Oral Supplementation of Sodium Butyrate Attenuates the Progression of Non-Alcoholic Steatohepatitis
Abstract
1. Introduction
2. Materials and Methods
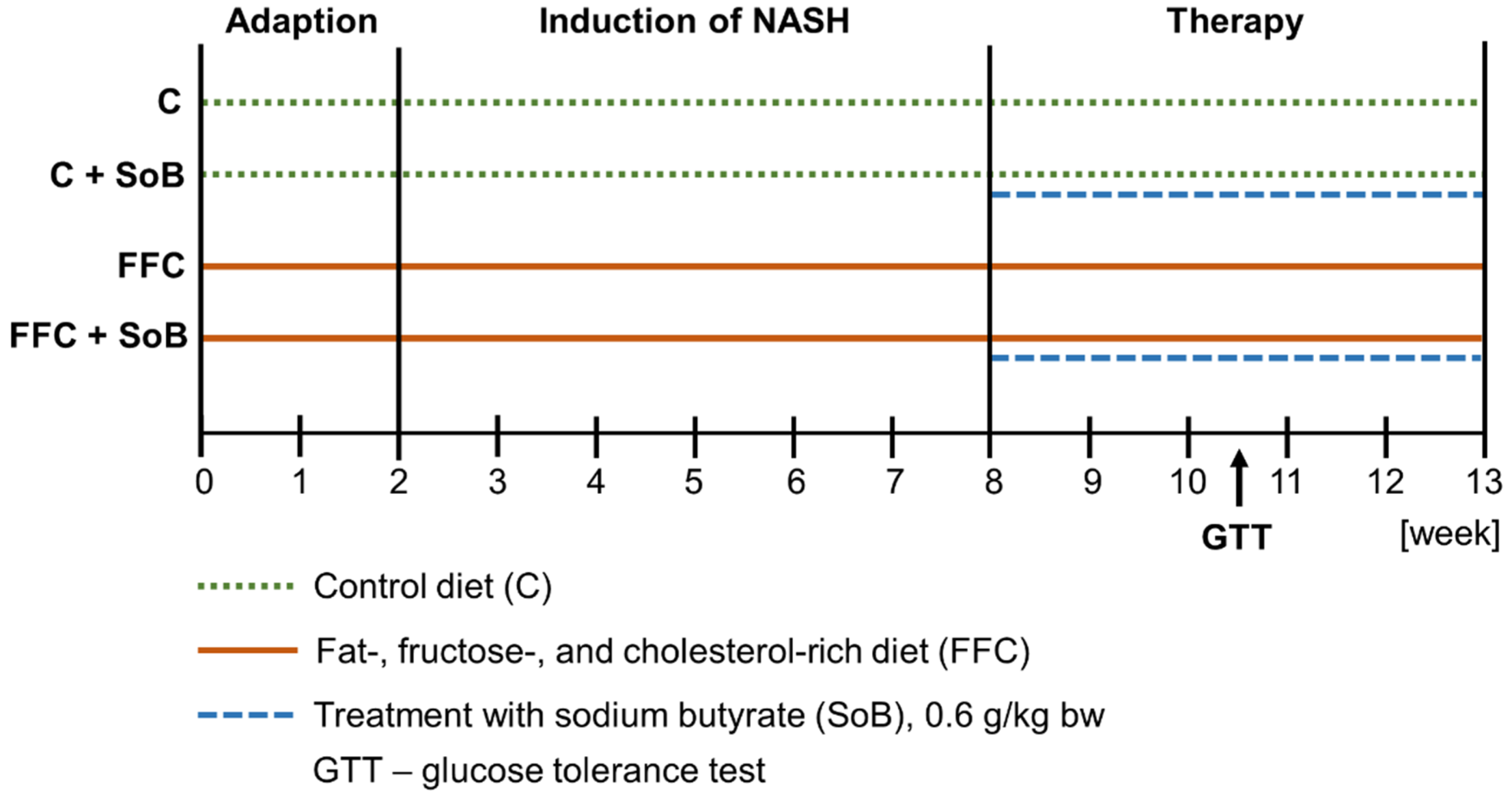
2.1. Animals and Treatments
2.2. Everted Gut Sac Model of Mice
2.3. Cell Culture
2.4. Histological Evaluation and Immunohistochemical Staining
2.5. Blood Parameters of Liver Damage
2.6. Endotoxin Assay
2.7. Griess Assay
2.8. RNA Isolation and Real-Time RT-PCR
2.9. ELISA and HDAC Enzymes Activity Assay
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
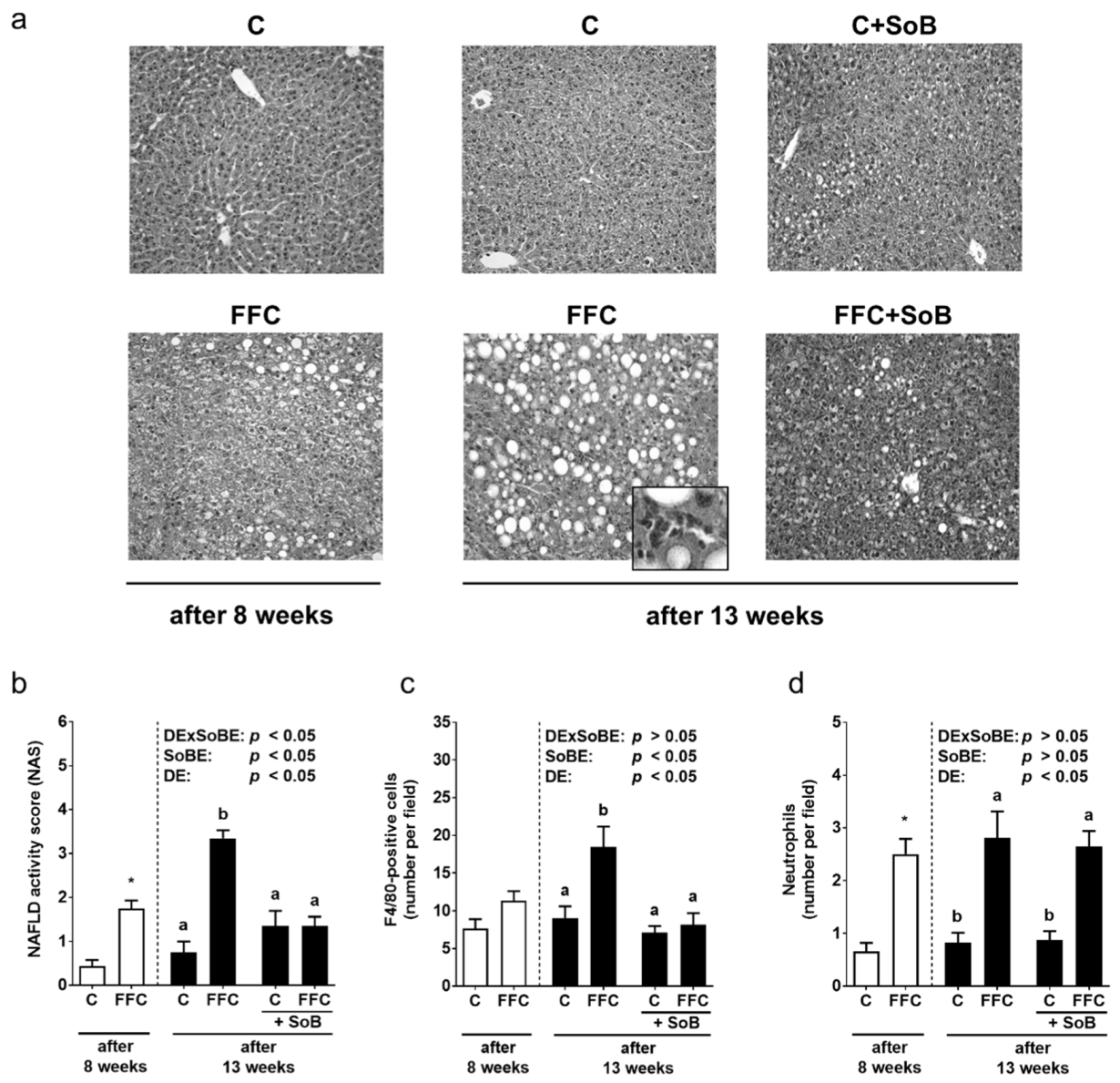
3.1. Body Weight and Markers of Liver Damage
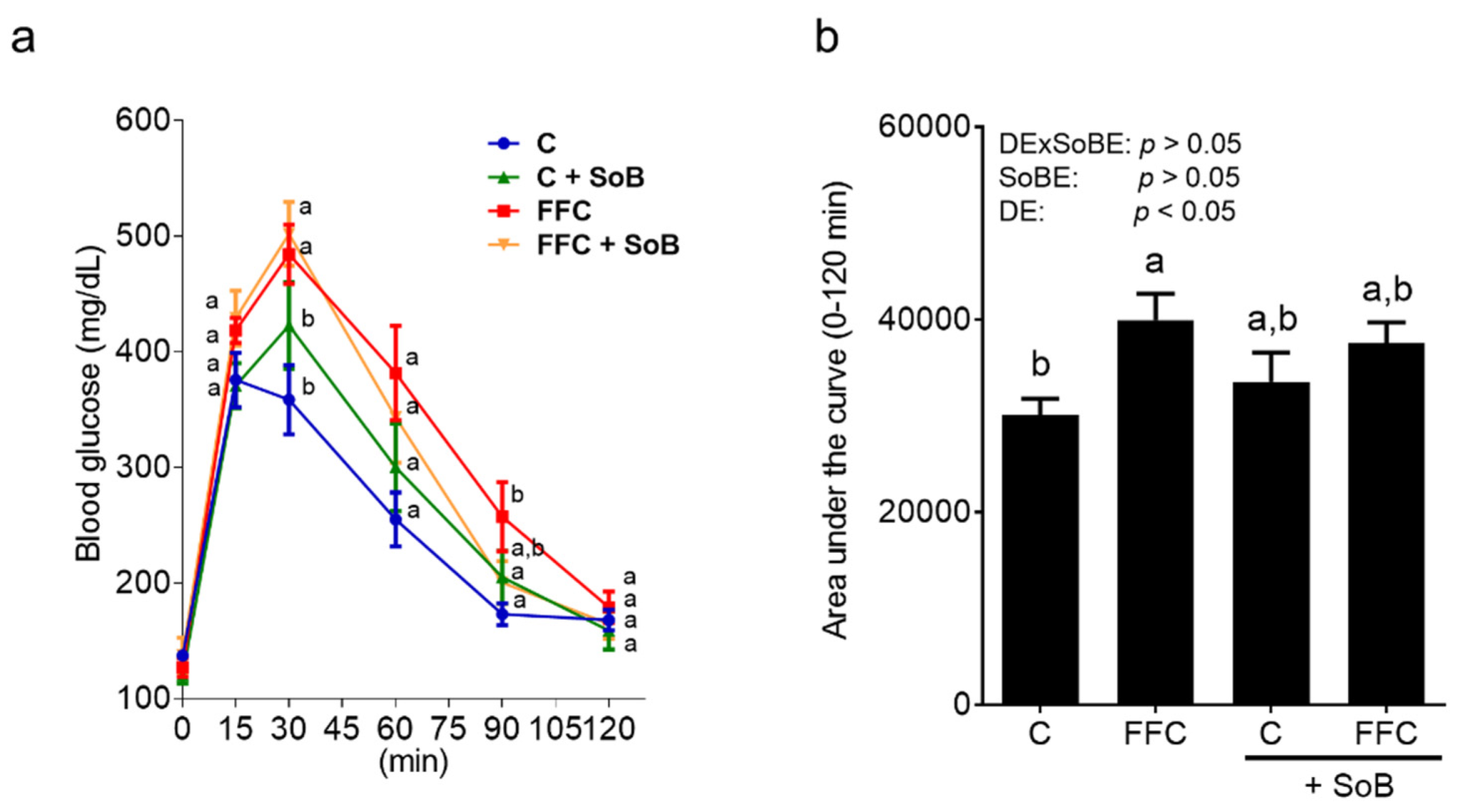
3.2. Parameters of Glucose Metabolism
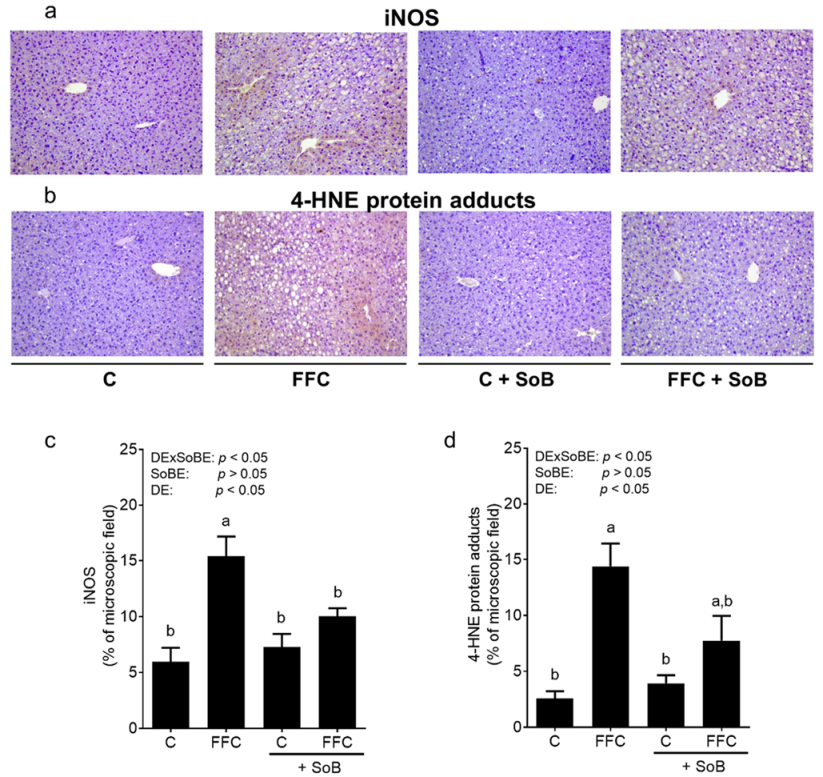
3.3. Markers of Lipid Peroxidation
3.4. Tight Junction Proteins, Portal Endotoxin and Tlr4-Dependent Signaling Pathway
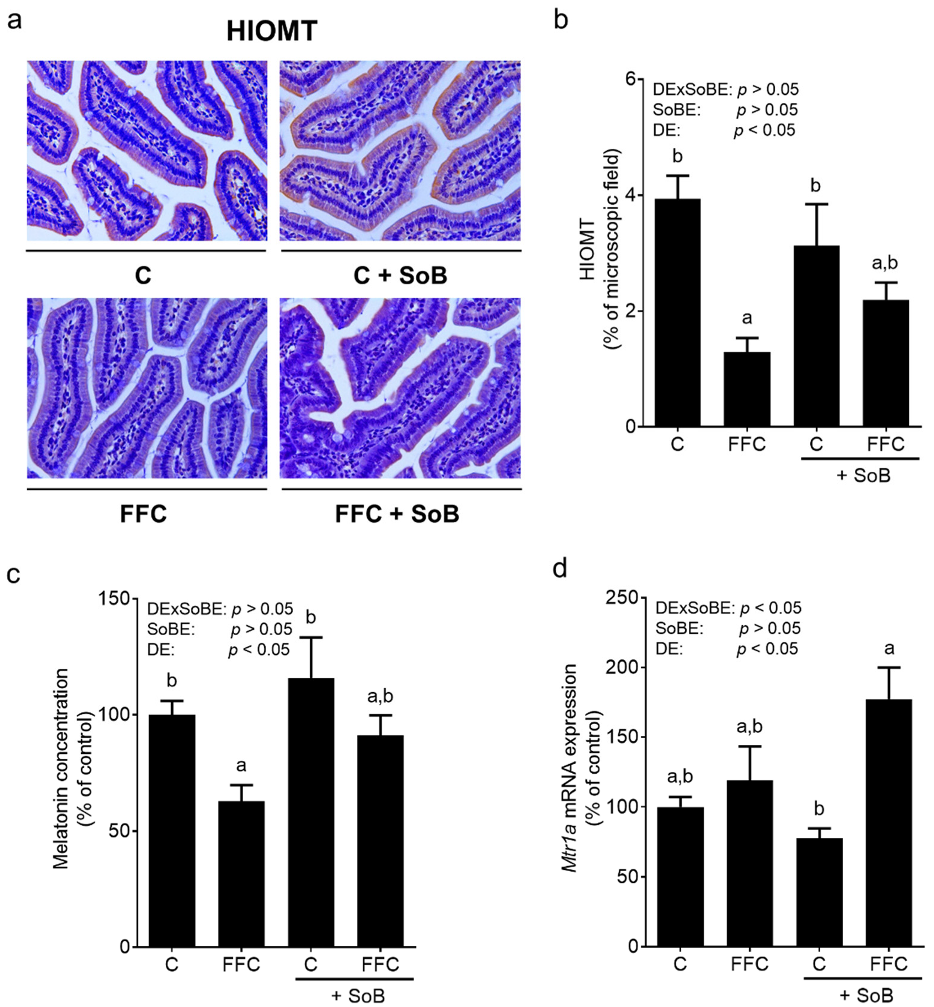
3.5. Intestinal Melatonin Metabolism and Melatonin Receptors in Liver Tissue
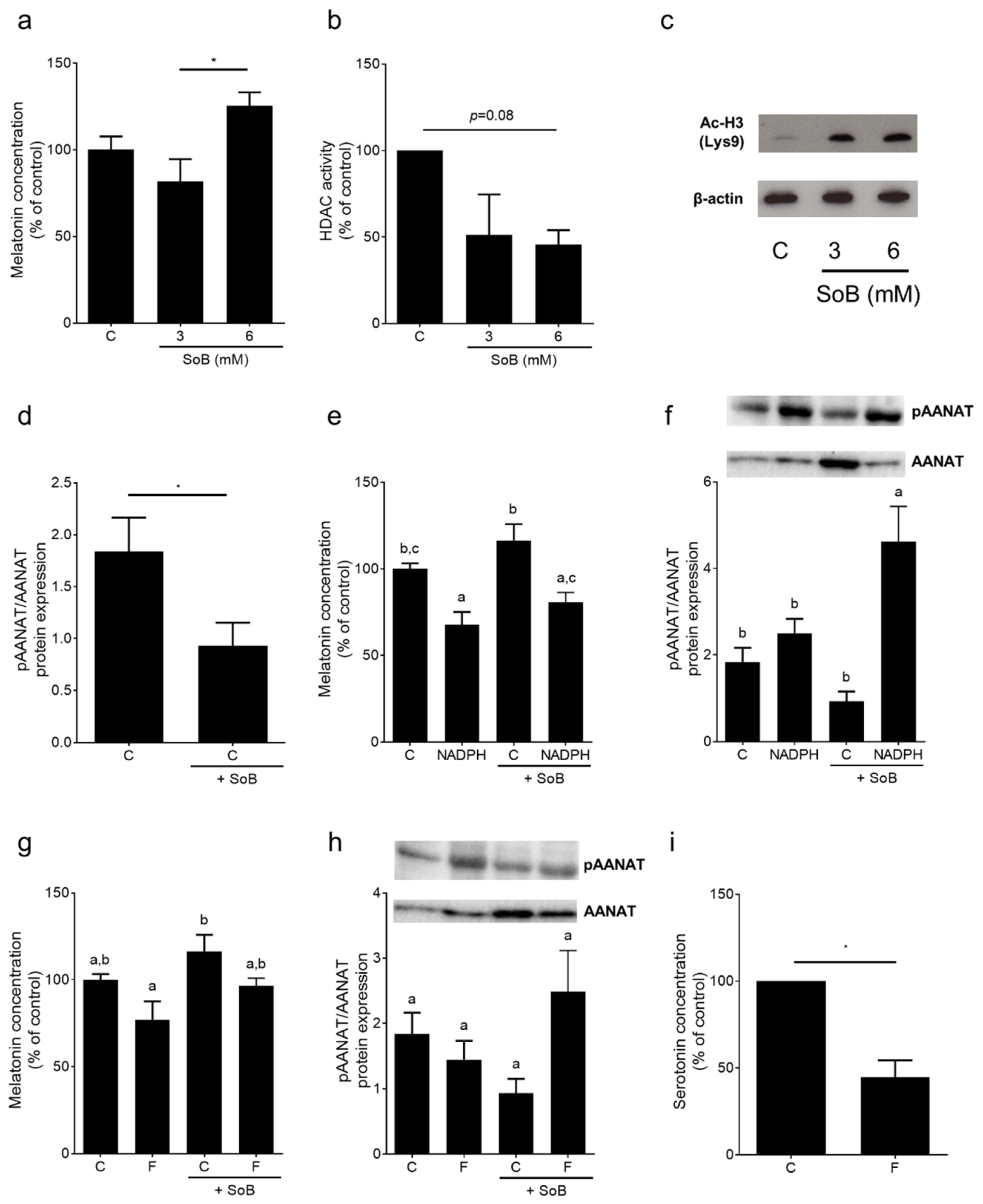
3.6. Melatonin Metabolism and Activity of Histone Deacetylases (HDAC) Enzymes in Small Intestinal Tissue: Ex Vivo Experiments Using an Everted Gut Sac Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Sass, D.A.; Chang, P.; Chopra, K.B. Nonalcoholic fatty liver disease: A clinical review. Dig. Dis. Sci. 2005, 50, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an aasld single topic conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef]
- Tsunoda, K.; Kai, Y.; Kitano, N.; Uchida, K.; Kuchiki, T.; Nagamatsu, T. Impact of physical activity on nonalcoholic steatohepatitis in people with nonalcoholic simple fatty liver: A prospective cohort study. Prev. Med. 2016, 88, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Edmison, J.; McCullough, A.J. Pathogenesis of non-alcoholic steatohepatitis: Human data. Clin. Liver Dis. 2007, 11, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.J.; Sellmann, C.; Engstler, A.J.; Ziegenhardt, D.; Bergheim, I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (nash). Br. J. Nutr. 2015, 114, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Brandl, K.; Schnabl, B. Intestinal microbiota and nonalcoholic steatohepatitis. Curr. Opin. Gastroenterol. 2017, 33, 128–133. [Google Scholar] [CrossRef]
- Kirpich, I.A.; Marsano, L.S.; McClain, C.J. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin. Biochem. 2015, 48, 923–930. [Google Scholar] [CrossRef]
- Zhu, J.Z.; Hollis-Hansen, K.; Wan, X.Y.; Fei, S.J.; Pang, X.L.; Meng, F.D.; Yu, C.H.; Li, Y.M. Clinical guidelines of non-alcoholic fatty liver disease: A systematic review. World J. Gastroenterol. 2016, 22, 8226–8233. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Zhou, D.; Fan, J.G. Microbial metabolites in non-alcoholic fatty liver disease. World J. Gastroenterol. 2019, 25, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Harig, J.M.; Soergel, K.H.; Komorowski, R.A.; Wood, C.M. Treatment of diversion colitis with short-chain-fatty acid irrigation. N. Engl. J. Med. 1989, 320, 23–28. [Google Scholar] [CrossRef]
- Cleophas, M.C.P.; Ratter, J.M.; Bekkering, S.; Quintin, J.; Schraa, K.; Stroes, E.S.; Netea, M.G.; Joosten, L.A.B. Effects of oral butyrate supplementation on inflammatory potential of circulating peripheral blood mononuclear cells in healthy and obese males. Sci. Rep. 2019, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Jia, Y.; Hong, J.; Sun, Q.; Gao, S.; Hu, Y.; Zhao, N.; Zhao, R. Sodium butyrate ameliorates high-fat-diet-induced non-alcoholic fatty liver disease through peroxisome proliferator-activated receptor alpha-mediated activation of beta oxidation and suppression of inflammation. J. Agric. Food Chem. 2018, 66, 7633–7642. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Lv, L.; Wu, W.; Li, Y.; Shi, D.; Fang, D.; Guo, F.; Jiang, H.; Yan, R.; Ye, W.; et al. Butyrate protects mice against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels. Front. Microbiol. 2018, 9, 1967. [Google Scholar] [CrossRef]
- Sheng, L.; Jena, P.K.; Hu, Y.; Liu, H.X.; Nagar, N.; Kalanetra, K.M.; French, S.W.; French, S.W.; Mills, D.A.; Wan, Y.Y. Hepatic inflammation caused by dysregulated bile acid synthesis is reversible by butyrate supplementation. J. Pathol. 2017, 243, 431–441. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, C.; Zhang, Y.; Deng, Y.; Liu, C.; Yang, Q. Probiotic mixture of lactobacillus and bifidobacterium alleviates systemic adiposity and inflammation in non-alcoholic fatty liver disease rats through gpr109a and the commensal metabolite butyrate. Inflammopharmacology 2018, 26, 1051–1055. [Google Scholar] [CrossRef]
- Jin, C.J.; Engstler, A.J.; Sellmann, C.; Ziegenhardt, D.; Landmann, M.; Kanuri, G.; Lounis, H.; Schroder, M.; Vetter, W.; Bergheim, I. Sodium butyrate protects mice from the development of the early signs of non-alcoholic fatty liver disease: Role of melatonin and lipid peroxidation. Br. J. Nutr. 2016, 116, 1682–1693. [Google Scholar] [CrossRef]
- Spruss, A.; Henkel, J.; Kanuri, G.; Blank, D.; Puschel, G.P.; Bischoff, S.C.; Bergheim, I. Female mice are more susceptible to nonalcoholic fatty liver disease: Sex-specific regulation of the hepatic amp-activated protein kinase-plasminogen activator inhibitor 1 cascade, but not the hepatic endotoxin response. Mol. Med. 2012, 18, 1346–1355. [Google Scholar] [CrossRef]
- Marin, V.; Rosso, N.; Dal Ben, M.; Raseni, A.; Boschelle, M.; Degrassi, C.; Nemeckova, I.; Nachtigal, P.; Avellini, C.; Tiribelli, C.; et al. An animal model for the juvenile non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. PLoS ONE 2016, 11, e0158817. [Google Scholar] [CrossRef] [PubMed]
- Sellmann, C.; Baumann, A.; Brandt, A.; Jin, C.J.; Nier, A.; Bergheim, I. Oral supplementation of glutamine attenuates the progression of nonalcoholic steatohepatitis in c57bl/6j mice. J. Nutr. 2017, 147, 2041–2049. [Google Scholar] [CrossRef]
- Hamilton, K.L.; Butt, A.G. Glucose transport into everted sacs of the small intestine of mice. Adv. Physiol. Educ. 2013, 37, 415–426. [Google Scholar] [CrossRef]
- Sellmann, C.; Priebs, J.; Landmann, M.; Degen, C.; Engstler, A.J.; Jin, C.J.; Garttner, S.; Spruss, A.; Huber, O.; Bergheim, I. Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J. Nutr. Biochem. 2015, 26, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, I.; Guo, L.; Davis, M.A.; Duveau, I.; Arteel, G.E. Critical role of plasminogen activator inhibitor-1 in cholestatic liver injury and fibrosis. J. Pharmacol. Exp. Ther. 2006, 316, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Spruss, A.; Kanuri, G.; Uebel, K.; Bischoff, S.C.; Bergheim, I. Role of the inducible nitric oxide synthase in the onset of fructose-induced steatosis in mice. Antioxid. Redox. Signal. 2011, 14, 2121–2135. [Google Scholar] [CrossRef]
- Konturek, S.J.; Konturek, P.C.; Brzozowski, T.; Bubenik, G.A. Role of melatonin in upper gastrointestinal tract. J. Physiol. Pharmacol. 2007, 58 (Suppl. S6), 23–52. [Google Scholar]
- Vogelauer, M.; Krall, A.S.; McBrian, M.A.; Li, J.Y.; Kurdistani, S.K. Stimulation of histone deacetylase activity by metabolites of intermediary metabolism. J. Biol. Chem. 2012, 287, 32006–32016. [Google Scholar] [CrossRef]
- Vriend, J.; Liu, W.; Reiter, R.J. The pineal gland: A model for adrenergic modulation of ubiquitin ligases. PLoS ONE 2017, 12, e0172441. [Google Scholar] [CrossRef]
- Tosello-Trampont, A.C.; Landes, S.G.; Nguyen, V.; Novobrantseva, T.I.; Hahn, Y.S. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J. Biol. Chem. 2012, 287, 40161–40172. [Google Scholar] [CrossRef]
- Ye, D.; Yang, K.; Zang, S.; Lin, Z.; Chau, H.T.; Wang, Y.; Zhang, J.; Shi, J.; Xu, A.; Lin, S.; et al. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil-macrophage crosstalk via the induction of cxcr2. J. Hepatol. 2016, 65, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Mridha, A.R.; Haczeyni, F.; Yeh, M.M.; Haigh, W.G.; Ioannou, G.N.; Barn, V.; Ajamieh, H.; Adams, L.; Hamdorf, J.M.; Teoh, N.C.; et al. Tlr9 is up-regulated in human and murine nash: Pivotal role in inflammatory recruitment and cell survival. Clin. Sci. (Lond.) 2017, 131, 2145–2159. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.C.; da Silva, J.F.; Navia-Pelaez, J.M.; Leonel, A.J.; Lopes, L.G.; Menezes-Garcia, Z.; Ferreira, A.V.M.; Capettini, L.; Teixeira, L.G.; Lemos, V.S.; et al. Sodium butyrate modulates adipocyte expansion, adipogenesis, and insulin receptor signaling by upregulation of ppar-gamma in obese apo e knockout mice. Nutrition 2018, 47, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Matheus, V.A.; Monteiro, L.; Oliveira, R.B.; Maschio, D.A.; Collares-Buzato, C.B. Butyrate reduces high-fat diet-induced metabolic alterations, hepatic steatosis and pancreatic beta cell and intestinal barrier dysfunctions in prediabetic mice. Exp. Biol. Med. (Maywood) 2017, 242, 1214–1226. [Google Scholar] [CrossRef]
- Li, H.P.; Chen, X.; Li, M.Q. Butyrate alleviates metabolic impairments and protects pancreatic beta cell function in pregnant mice with obesity. Int. J. Clin. Exp. Pathol. 2013, 6, 1574–1584. [Google Scholar]
- Kanuri, G.; Ladurner, R.; Skibovskaya, J.; Spruss, A.; Konigsrainer, A.; Bischoff, S.C.; Bergheim, I. Expression of toll-like receptors 1–5 but not tlr 6–10 is elevated in livers of patients with non-alcoholic fatty liver disease. Liver Int. 2015, 35, 562–568. [Google Scholar] [CrossRef]
- Mattace Raso, G.; Simeoli, R.; Russo, R.; Iacono, A.; Santoro, A.; Paciello, O.; Ferrante, M.C.; Canani, R.B.; Calignano, A.; Meli, R. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS ONE 2013, 8, e68626. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Laerke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Correa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Wagnerberger, S.; Spruss, A.; Kanuri, G.; Volynets, V.; Stahl, C.; Bischoff, S.C.; Bergheim, I. Toll-like receptors 1-9 are elevated in livers with fructose-induced hepatic steatosis. Br. J. Nutr. 2012, 107, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Barker, J. Diverse pro-inflammatory endotoxin recognition systems of mammalian innate immunity. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Iimuro, Y.; Frankenberg, M.V.; Arteel, G.E.; Bradford, B.U.; Wall, C.A.; Thurman, R.G. Female rats exhibit greater susceptibility to early alcohol-induced liver injury than males. Am. J. Physiol. 1997, 272, G1186–G1194. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.H.; Lopez, G.-C.; Lynch, H.J.; Wurtman, R.J. Melatonin and its precursors in y79 human retinoblastoma cells: Effect of sodium butyrate. Brain Res. 1991, 561, 274–278. [Google Scholar] [CrossRef]
- Wiechmann, A.F.; Burden, M.A. Regulation of aa-nat and hiomt gene expression by butyrate and cyclic amp in y79 human retinoblastoma cells. J. Pineal Res. 1999, 27, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Haub, S.; Ritze, Y.; Ladel, I.; Saum, K.; Hubert, A.; Spruss, A.; Trautwein, C.; Bischoff, S.C. Serotonin receptor type 3 antagonists improve obesity-associated fatty liver disease in mice. J. Pharmacol. Exp. Ther. 2011, 339, 790–798. [Google Scholar] [CrossRef]
- Hatzis, G.; Ziakas, P.; Kavantzas, N.; Triantafyllou, A.; Sigalas, P.; Andreadou, I.; Ioannidis, K.; Chatzis, S.; Filis, K.; Papalampros, A.; et al. Melatonin attenuates high fat diet-induced fatty liver disease in rats. World J. Hepatol. 2013, 5, 160–169. [Google Scholar] [CrossRef]
- Celinski, K.; Konturek, P.C.; Slomka, M.; Cichoz-Lach, H.; Brzozowski, T.; Konturek, S.J.; Korolczuk, A. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease—14 months follow up. J. Physiol. Pharmacol. 2014, 65, 75–82. [Google Scholar]
- Chamanara, M.; Rashidian, A.; Mehr, S.E.; Dehpour, A.R.; Shirkohi, R.; Akbarian, R.; Abdollahi, A.; Rezayat, S.M. Melatonin ameliorates tnbs-induced colitis in rats through the melatonin receptors: Involvement of tlr4/myd88/nf-kappab signalling pathway. Inflammopharmacology 2019, 27, 361–371. [Google Scholar] [CrossRef]
- Yao, L.; Lu, P.; Ling, E.A. Melatonin suppresses toll like receptor 4-dependent caspase-3 signaling activation coupled with reduced production of proinflammatory mediators in hypoxic microglia. PLoS ONE 2016, 11, e0166010. [Google Scholar] [CrossRef]
- Hu, Z.P.; Fang, X.L.; Fang, N.; Wang, X.B.; Qian, H.Y.; Cao, Z.; Cheng, Y.; Wang, B.N.; Wang, Y. Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the tlr4/nf-kappab system in high-fat-fed rabbits. J. Pineal Res. 2013, 55, 388–398. [Google Scholar] [PubMed]
- Boffa, L.C.; Vidali, G.; Mann, R.S.; Allfrey, V.G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J. Biol. Chem. 1978, 253, 3364–3366. [Google Scholar] [PubMed]
- Lumsden, A.L.; Martin, A.M.; Sun, E.W.; Schober, G.; Isaacs, N.J.; Pezos, N.; Wattchow, D.A.; de Fontgalland, D.; Rabbitt, P.; Hollington, P.; et al. Sugar responses of human enterochromaffin cells depend on gut region, sex, and body mass. Nutrients 2019, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Haub, S.; Kanuri, G.; Volynets, V.; Brune, T.; Bischoff, S.C.; Bergheim, I. Serotonin reuptake transporter (sert) plays a critical role in the onset of fructose-induced hepatic steatosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G335–G344. [Google Scholar] [CrossRef]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Ghavami, A.; Rahbar Saadat, Y.; Mesri Alamdari, N.; Alipour, S.; Dastouri, M.R.; Ostadrahimi, A. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of akkermansia muciniphila abundance in type 2 diabetes; a randomized, double-blind, placebo-controlled trial. J. Cardiovasc. Thorac. Res. 2017, 9, 183–190. [Google Scholar] [CrossRef]
- Bouter, K.; Bakker, G.J.; Levin, E.; Hartstra, A.V.; Kootte, R.S.; Udayappan, S.D.; Katiraei, S.; Bahler, L.; Gilijamse, P.W.; Tremaroli, V.; et al. Differential metabolic effects of oral butyrate treatment in lean versus metabolic syndrome subjects. Clin. Transl. Gastroenterol. 2018, 9, 155. [Google Scholar] [CrossRef]

| Diet Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–8 Weeks | 8–13 Weeks | p (Two-Way ANOVA) | |||||||
| C | FFC | C | FFC | C+SoB | FFC+SoB | DE × SoBE | SoBE | DE | |
| Caloric intake (kcal/g bw/d) | 0.49 ± 0.01 | 0.49 ± 0.01 | 0.47 ± 0.01 a | 0.49 ± 0.01 a | 0.47 ± 0.01 a | 0.48 ± 0.01 a | >0.05 | >0.05 | >0.05 |
| Absolute body weight gain (g) | 2.9 ± 0.2 | 3.6 ± 0.4 | 4.3 0.4 a,b | 5.8 ± 0.4 a | 4.5 ± 0.6 b | 5.3 ± 0.4 a,b | >0.05 | >0.05 | <0.05 |
| Absolute body weight (g) | 20 ± 0.4 | 22 ± 0.2 * | 22.3 ± 0.5 a | 23.4 ± 0.5 a | 22.4 ± 0.3 a | 23.3 ± 0.6 a | >0.05 | >0.05 | >0.05 |
| Liver weight (g) | 0.9 ± 0 | 1.4 ± 0 * | 1 ± 0.1 b | 1.5 ± 0 a | 1.1 ± 0 b | 1.4 ± 0.1 a | <0.05 | >0.05 | <0.05 |
| Liver/body weight ratio (%) | 4.5 ± 0.1 | 6.2 ± 0.1 * | 4.7 ± 0.2 b | 6.3 ± 0.2 a | 5 ± 0.2 b | 6.2 ± 0.2 a | >0.05 | >0.05 | <0.05 |
| ALT (U/L) | 22.5 ± 2.5 | 38.5 8.7 | 12.8 0.5 b | 34.9 ± 9.1 a | 14.5 ± 1.7 b | 40.4 ± 5 a | >0.05 | >0.05 | <0.05 |
| AST (U/L) | 49.8 ± 4.6 | 68.4 ± 11.3 | 35.4 ± 1.4 b | 82.4 ± 19.1 a | 38.8 ± 3.6 b | 81.2 ± 9.8 a | >0.05 | >0.05 | <0.05 |
| TNFα (pg/mg protein) | n.d. | n.d. | 26.7 ± 0.9 b | 44.4 ± 5.6 a | 26.2 ± 2.2 b | 28.4 ± 1.1 b | <0.05 | <0.05 | <0.05 |
| IL-6 (pg/mg protein) | n.d. | n.d. | 96.7 ± 5.3 b | 135.3 ± 4.1 a | 99.7 ± 4.3 b | 107.6 ± 4.4 b | <0.05 | <0.05 | <0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumann, A.; Jin, C.J.; Brandt, A.; Sellmann, C.; Nier, A.; Burkard, M.; Venturelli, S.; Bergheim, I. Oral Supplementation of Sodium Butyrate Attenuates the Progression of Non-Alcoholic Steatohepatitis. Nutrients 2020, 12, 951. https://doi.org/10.3390/nu12040951
Baumann A, Jin CJ, Brandt A, Sellmann C, Nier A, Burkard M, Venturelli S, Bergheim I. Oral Supplementation of Sodium Butyrate Attenuates the Progression of Non-Alcoholic Steatohepatitis. Nutrients. 2020; 12(4):951. https://doi.org/10.3390/nu12040951
Chicago/Turabian StyleBaumann, Anja, Cheng Jun Jin, Annette Brandt, Cathrin Sellmann, Anika Nier, Markus Burkard, Sascha Venturelli, and Ina Bergheim. 2020. "Oral Supplementation of Sodium Butyrate Attenuates the Progression of Non-Alcoholic Steatohepatitis" Nutrients 12, no. 4: 951. https://doi.org/10.3390/nu12040951
APA StyleBaumann, A., Jin, C. J., Brandt, A., Sellmann, C., Nier, A., Burkard, M., Venturelli, S., & Bergheim, I. (2020). Oral Supplementation of Sodium Butyrate Attenuates the Progression of Non-Alcoholic Steatohepatitis. Nutrients, 12(4), 951. https://doi.org/10.3390/nu12040951








