Randomised Double-Blind Placebo-Controlled Trial of Inulin with Metronidazole in Non-Alcoholic Fatty Liver Disease (NAFLD)
Abstract
1. Introduction
2. Patients and Methods
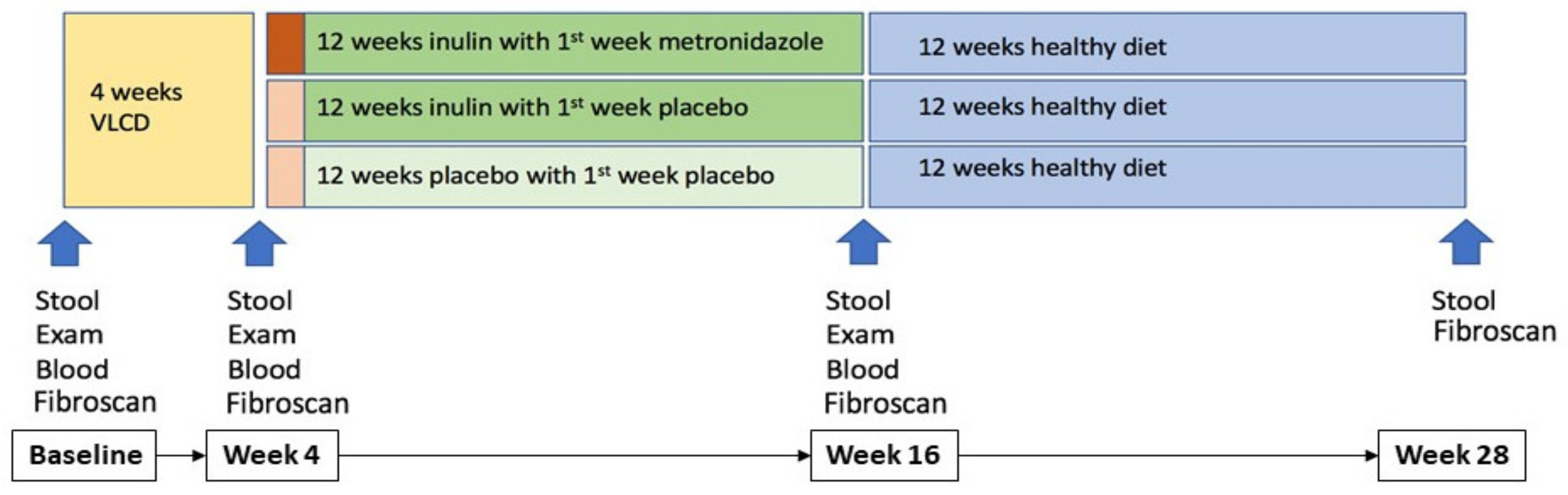
2.1. Study Design
2.2. Randomisation and Treatment Groups
2.3. Stool Sample Collection
2.4. 16S rRNA Gene Amplicon Sequencing
2.5. 16S rRNA Amplicon Sequence Analyses
2.6. Primary and Secondary Outcomes
2.7. Statistical Analysis
2.8. Statistical Methods for Microbiota Analysis
3. Results
3.1. Participants
3.2. Primary and Secondary Outcomes
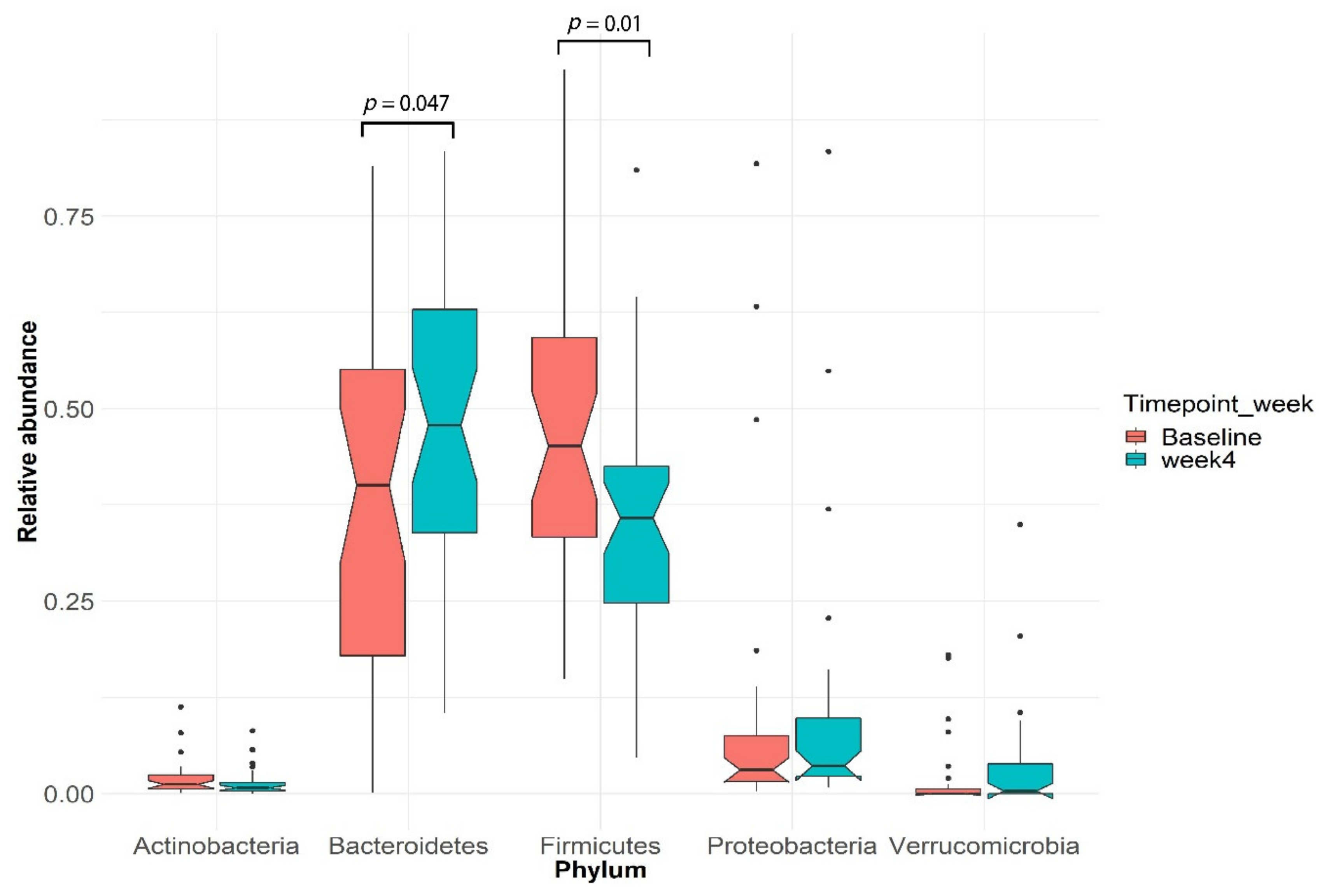
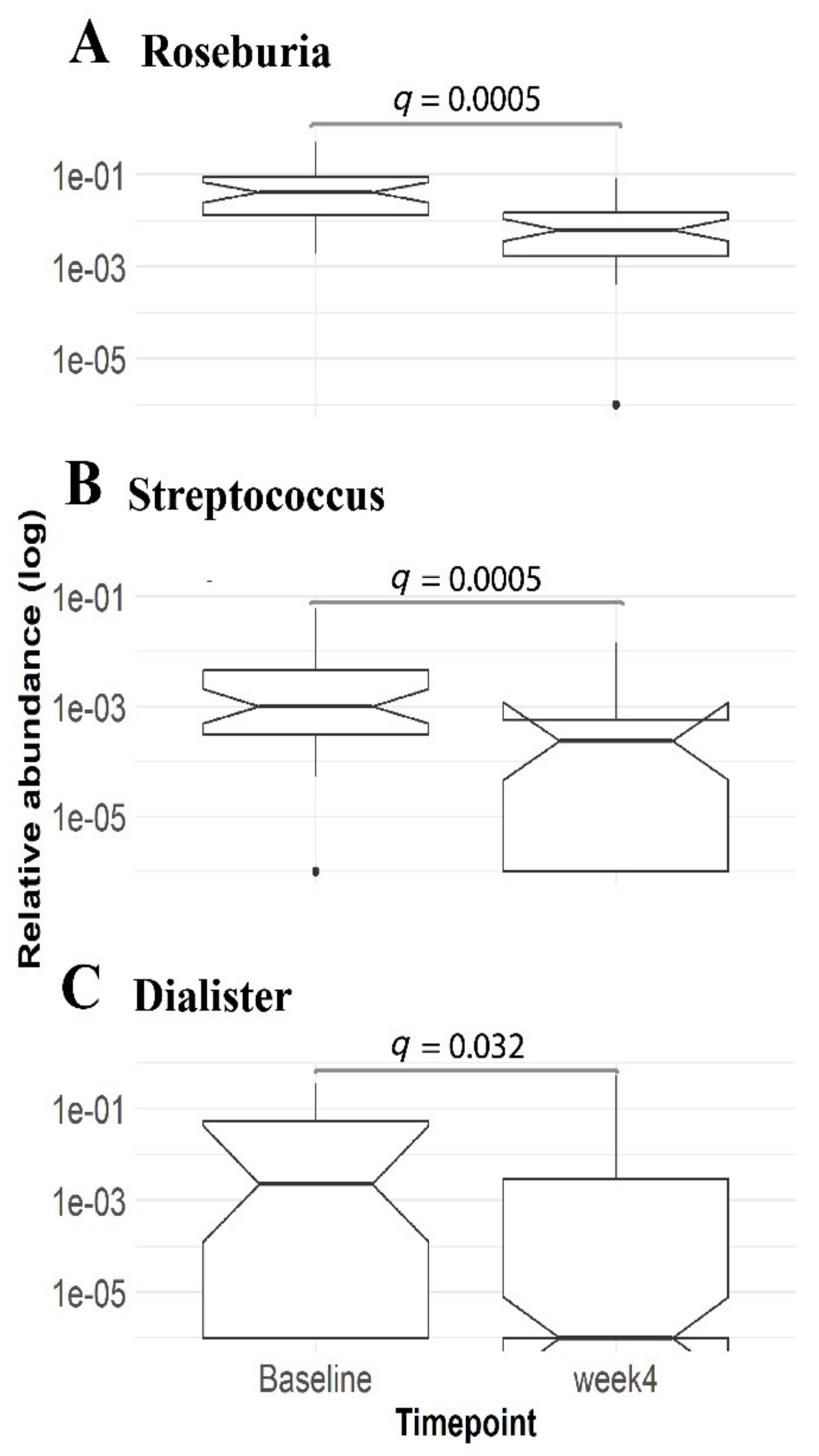
3.3. Gut Microbial Changes in Our Study Cohort
3.4. Enrichment of Distinct Gut Microbial Profile in Our Study Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VLCD | Very low calorie diet |
| NAFLD | Non-alcoholic fatty liver disease |
| ALT | Alanine aminotransferase |
| ASVs | Amplicon Sequence Variants |
Appendix A
| Clinical Characteristics | Metronidazole-Inulin (n = 20) | Placebo-Inulin (n = 20) | Placebo-Placebo (n = 20) | Total (n = 60) |
|---|---|---|---|---|
| Age (y) | 50.6 (10.4) | 51.5 (13.5) | 46.7 (11.2) | 49.6 (11.8) |
| Sex (M:F) | 10:10 | 8:12 | 13:7 | 31:29 |
| Body mass index (kg/m2) | ||||
| Baseline Week 4 | 31.4 (3.4) 29.0 (3.4) | 30.9 (3.5) 27.1 (7.3) | 32.6 (4.3) 29.9 (3.8) | 31.6 (3.8) 29.1 (3.6) *** |
| Weight (kg) | ||||
| Baseline Week 4 | 89.8 (12.4) 83.0 (12.0) | 85.6 (15.5) 73.7 (21.6) | 94.2 (15.9) 86.4 (13.4) | 89.9 (14.9) 82.7 (13.3) *** |
| Waist:hip ratio | ||||
| Baseline Week 4 | 0.98 (0.07) 0.93 (0.06) | 0.99 (0.04) 0.94 (0.05) | 0.98 (0.06) 0.92 (0.08) | 0.98 (0.05) 0.96 (0.06) *** |
| Blood pressure (mm Hg) | ||||
| Systolic at Baseline Systolic at Week 4 Diastolic at Baseline Diastolic at Week 4 | 132 (16) 121 (13) 83 (10) 76 (10) | 129 (14) 121 (16) 77 (10) 74 (10) | 125 (17) 121 (21) 77 (11) 74 (14) | 129 (16) 121 (17) *** 79 (11) 75 (11) *** |
| ALT (U/L) | ||||
| Baseline Week 4 | 64.0 (28.6) 59.8 (25.0) | 70.0 (26.1) 53.3 (22.0) | 60.2 (28.1) 46.2 (21.1) | 64.7 (27.4) 53.2 (23.1) ** |
| AST (U/L) | ||||
| Baseline Week 4 | 34 (25, 46) 41 (31, 55) | 41 (31, 47) 36 (30, 47) | 33 (27, 40) 30 (27, 37) | 36 (29, 45) 36(29, 43) |
| GGT (U/L) | ||||
| Baseline Week 4 | 101 (61, 141) 49 (29, 73) | 164 (76, 246) 83 (35, 124) | 71 (42, 134) 35 (22, 65) | 93 (57, 163) 46(27, 88) *** |
| Bilirubin (mol/L) | ||||
| Baseline Week 4 | 9.5 (7.5, 13.0) 11.0 (8.5,12.0) | 9.5 (6.5, 3.5) 10.0 (6.0,15.0) | 9.5 (7.5, 12.0) 11.0 (8.0,15.0) | 9.5 (7, 13) 10 (8, 14) * |
| Albumin (g/L) | ||||
| Baseline Week 4 | 46.0 (2.5) 45.5 (2.7) | 47.4 (2.8) 47.3 (2.6) | 45.3 (2.1) 46.1 (2.2) | 46.2 (2.6) 46.3 (2.6) |
| Ferritin (μg/L) | ||||
| Baseline Week 4 | 228 (172) 295 (255) | 214 (190) 221 (166) | 254 (222) 241 (204) | 232 (194) 251(208) |
| Cholesterol (mmol/L) | ||||
| Total (Baseline) Total (Week 4) High-density (Baseline) High-density (Week 4) Low-density (Baseline) Low–density (Week 4) | 4.77 (0.98) 3.77 (1.05) 1.35 (0.43) 1.32 (0.40) 2.54 (0.74) 1.85 (0.72) | 5.16 (1.29) 3.78 (1.00) 1.41 (0.36) 1.37 (0.32) 2.88 (1.28) 1.94 (0.91) | 5.01 (0.86) 3.64 (0.76) 1.26 (0.27 1.18 (0.21) 2.96 (0.76) 1.96 (0.72) | 4.98 (1.06) 3.73 (0.93) *** 1.34 (0.36) 1.29 (0.32) * 2.79 (0.97) 1.92 (0.78) *** |
| Triglycerides (mmol/L) | ||||
| Baseline Week 4 | 2.01 (1.07) 0.98 (0.49) | 1.96 (0.80) 1.06 (0.46) | 1.74 (0.79) 1.07 (0.54) | 1.91 (0.89) 1.04 (0.49) *** |
| Type 2 diabetes | 7/20 | 7/20 | 7/20 | 21/60 |
| Fasting glucose (mmol/L) | ||||
| Baseline Week 4 | 6.32 (1.80) 5.70 (1.46) | 6.02 (1.70) 5.50 (1.39) | 6.07 (1.85) 5.19 (0.77) | 6.17 (1.77) 5.46 (1.23) *** |
| HbA1c (mmol/mol) | ||||
| Baseline Week 4 | 44.3 (11.5) 40.4 (8.3) | 48.1 (12.9) 42.5 (8.6) | 44.6 (11.5) 39.3 (7.4) | 45.6 (11.9) 40.7 (8.1) *** |
| CRP (mg/L) | ||||
| Baseline Week 4 | 2.0 (0.5, 4.0) 1.0 (0.5, 4.0) | 2.0 (1.0, 5.0) 0.8 (0.5, 3.0) | 2.5 (0.5, 6.0) 1.5 (0.5, 3.0) | 2.0 (0.5, 5.0) 1.0 (0.5, 3.0) ** |
| Fibroscan® CAP (dB/m) | ||||
| Baseline Week 4 | 307 (51) 267 (55) | 299 (58) 246 (51) | 296 (63) 258 (69) | 301 (57) 257 (58) *** |
Appendix B
| Clinical Characteristics | Group MI: Metronidazole-Inulin (n = 19) | Group PI: Placebo-Inulin (n = 19) | Group PP: Placebo-Placebo (n = 18) | p-Value Group MI vs. PP | p-Value Group PI vs. PP |
|---|---|---|---|---|---|
| Body mass index (kg/m2) | 0.10 (1.20) | 0.12 (1.80) | 0.21 (1.36) | 0.792 | 0.859 |
| Weight (kg) | 0.35 (3.38) | 0.29 (5.02) | 0.47 (4.10) | 0.922 | 0.910 |
| Waist:hip ratio | −0.00 (0.04) | −0.01 (0.04) | −0.00 (0.06) | 0.893 | 0.604 |
| Blood pressure (mm Hg) | |||||
| Systolic Diastolic | 3.4 (11.3) −1.4 (10.2) | 2.2 (16.7) 0.4 (12.0) | −7.2 (18.4) 0.5 (13.8) | 0.043 0.641 | 0.117 0.981 |
| ALT (U/L) | −19.6 (25.5) | −2.2 (16.7) | −0.2 (24.5) | 0.026 | 0.856 |
| AST (U/L) | −14 (−32, −7) | −3 (−22, 2) | −4 (−9, 5) | 0.006 | 0.849 |
| GGT (U/L) | 13 (−2, 29) | 26 (−1, 91) | 17 (8, 62) | 0.274 | 0.693 |
| Bilirubin (µmol/L) | −1 (−3, 1) | −2 (−3, 0) | 1 (−2, 2) | 0.115 | 0.033 |
| Albumin (g/L) | 0.13 (1.81) | −1.17 (2.46) | −1.14 (2.57) | 0.131 | 0.979 |
| Ferritin (µg/L) | −56.3 (60.1) | −27.6 (63.5) | −1.3 (72.7) | 0.031 | 0.291 |
| Cholesterol (mmol/L) | |||||
| Total HDL LDL | 1.09 (1.10) 0.16 (0.15) 2.28 (5.56) | 1.32 (1.13) 0.20 (0.24) 0.83 (0.77) | 0.91 (0.57) 0.13 (0.17) 0.55 (0.49) | 0.560 0.638 0.181 | 0.208 0.344 0.246 |
| Triglycerides (mmol/L) | 0.52 (0.32) | 0.50 (0.79) | 0.56 (0.64) | 0.831 | 0.814 |
| Fasting glucose (mmol/L) | 0.39 (1.32) | 0.18 (0.70) | 1.14 (2.05) | 0.228 | 0.077 |
| HbA1c (mmol/mol) | 1.94 (4.25) | 0 (5.79) | 2.06 (6.02) | 0.947 | 0.316 |
| CRP (mg/L) | 0.0 (−1.0, 0.75) | 1.0 (0.0, 1.5) | 0.0 (0.0, 1.0) | 0.884 | 0.235 |
| Fibroscan CAP (dB/m) | 14.5 (65.6) | 22.4 (71.6) | 10.8 (42.6) | 0.859 | 0.605 |
Appendix C
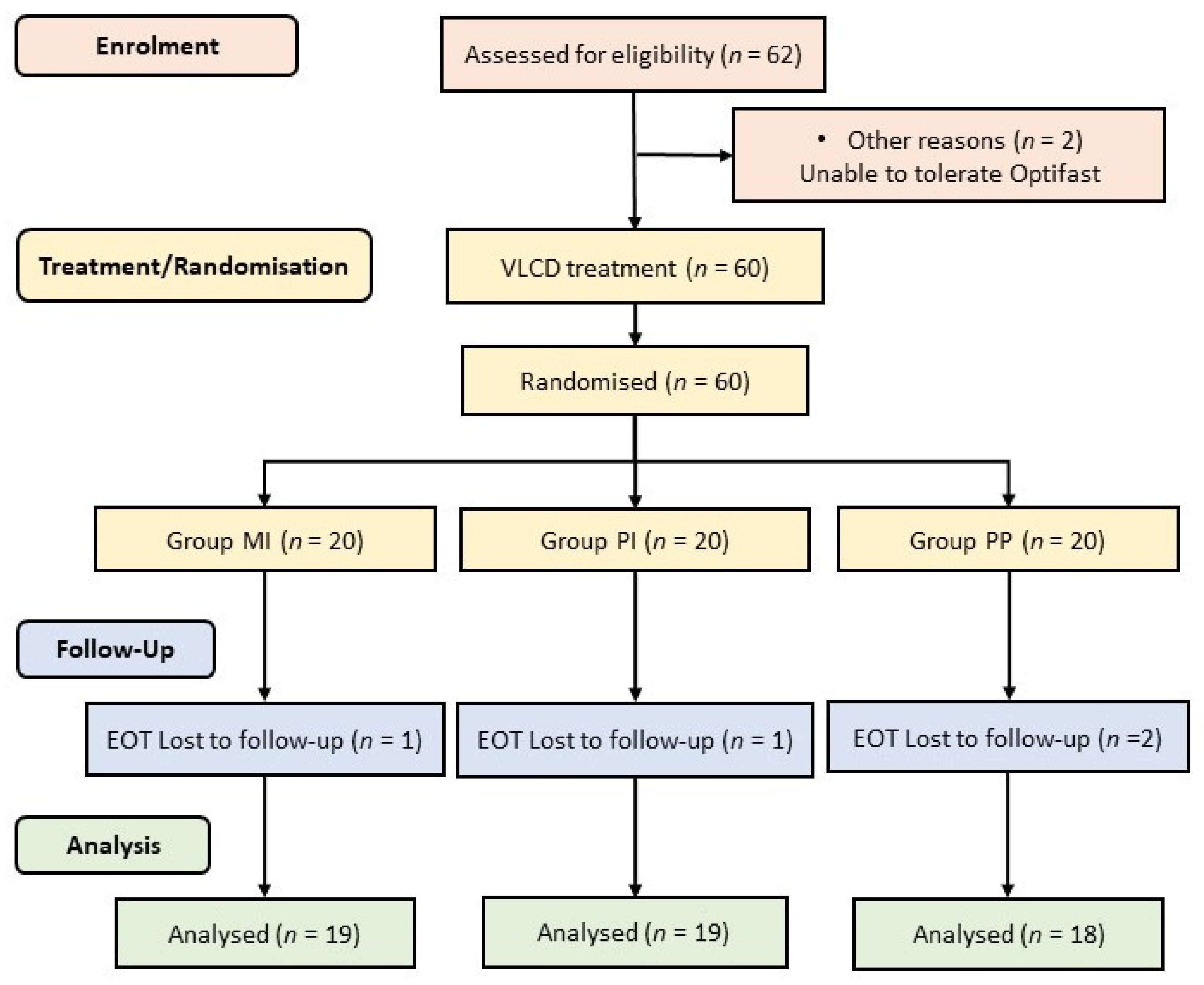
Appendix D
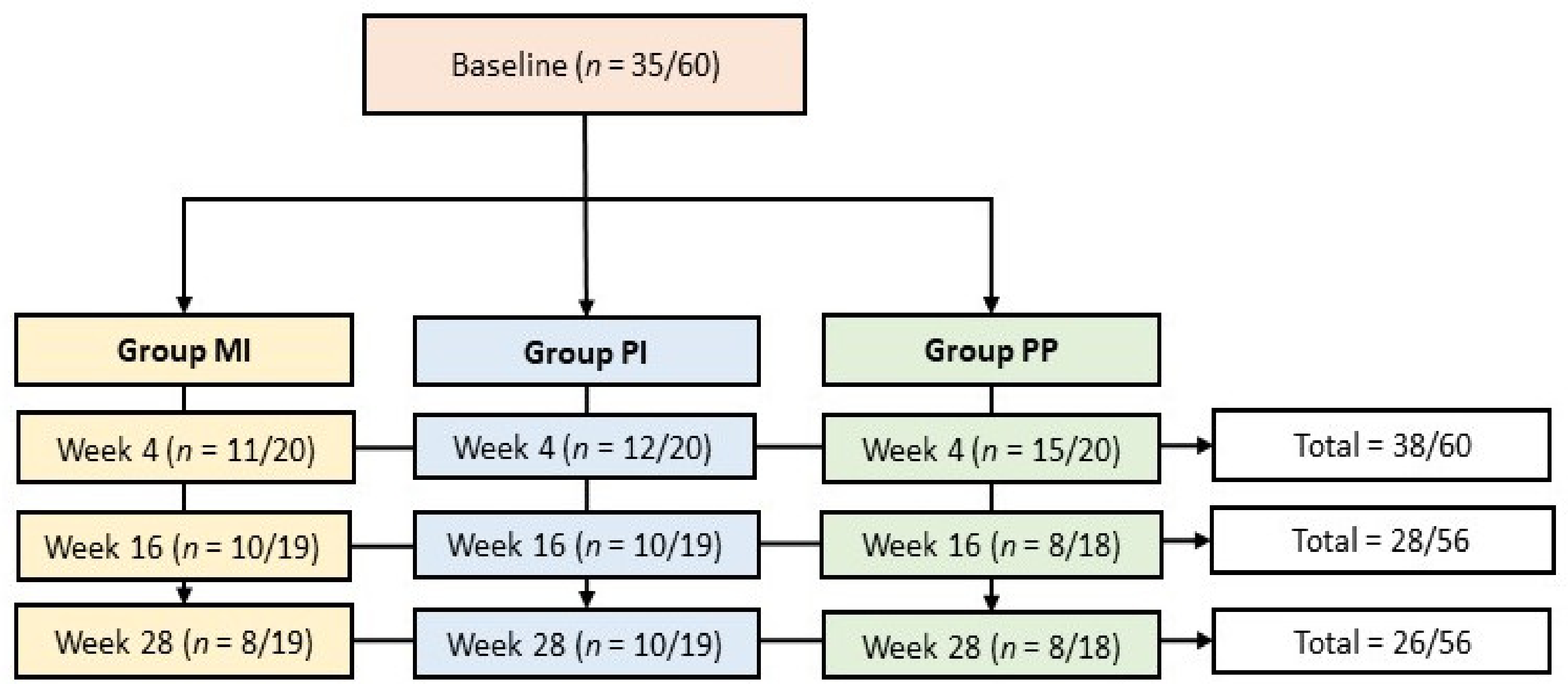
References
- Shouhed, D.; Steggerda, J.; Burch, M.; Noureddin, M. The role of bariatric surgery in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 797–811. [Google Scholar] [CrossRef]
- Calzadilla Bertot, L.; Adams, L. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Nasr, P.; Kechagias, S. Natural History of NAFLD/NASH. Curr. Hepatol. Rep. 2017, 16, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Gaborit, B.; Dutour, A.; Clement, K. Gut microbiota and non-alcoholic fatty liver disease: New insights. Clin. Microbiol. Infect. 2013, 19, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Drenick, E.J.; Fisler, J.; Johnson, D. Hepatic Steatosis After Intestinal Bypass—Prevention and Reversal by Metronidazole, Irrespective of Protein-Calorie Malnutrition. Gastroenterology 1982, 82, 535–548. [Google Scholar] [CrossRef]
- Di Stefano, M.; Miceli, E.; Missanelli, A.; Mazzocchi, S.; Corazza, G.R. Absorbable vs. non-absorbable antibiotics in the treatment of small intestine bacterial overgrowth in patients with blind-loop syndrome. Aliment. Pharmacol. Ther. 2005, 21, 985–992. [Google Scholar] [CrossRef]
- Wigg, A.J. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut 2001, 48, 206–211. [Google Scholar] [CrossRef]
- Shanab, A.A.; Scully, P.; Crosbie, O.; Buckley, M.; O’Mahony, L.; Shanahan, F.; Gazareen, S.; Murphy, E.; Quigley, E.M.M. Small Intestinal Bacterial Overgrowth in Nonalcoholic Steatohepatitis: Association with Toll-Like Receptor 4 Expression and Plasma Levels of Interleukin 8. Dig. Dis. Sci. 2011, 56, 1524–1534. [Google Scholar] [CrossRef]
- Rafiei, R.; Bemanian, M.; Rafiei, F.; Bahrami, M.; Fooladi, L.; Ebrahimi, G.; Hemmat, A.; Torabi, Z. Liver disease symptoms in non-alcoholic fatty liver disease and small intestinal bacterial overgrowth. Rom. J. Intern. Med. 2018, 56, 85–89. [Google Scholar] [CrossRef]
- Kapil, S.; Duseja, A.; Sharma, B.K.; Singla, B.; Chakraborti, A.; Das, A.; Ray, P.; Dhiman, R.K.; Chawla, Y. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2016, 31, 213–221. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Knop, F.K.; Taylor, R. Mechanism of Metabolic Advantages After Bariatric Surgery: It’s all gastrointestinal factors versus it’s all food restriction. Diabetes Care 2013, 36, S287–S291. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.K.; O’Sullivan, J.M.; Plank, L.D.; Murphy, R. Altered gut microbiome after bariatric surgery and its association with metabolic benefits: A systematic review. Surg. Obes. Relat. Dis. 2019, 15, 656–665. [Google Scholar] [CrossRef]
- Damms-Machado, A.; Mitra, S.; Schollenberger, A.E.; Kramer, K.M.; Meile, T.; Königsrainer, A.; Huson, D.H.; Bischoff, S.C. Effects of Surgical and Dietary Weight Loss Therapy for Obesity on Gut Microbiota Composition and Nutrient Absorption. Biomed. Res. Int. 2015, 1–12. [Google Scholar] [CrossRef]
- Heinsen, F.A.; Fangmann, D.; Müller, N.; Schulte, D.M.; Rühlemann, M.C.; Türk, K.; Settgast, U.; Lieb, W.; Baines, J.F.; Schreiber, S.; et al. Beneficial Effects of a Dietary Weight Loss Intervention on Human Gut Microbiome Diversity and Metabolism Are Not Sustained during Weight Maintenance. Obes. Facts 2016, 9, 379–391. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Metabolic benefits of dietary prebiotics in human subjects: A systematic review of randomised controlled trials. Br. J. Nutr. 2014, 111, 1147–1161. [Google Scholar] [CrossRef]
- Loman, B.R.; Hernández-Saavedra, D.; An, R.; Rector, R.S. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutr. Rev. 2018, 76, 822–839. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Feng, Z.; Yi, X. A general introduction to adjustment for multiple comparisons. J. Thorac. Dis. 2017, 9, 1725–1729. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Possemiers, S.; Verstraete, W.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin–glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J. Nutr. Biochem. 2012, 23, 51–59. [Google Scholar] [CrossRef]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br. J. Nutr. 2013, 110, 1253–1262. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- Hold, G.L.; Schwiertz, A.; Aminov, R.I.; Blaut, M.; Flint, H.J. Oligonucleotide Probes That Detect Quantitatively Significant Groups of Butyrate-Producing Bacteria in Human Feces. Appl. Environ. Microbiol. 2003, 69, 4320–4324. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, D.; He, Y.; Li, Y.; Yang, Z.; Zhao, X.; Liu, Y.; Wang, Y.; Sun, J.; Feng, X.; et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci. Rep. 2019, 9, 13424. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Niioka, M.; Kobayashi, N.; Tanaka, M.; Watanabe, T. Butyrate-Producing Probiotics Reduce Nonalcoholic Fatty Liver Disease Progression in Rats: New Insight into the Probiotics for the Gut-Liver Axis. PLoS ONE 2013, 8, e63388. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Bennuri, S.C.; Davis, J.E.; Wynne, R.; Slattery, J.C.; Tippett, M.; Delhey, L.; Melnyk, S.; Kahler, S.G.; MacFabe, D.F.; et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl. Psychiatry 2018, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef]
- Roediger, W.E. The colonic epithelium in ulcerative colitis: An energy-deficiency disease? Lancet 1980, 316, 712–715. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Lührs, H.; Gerke, T.; Schauber, J.; Dusel, G.; Melcher, R.; Scheppach, W.; Menzel, T. Cytokine-activated degradation of inhibitory κB protein α is inhibited by the short-chain fatty acid butyrate. Int. J. Colorectal Dis. 2001, 16, 195–201. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef]
- Raman, M.; Ahmed, I.; Gillevet, P.M.; Probert, C.S.; Ratcliffe, N.M.; Smith, S.; Greenwood, R.; Sikaroodi, M.; Lam, V.; Crotty, P.; et al. Fecal Microbiome and Volatile Organic Compound Metabolome in Obese Humans With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2013, 11, 868–875.e3. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced Dietary Intake of Carbohydrates by Obese Subjects Results in Decreased Concentrations of Butyrate and Butyrate-Producing Bacteria in Feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef]
- Alemán, J.O.; Bokulich, N.A.; Swann, J.R.; Walker, J.M.; De Rosa, J.C.; Battaglia, T.; Costabile, A.; Pechlivanis, A.; Liang, Y.; Breslow, J.L.; et al. Fecal microbiota and bile acid interactions with systemic and adipose tissue metabolism in diet-induced weight loss of obese postmenopausal women. J. Transl. Med. 2018, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Chassard, C.; Goumy, V.; Leclerc, M.; Del’homme, C.; Bernalier-Donadille, A. Characterization of the xylan-degrading microbial community from human faeces. FEMS Microbiol. Ecol. 2007, 61, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Nistal, E.; Sáenz de Miera, L.E.; Ballesteros Pomar, M.; Sánchez Campos, S.; García Mediavilla, M.V.; Álvarez Cuenllas, B.; Linares, P.; Olcoz, J.L.; Arias Loste, M.T.; García Lobo, J.M.; et al. An altered fecal microbiota profile in patients with non-alcoholic fatty liver disease (NAFLD) associated with obesity. Rev. Esp. Enferm. Dig. 2019, 111, 275–282. [Google Scholar] [CrossRef]
- Caussy, C.; Tripathi, A.; Humphrey, G.; Bassirian, S.; Singh, S.; Faulkner, C.; Bettencourt, R.; Rizo, E.; Richards, L.; Xu, Z.Z.; et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat. Commun. 2019, 10, 1406. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Paroni Sterbini, F.; Petito, V.; et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef]
- Shen, F.; Zheng, R.D.; Sun, X.Q.; Ding, W.J.; Wang, X.Y.; Fan, J.G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef]
- Dimova, L.G.; Zlatkov, N.; Verkade, H.J.; Uhlin, B.E.; Tietge, U.J.F. High-cholesterol diet does not alter gut microbiota composition in mice. Nutr. Metab. 2017, 14, 15. [Google Scholar] [CrossRef]
- Arguello, G.; Balboa, E.; Arrese, M.; Zanlungo, S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1765–1778. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, K.P.; Chanin, R.; McDonald, C. Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes. 2015, 6, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.J.; Stote, K.S.; Henderson, T.; Paul, D.R.; Okuma, K.; Tagami, H.; Kanahori, S.; Gordon, D.T.; Rumpler, W.V.; Ukhanova, M.; et al. The metabolizable energy of dietary resistant maltodextrin is variable and alters fecal microbiota composition in adult men. J. Nutr. 2014, 177, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.R.; Chassaing, B. Maltodextrin, modern stressor of the intestinal environment. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 475–476. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, C.Y.L.; Orr, D.; Plank, L.D.; Vatanen, T.; O'Sullivan, J.M.; Murphy, R. Randomised Double-Blind Placebo-Controlled Trial of Inulin with Metronidazole in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 937. https://doi.org/10.3390/nu12040937
Chong CYL, Orr D, Plank LD, Vatanen T, O'Sullivan JM, Murphy R. Randomised Double-Blind Placebo-Controlled Trial of Inulin with Metronidazole in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients. 2020; 12(4):937. https://doi.org/10.3390/nu12040937
Chicago/Turabian StyleChong, Clara Yieh Lin, David Orr, Lindsay D. Plank, Tommi Vatanen, Justin M. O'Sullivan, and Rinki Murphy. 2020. "Randomised Double-Blind Placebo-Controlled Trial of Inulin with Metronidazole in Non-Alcoholic Fatty Liver Disease (NAFLD)" Nutrients 12, no. 4: 937. https://doi.org/10.3390/nu12040937
APA StyleChong, C. Y. L., Orr, D., Plank, L. D., Vatanen, T., O'Sullivan, J. M., & Murphy, R. (2020). Randomised Double-Blind Placebo-Controlled Trial of Inulin with Metronidazole in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients, 12(4), 937. https://doi.org/10.3390/nu12040937






