16.44 | 0.00 | 333 | +2.80 | 16.44 | 0.00 | 0.94 |
| Mean platelet volume, fL | 3997 | +2.13 | 8.41 | 0.00 | 3660 | +2.13 | 8.41 | 0.00 | 337 | +2.12 | 8.331 | 0.01 | 0.30 |
| Lead, µmol/L | 3999 | −2.13 | 0.118 | 0.03 | 3662 | −2.15 | 0.116 | 0.04 | 337 | −1.82 | 0.162 | 0.06 | <0.001 |
| Erythrocyte protoporphyrin, SI | 3999 | −0.19 | 0.83 | 0.01 | 3662 | −0.18 | 0.84 | 0.01 | 337 | −0.25 | 0.779 | 0.03 | 0.029 |
| Serum iron, µmol/L | 4000 | +2.71 | 15.02 | 0.01 | 3663 | +2.71 | 15.03 | 0.01 | 337 | +2.73 | 15.33 | 0.04 | 0.67 |
| Serum TIBC, µmol/L | 3997 | +4.16 | 64.07 | 0.01 | 3660 | +4.15 | 63.43 | 0.01 | 337 | +4.18 | 65.37 | 0.01 | 0.067 |
| Serum ferritin, µmol/L | 3998 | +4.43 | 83.93 | 0.03 | 3661 | +4.41 | 82.27 | 0.03 | 337 | +4.55 | 94.63 | 0.09 | 0.14 |
| Serum folate, nmol/L | 4000 | +2.57 | 13.07 | 0.04 | 3663 | +2.61 | 13.60 | 0.04 | 337 | +2.03 | 7.614 | 0.07 | <0.001 |
| RBC folate, nmol/L | 3952 | +6.03 | 415.7 | 0.03 | 3615 | +6.05 | 424.1 | 0.02 | 337 | +5.73 | 307.9 | 0.06 | <0.001 |
| Serum vitamin B-12, pmol/L | 3999 | +5.79 | 327.0 | 0.01 | 3662 | +5.81 | 333.6 | 0.01 | 337 | +5.52 | 249.6 | 0.04 | <0.001 |
| Serum vitamin C, nmol/L | 3841 | +3.50 | 33.11 | 0.04 | 3510 | +3.54 | 34.47 | 0.04 | 331 | +3.11 | 22.42 | 0.09 | <0.001 |
| Serum normalized calcium, mmol/L | 3709 | +0.21 | 1.233 | 0.00 | 3410 | +0.21 | 1.234 | 0.00 | 308 | +0.21 | 1.234 | 0.00 | 0.55 |
| Serum total calcium, nmol/L | 3993 | +0.84 | 2.316 | 0.00 | 3657 | +0.84 | 2.316 | 0.00 | 336 | +0.84 | 2.314 | 0.00 | 0.029 |
| Serum selenium, nmol/L | 3977 | +0.47 | 1.599 | 0.01 | 3642 | +0.47 | 1.600 | 0.01 | 335 | +0.51 | 1.665 | 0.02 | 0.004 |
| Serum vitamin A, µmol/L | 3993 | +0.66 | 1.934 | 0.01 | 3656 | +0.66 | 1.935 | 0.01 | 337 | +0.67 | 1.954 | 0.02 | 0.57 |
| Serum vitamin E, µmol/L | 3993 | +3.26 | 26.05 | 0.01 | 3656 | +3.27 | 26.31 | 0.01 | 337 | +3.16 | 23.571 | 0.02 | <0.001 |
| Serum alpha carotene, µmol/L | 3993 | −2.62 | 0.073 | 0.028 | 3622 | −2.60 | 0.074 | 0.030 | 322 | −2.87 | 0.057 | 0.08 | 0.003 |
| Serum beta carotene, µmol/L | 3991 | −1.24 | 0.289 | 0.02 | 3656 | −1.22 | 0.295 | 0.02 | 335 | −1.56 | 0.210 | 0.07 | <0.001 |
| Serum beta-cryptoxanthin, µmol/L | 3991 | −1.94 | 0.143 | 0.02 | 3655 | −1.92 | 0.147 | 0.02 | 336 | −2.17 | 0.114 | 0.05 | <0.001 |
| Serum lutein/zeaxanthin, µmol/L | 3992 | −1.07 | 0.343 | 0.01 | 3656 | −1.07 | 0.343 | 0.01 | 336 | −1.15 | 0.317 | 0.03 | 0.003 |
| Serum lycopene, µmol/L | 3990 | −0.91 | 0.402 | 0.02 | 3655 | −0.90 | 0.407 | 0.02 | 335 | −1.00 | 0.368 | 0.03 | 0.012 |
| Serum retinyl esters, µmol/L | 3974 | −1.78 | 0.169 | 0.02 | 3641 | −1.75 | 0.174 | 0.02 | 333 | −2.09 | 0.124 | 0.05 | <0.001 |
| Serum cholesterol, mmol/L | 3994 | +1.65 | 5.206 | 0.01 | 3657 | +1.65 | 5.207 | 0.01 | 337 | +1.66 | 5.259 | 0.02 | 0.56 |
| Serum triglycerides, mmol/L | 3994 | +0.33 | 1.391 | 0.02 | 3657 | +0.32 | 1.377 | 0.02 | 337 | +0.39 | 1.476 | 0.05 | 0.30 |
| Serum HDL-cholesterol, mmol/L | 3972 | +0.20 | 1.221 | 0.01 | 3640 | +0.21 | 1.234 | 0.01 | 332 | +0.17 | 1.185 | 0.02 | 0.12 |
| Serum C-reactive protein, mg/dL | 3983 | −1.20 | 0.301 | 0.02 | 3646 | −1.21 | 0.298 | 0.02 | 337 | −1.19 | 0.304 | 0.04 | 0.65 |
| Serum hepatitis A Ab | 4000 | +0.47 | 1.600 | 0.01 | 3663 | +0.47 | 1.600 | 0.01 | 337 | +0.48 | 1.616 | 0.03 | 0.67 |
| Serum hepatitis B core Ab | 4000 | +0.65 | 1.915 | 0.00 | 3663 | +0.65 | 1.916 | 0.00 | 337 | +0.64 | 1.896 | 0.02 | 0.66 |
| Serum hepatitis C Ab | 4000 | +0.67 | 1.954 | 0.00 | 3663 | +0.68 | 1.974 | 0.00 | 337 | +0.67 | 1.954 | 0.01 | 0.46 |
| Serum rubella Ab, IU | 3885 | +4.31 | 74.44 | 0.04 | 3555 | +4.31 | 74.44 | 0.04 | 330 | +4.26 | 70.81 | 0.13 | 0.74 |
| Serum sodium, mmol/L | 3997 | +4.95 | 141.1 | 0.00 | 3641 | +4.94 | 139.77 | 0.00 | 336 | +4.95 | 141.1 | 0.00 | 0.81 |
| Serum potassium, mmol/L | 3977 | +1.41 | 4.095 | 0.00 | 3641 | +1.41 | 4.096 | 0.00 | 336 | +1.40 | 4.055 | 0.01 | 0.30 |
| Serum chloride, mmol/L | 3977 | +4.64 | 103.5 | 0.00 | 3641 | +4.64 | 103.5 | 0.00 | 336 | +4.64 | 103.5 | 0.00 | 0.35 |
| Serum bicarbonate, mmol/L | 4000 | +3.30 | 27.11 | 0.01 | 3663 | +3.30 | 27.11 | 0.01 | 337 | +3.30 | 27.11 | 0.02 | 0.91 |
| Serum total calcium, mmol/L | 3977 | +0.83 | 2.293 | 0.00 | 3641 | +0.83 | 2.290 | 0.00 | 336 | +0.84 | 2.320 | 0.00 | 0.021 |
| Serum phosphorus, mmol/L | 3977 | +0.09 | 1.094 | 0.01 | 3641 | +0.08 | 1.083 | 0.01 | 336 | +0.10 | 1.105 | 0.01 | 0.10 |
| Serum uric acid, µmol/L | 3977 | +5.72 | 304.9 | 0.01 | 3641 | +5.71 | 301.87 | 0.01 | 336 | +5.82 | 336.97 | 0.02 | <0.001 |
| Serum glucose, mmol/L | 3974 | +1.68 | 5.366 | 0.01 | 3639 | +1.68 | 5.366 | 0.01 | 335 | +1.69 | 5.419 | 0.03 | 0.66 |
| Serum blood urea nitrogen, SI | 3977 | +1.55 | 4.711 | 0.01 | 3641 | +1.55 | 4.711 | 0.01 | 336 | +1.54 | 4.664 | 0.02 | 0.49 |
| Serum total bilirubin, µmol/L | 3977 | +2.25 | 9.487 | 0.02 | 3641 | +2.25 | 9.487 | 0.02 | 336 | +2.30 | 9.974 | 0.04 | 0.18 |
| Serum creatinine, µmol/L | 3977 | +4.52 | 91.83 | 0.00 | 3641 | +4.52 | 91.83 | 0.00 | 336 | +4.61 | 100.48 | 0.02 | <0.001 |
| Serum iron, µmol/L | 3977 | +2.66 | 14.29 | 0.01 | 3641 | +2.66 | 14.30 | 0.01 | 336 | +2.68 | 14.58 | 0.04 | 0.65 |
| Serum cholesterol, mmol/L | 3977 | +1.68 | 5.365 | 0.01 | 3641 | +1.68 | 5.366 | 0.01 | 336 | +1.69 | 5.419 | 0.02 | 0.40 |
| Serum triglycerides, mmol/L | 3977 | +0.29 | 1.336 | 0.02 | 3641 | +0.29 | 1.336 | 0.02 | 336 | +0.35 | 1.419 | 0.06 | 0.34 |
| Aspartate aminotransferase, U/L | 3977 | +3.02 | 20.49 | 0.01 | 3641 | +3.02 | 20.49 | 0.01 | 336 | +3.02 | 20.49 | 0.05 | 0.90 |
| Alanine aminotransferase, U/L | 3977 | +2.86 | 17.46 | 0.02 | 3641 | +2.87 | 17.63 | 0.02 | 336 | +2.73 | 15.33 | 0.07 | 0.058 |
| Gamma glutamyl transferase, U/L | 3976 | +3.17 | 23.80 | 0.02 | 3640 | +3.16 | 23.57 | 0.02 | 336 | +3.31 | 27.38 | 0.07 | 0.056 |
| Serum lactate dehydrogenase, U/L | 3976 | +5.10 | 164.0 | 0.01 | 3641 | +5.10 | 164.02 | 0.01 | 335 | +5.10 | 164.02 | 0.01 | 0.86 |
| Serum alkaline phosphatase, U/L | 3977 | +4.37 | 79.04 | 0.01 | 3641 | +4.36 | 78.26 | 0.01 | 336 | +4.52 | 91.83 | 0.02 | <0.001 |
| Serum total protein, g/L | 3977 | +4.29 | 72.96 | 0.00 | 3641 | +4.29 | 72.97 | 0.00 | 336 | +4.29 | 72.97 | 0.01 | 0.80 |
| Serum albumin, g/L | 3977 | +3.71 | 40.85 | 0.00 | 3641 | +3.71 | 40.85 | 0.00 | 336 | +3.72 | 41.26 | 0.01 | 0.049 |
| Serum globulin, g/L | 3977 | +3.46 | 31.81 | 0.01 | 3641 | +3.46 | 31.82 | 0.01 | 336 | +3.45 | 31.50 | 0.01 | 0.22 |
| Serum osmolality, mmol/kg | 3977 | +5.64 | 281.4 | 0.00 | 3641 | +5.64 | 281.46 | 0.00 | 336 | +5.64 | 281.46 | 0.00 | 0.68 |
| Glycated hemoglobin, % | 3995 | +1.68 | 5.365 | 0.01 | 3658 | +1.68 | 5.365 | 0.01 | 337 | +1.69 | 5.419 | 0.02 | 0.45 |
| Plasma glucose, mmol/L | 3996 | +1.67 | 5.312 | 0.01 | 3659 | +1.67 | 5.312 | 0.01 | 337 | +1.68 | 5.365 | 0.02 | 0.66 |
| Urinary cadmium, nmol/L | 3964 | +1.21 | 3.353 | 0.05 | 3637 | +1.19 | 3.287 | 0.05 | 327 | +1.47 | 4.349 | 0.10 | 0.003 |
| Urinary creatinine, mmol/L | 3960 | +2.18 | 8.846 | 0.02 | 3635 | +2.17 | 8.758 | 0.02 | 325 | +2.30 | 9.974 | 0.06 | 0.048 |
| Urinary albumin, µg/L | 3960 | +1.58 | 4.854 | 0.06 | 3635 | +1.55 | 4.711 | 0.06 | 325 | +1.91 | 6.753 | 0.16 | 0.041 |
| Urinary iodine, µg/L | 3956 | +2.54 | 12.67 | 0.05 | 3631 | +2.54 | 12.68 | 0.05 | 325 | +2.51 | 12.30 | 0.07 | 0.68 |
| n | Mean | % | SE | n | Mean | % | SE | n | Mean | % | SE | PHcy | |
| NHANES 1999–2006 | 10,151 | 100.0 | 0.0 | 9704 | 95.9 | 0.3 | 447 | 4.1 | 0.3 | ||||
| Age (y) | 10,151 | 45.8 | 0.20 | 9704 | 45.6 | 0.20 | 447 | 50.4 | 0.6 | <0.001 | |||
| Sex, % men | 10,151 | 48.6 | 0.4 | 9704 | 48.2 | 0.4 | 447 | 59.9 | 0.4 | 0.005 | |||
| Race/ethnicity | 7605 | 7260 | 0.50 | ||||||||||
| NH white | 73.0 | 2.1 | 73.1 | 2.0 | 345 | 72.1 | 4.1 | ||||||
| NH black | 10.9 | 1.1 | 10.7 | 1.0 | 16.5 | 2.5 | |||||||
| MA | 6.8 | 1.0 | 7.0 | 1.0 | 3.0 | 0.6 | |||||||
| Other | 9.2 | 1.1 | 9.3 | 1.1 | 8.4 | 2.6 | |||||||
| Poverty status | 9471 | 9060 | <0.001 | ||||||||||
| PIR ≥ 125% | 81.2 | 0.7 | 84.6 | 0.7 | 411 | 75.1 | 2.4 | ||||||
| PIR < 125% | 15.8 | 0.7 | 15.4 | 0.7 | 24.9 | 2.4 | |||||||
| Hcy, Loge | 10,151 | +2.08 | 0.01 | 9704 | +2.04 | 0.01 | 447 | +2.94 | 0.02 | <0.001 | |||
| n | Mean | % | SE | n | Mean | % | SE | n | Mean | % | SE | PHcy | |
| HANDLS 2004–2018 | 245 | 100.0 | 220 | 89.8 | 25 | 10.2 | |||||||
| Age (y) | 245 | 49.2 | 0.56 | 220 | 48.6 | 0.59 | 25 | 54.3 | 1.5 | 0.002 | |||
| Sex, % men | 245 | 51.0 | 220 | 48.6 | 25 | 72.0 | 0.032 | ||||||
| Race/ethnicity | 245 | 220 | 25 | ||||||||||
| Whites | 29.8 | 31.8 | 12.0 | 0.052 | |||||||||
| AA | 70.2 | 68.2 | 88.0 | ||||||||||
| Poverty status | 245 | 220 | 25 | 0.069 | |||||||||
| PIR ≥ 125% | 37.1 | 39.1 | 20.0 | ||||||||||
| PIR < 125% | 62.9 | 60.9 | 80.0 | ||||||||||
| Hcy, Loge | 245 | +2.26 | 0.02 | 220 | +2.19 | 0.02 | 25 | +2.90 | 0.05 | <0.001 | |||
| Elevated Homocysteine | |||
|---|---|---|---|
| OR | 95% CI | p-Value | |
| Model 1: Binary predictors, (n = 14,739) | |||
| Lower serum folate 2 | 3.49 | (2.63,4.63) | <0.001 |
| Higher serum creatinine 3 | 1.86 | (1.51,2.29) | <0.001 |
| Older age 4 | 1.95 | (1.56,2.44) | <0.001 |
| Lower serum vitamin B-12 5 | 2.52 | (1.98,3.21) | <0.001 |
| Higher MCH 6 | 1.60 | (1.28,2.02) | <0.001 |
| Higher RDW 7 | 1.24 | (1.01,1.54) | 0.044 |
| Higher SUA 8 | 1.67 | (1.35,2.06) | <0.001 |
| Higher alkaline phosphatase 9 | 1.71 | (1.35,2.15) | <0.001 |
| Higher serum cotinine 10 | 1.77 | (1.44,2.17) | <0.001 |
| Model 2: Index I ≥ 5 (n = 14,739) | 7.43 | (5.75,9.61) | <0.001 |
| Model 3: Index II ≥ 5, (n = 14,829) | 6.90 | (5.37,8.84) | <0.001 |
| Outcome | Intercept | Time | Hcy | Hcy × Time | (n) k | ||||
|---|---|---|---|---|---|---|---|---|---|
| γ00 ± SE | p | γ10 ± SE | p | γ0a ± SE | p | γ1a ± SE | p | ||
| Serum folate, nmol/L | |||||||||
| Model 1: Age-adjusted | +33.6 ± 1.2 | <0.001 | +0.11 ± 0.16 | 0.47 | −0.21 ± 0.32 | 0.51 | −0.05 ± 0.05 | 0.36 | (243) k = 2.4 |
| Model 2: Socio-demographic adjusted | +29.8 ± 6.0 | <0.001 | +2.07 ± 0.83 | 0.012 | −0.25 ± 0.34 | 0.45 | −0.05 ± 0.05 | 0.37 | (243) k = 2.4 |
| Model 3: Multivariable-adjusted | +35.1 ± 6.2 | <0.001 | +1.39 ± 0.87 | 0.11 | −1.41 ± 0.43 | 0.001 | +0.03 ± 0.07 | 0.69 | (227) k = 2.4 |
| Serum creatinine, µmol/L | |||||||||
| Model 1: Age-adjusted | +107.9 ± 7.1 | <0.001 | −1.18 ± 0.30 | <0.001 | +18.3 ± 1.89 | <0.001 | +0.11 ± 0.10 | 0.24 | (243) k = 2.4 |
| Model 2: Socio-demographic adjusted | +100.1 ± 37.1 | 0.007 | −3.40 ± 1.50 | 0.024 | +18.3 ± 2.00 | <0.001 | +0.14 ± 0.10 | 0.16 | (243) k = 2.4 |
| Model 3: Multivariable-adjusted | +117.7 ± 35.1 | 0.001 | −3.93 ± 1.60 | 0.014 | +19.8 ± 2.01 | <0.001 | +0.24 ± 0.12 | 0.045 | (227) k = 2.4 |
| Serum vitamin B-12, µmol/L | |||||||||
| Model 1: Age-adjusted | +472 ± 13 | <0.001 | −11.2 ± 1.6 | <0.001 | −4.64 ± 3.69 | 0.20 | +0.85 ± 0.53 | 0.11 | (243) k = 2.4 |
| Model 2: Socio-demographic adjusted | +334 ± 68 | <0.001 | +6.1 ± 8.6 | 0.48 | −5.19 ± 3.80 | 0.17 | +0.87 ± 0.55 | 0.11 | (243) k = 2.4 |
| Model 3: Multivariable-adjusted | +307 ± 69 | <0.001 | −8.0 ± 8.9 | 0.37 | −11.0 ± 4.9 | 0.023 | +1.66 ± 0.69 | 0.015 | (227) k = 2.4 |
| Mean cell hemoglobin, pg | |||||||||
| Model 1: Age-adjusted | +29.6 ± 0.2 | <0.001 | −0.003 ± 0.002 | 0.56 | +0.03 ± 0.05 | 0.53 | −0.003 ± 0.005 | 0.56 | (244) k = 2.4 |
| Model 2: Socio-demographic adjusted | +30.9 ± 0.9 | <0.001 | −0.04 ± 0.10 | 0.67 | +0.02 ± 0.05 | 0.72 | −0.004 ± 0.005 | 0.49 | (244) k = 2.4 |
| Model 3: Multivariable-adjusted | +29.2 ± 0.97 | <0.001 | +0.03 ± 0.10 | 0.78 | +0.04 ± 0.06 | 0.50 | +0.01 ± 0.01 | 0.42 | (227) k = 2.4 |
| Red cell distribution width, % | |||||||||
| Model 1: Age-adjusted | +13.8 ± 0.11 | <0.001 | +0.12 ± 0.01 | <0.001 | +0.05 ± 0.03 | 0.079 | +0.007 ± 0.003 | 0.035 | (244) k = 2.4 |
| Model 2: Socio-demographic adjusted | +12.7 ± 0.57 | <0.001 | +0.18 ± 0.06 | 0.002 | +0.04 ± 0.03 | 0.16 | +0.008 ± 0.004 | 0.021 | (244) k = 2.4 |
| Model 3: Multivariable-adjusted | +12.7 ± 0.5 | <0.001 | +0.19 ± 0.06 | 0.002 | −0.04 ± 0.04 | 0.20 | +0.009 ± 0.005 | 0.056 | (227) k = 2.4 |
| Serum uric acid, µmol/L | |||||||||
| Model 1: Age-adjusted | +314.8 ± 5.3 | <0.001 | +3.79 ± 0.7 | <0.001 | +7.48 ± 1.45 | <0.001 | −0.20 ± 0.22 | 0.36 | (243) k = 2.4 |
| Model 2: Socio-demographic adjusted | +226.8 ± 26.7 | <0.001 | +2.80 ± 3.51 | 0.42 | +5.30 ± 1.48 | <0.001 | −0.12 ± 0.23 | 0.62 | (243) k = 2.4 |
| Model 3: Multivariable-adjusted | +228.6 ± 27.0 | <0.001 | +2.01 ± 3.61 | 0.58 | +8.11 ± 1.88 | <0.001 | −0.01 ± 0.29 | 0.97 | (227) k = 2.4 |
| Serum alkaline phosphatase, U/L | |||||||||
| Model 1: Age-adjusted | +90.8 ± 2.1 | <0.001 | −0.88 ± 0.22 | <0.001 | +2.66 ± 0.56 | <0.001 | −0.03 ± 0.07 | 0.70 | (243) k = 2.4 |
| Model 2: Socio-demographic adjusted | +70.7 ± 10.5 | <0.001 | −0.39 ± 1.19 | 0.74 | +2.54 ± 0.58 | <0.001 | −0.002 ± 0.08 | 0.98 | (243) k = 2.4 |
| Model 3: Multivariable-adjusted | +75.2 ± 10.7 | <0.001 | −0.27 ± 1.23 | 0.83 | +2.72 ± 0.74 | <0.001 | +0.16 ± 0.10 | 0.095 | (227) k = 2.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beydoun, M.A.; Beydoun, H.A.; MacIver, P.H.; Hossain, S.; Canas, J.A.; Evans, M.K.; Zonderman, A.B. Biochemical and Hematological Correlates of Elevated Homocysteine in National Surveys and a Longitudinal Study of Urban Adults. Nutrients 2020, 12, 950. https://doi.org/10.3390/nu12040950
Beydoun MA, Beydoun HA, MacIver PH, Hossain S, Canas JA, Evans MK, Zonderman AB. Biochemical and Hematological Correlates of Elevated Homocysteine in National Surveys and a Longitudinal Study of Urban Adults. Nutrients. 2020; 12(4):950. https://doi.org/10.3390/nu12040950
Chicago/Turabian StyleBeydoun, May A., Hind A. Beydoun, Peter H. MacIver, Sharmin Hossain, Jose A. Canas, Michele K. Evans, and Alan B. Zonderman. 2020. "Biochemical and Hematological Correlates of Elevated Homocysteine in National Surveys and a Longitudinal Study of Urban Adults" Nutrients 12, no. 4: 950. https://doi.org/10.3390/nu12040950
APA StyleBeydoun, M. A., Beydoun, H. A., MacIver, P. H., Hossain, S., Canas, J. A., Evans, M. K., & Zonderman, A. B. (2020). Biochemical and Hematological Correlates of Elevated Homocysteine in National Surveys and a Longitudinal Study of Urban Adults. Nutrients, 12(4), 950. https://doi.org/10.3390/nu12040950





