Bioelectrical Impedance Analysis—An Easy Tool for Quantifying Body Composition in Infancy?
Abstract
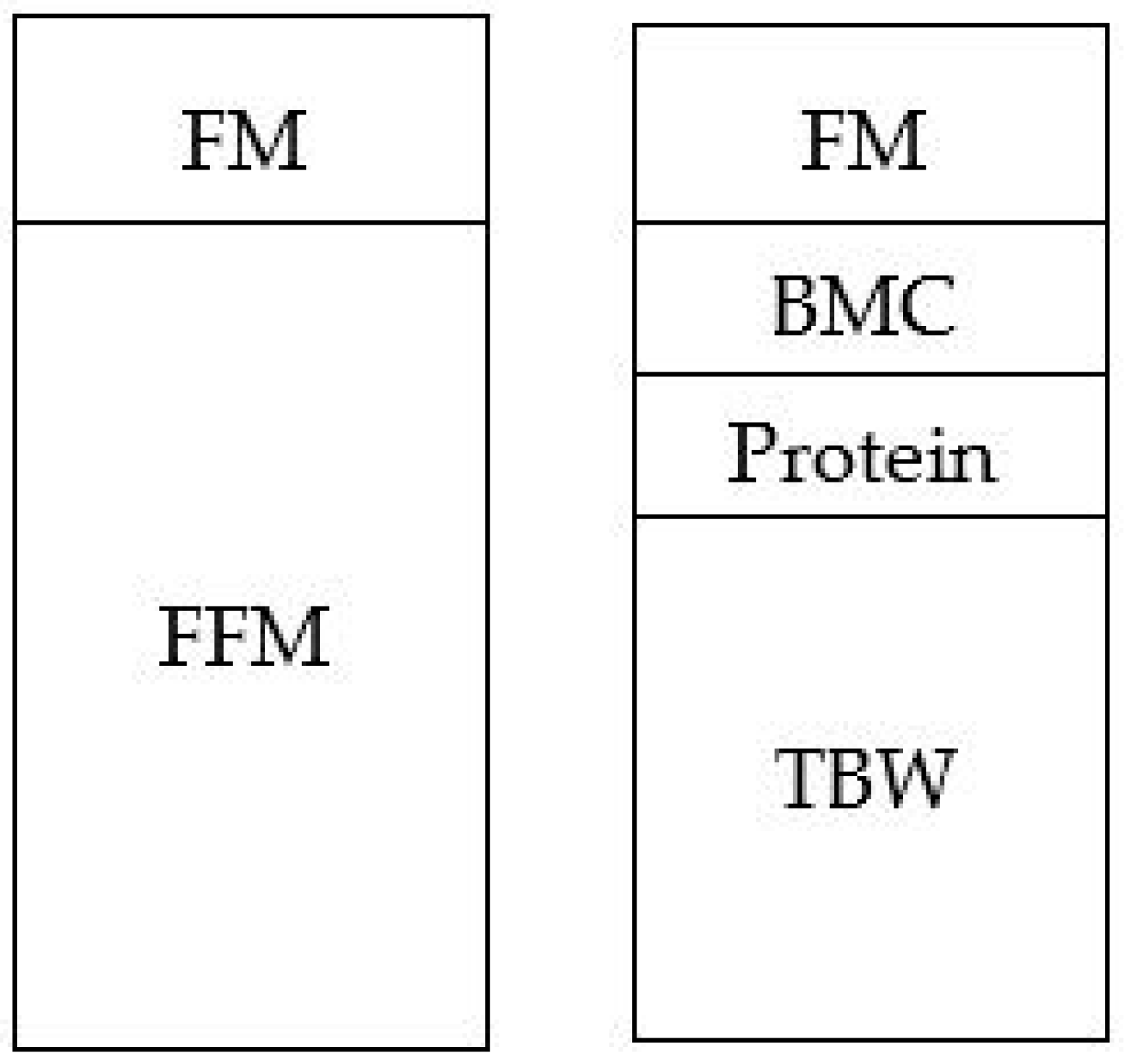
1. Body composition in infancy
2. Moving Away from a Two-Compartment Model
3. Bioelectrical Impedance Analysis—An Inexpensive, Portable and Easy Tool
4. Limitations of Bioelectrical Impedance Technologies
4.1. Is it Actually That Easy?
4.2. A Call for Standardization
- What is the optimal size and placement of electrodes?
- Can “normal” hydration be standardized?
- How does time spent supine influence water distribution within the body?
4.2.1. Electrodes
4.2.2. Hydration
4.2.3. Movement
4.3. Validating against a TRUE Criterion Method
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- von Bezold, A. Das chemische Skelett der Wirbelthiere. Z. Fur Wiss. Zool. 1858, 9, 240–269. [Google Scholar]
- Fomon, S.J.; Haschke, F.; Ziegler, E.E.; Nelson, S.E. Body composition of reference children from birth to age 10 years. Am. J. Clin. Nutr. 1982, 35, 1169–1175. [Google Scholar] [CrossRef]
- Butte, N.F.; Hopkinson, J.M.; Wong, W.W.; Smith, E.O.B.; Ellis, K.J. Body Composition during the First 2 Years of Life: An Updated Reference. Pediatric Res. 2000, 47, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Rolland-Cachera, M.F.; Deheeger, M.; Bellisle, F.; Sempé, M.; Guilloud-Bataille, M.; Patois, E. Adiposity rebound in children: A simple indicator for predicting obesity. Am. J. Clin. Nutr. 1984, 39, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, E.I.d.S.; Lima, N.P.; Menezes, A.M.B.; Gonçalves, H.; Wehrmeister, F.C.; Assunção, M.C.F.; Horta, B.L. Maternal smoking during pregnancy and offspring body composition in adulthood: Results from two birth cohort studies. BMJ Open 2019, 9, e023852. [Google Scholar] [CrossRef] [PubMed]
- Landhuis, C.E.; Poulton, R.; Welch, D.; Hancox, R.J. Childhood sleep time and long-term risk for obesity: A 32-year prospective birth cohort study. Pediatrics 2008, 122, 955–960. [Google Scholar] [CrossRef]
- Lowe, W.L., Jr.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. Association of Gestational Diabetes With Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA 2018, 320, 1005–1016. [Google Scholar] [CrossRef]
- Rito, A.I.; Buoncristiano, M.; Spinelli, A.; Salanave, B.; Kunešová, M.; Hejgaard, T.; García Solano, M.; Fijałkowska, A.; Sturua, L.; Hyska, J.; et al. Association between Characteristics at Birth, Breastfeeding and Obesity in 22 Countries: The WHO European Childhood Obesity Surveillance Initiative—COSI 2015/2017. Obes. Facts 2019, 12, 226–243. [Google Scholar] [CrossRef]
- Taveras, E.M.; Rifas-Shiman, S.L.; Sherry, B.; Oken, E.; Haines, J.; Kleinman, K.; Rich-Edwards, J.W.; Gillman, M.W. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch. Pediatric Adolesc. Med. 2011, 165, 993–998. [Google Scholar] [CrossRef]
- Voerman, E.; Santos, S.; Patro Golab, B.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef]
- Whitaker, R.C.; Pepe, M.S.; Wright, J.A.; Seidel, K.D.; Dietz, W.H. Early adiposity rebound and the risk of adult obesity. Pediatrics 1998, 101, E5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deurenberg, P.; Wang, W.; Pietrobelli, A.; Baumgartner, R.N.; Heymsfield, S.B. Hydration of fat-free body mass: Review and critique of a classic body-composition constant. Am. J. Clin. Nutr. 1999, 69, 833–841. [Google Scholar] [CrossRef]
- Weber, D.R.; Leonard, M.B.; Zemel, B.S. Body composition analysis in the pediatric population. Pediatric Endocrinol. Rev. 2012, 10, 130–139. [Google Scholar]
- Fomon, S.J.; Nelson, S.E. Body composition of the male and female reference infants. Annu. Rev. Nutr. 2002, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatryod, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, C.M.; Rudnicka, A.R.; Owen, C.G.; Cook, D.G.; Whincup, P.H. Patterns of body size and adiposity among UK children of South Asian, black African-Caribbean and white European origin: Child Heart And health Study in England (CHASE Study). Int. J. Epidemiol. 2011, 40, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Fall, C.H.D.; Coyaji, K.J.; Hirve, S.S.; Rao, S.; Barker, D.J.P.; Joglekar, C.; Kellingray, S. Neonatal anthropometry: The thin-fat Indian baby. The Pune Maternal Nutrition Study. Int. J. Obes. 2003, 27, 173–180. [Google Scholar] [CrossRef]
- Demerath, E.W.; Fields, D.A. Body composition assessment in the infant. Am. J. Hum. Biol. 2014, 26, 291–304. [Google Scholar] [CrossRef]
- Lohman, T.G. Assessment of body composition in children. Pediatric Exerc. Sci. 1989, 1, 19–30. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Johnson, P.E.; Bolonchuk, W.W.; Lykken, G.I. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am. J. Clin. Nutr. 1985, 41, 810–817. [Google Scholar] [CrossRef]
- Ward, L.C. Bioelectrical impedance analysis for body composition assessment: Reflections on accuracy, clinical utility, and standardisation. Eur J. Clin. Nutr. 2019, 73, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Cornish, B.H.; Thomas, B.J.; Ward, L.C. Improved prediction of extracellular and total body water using impedance loci generated by multiple frequency bioelectrical impedance analysis. Phys. Med. Biol. 1993, 38, 337–346. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, A.; Andreoli, A.; Matthie, J.; Withers, P. Predicting body cell mass with bioimpedance by using theoretical methods: A technological review. J. Appl. Physiol. (1985) 1997, 82, 1542–1558. [Google Scholar] [CrossRef] [PubMed]
- Matthie, J.R. Bioimpedance measurements of human body composition: Critical analysis and outlook. Expert Rev. Med. Devices 2008, 5, 239–261. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Lingwood, B.E. Bioelectrical impedance analysis for assessment of fluid status and body composition in neonates--the good, the bad and the unknown. Eur. J. Clin. Nutr. 2013, 67, S28–S33. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Collins, C.T.; Reid, J.; Makrides, M.; Lingwood, B.E.; McPhee, A.J.; Morris, S.A.; Gibson, R.A.; Ward, L.C. Prediction of body water compartments in preterm infants by bioelectrical impedance spectroscopy. Eur. J. Clin. Nutr. 2013, 67, S47–S53. [Google Scholar] [CrossRef]
- Lingwood, B.E.; Storm van Leeuwen, A.M.; Carberry, A.E.; Fitzgerald, E.C.; Callaway, L.K.; Colditz, P.B.; Ward, L.C. Prediction of fat-free mass and percentage of body fat in neonates using bioelectrical impedance analysis and anthropometric measures: Validation against the PEA POD. Br. J. Nutr. 2012, 107, 1545–1552. [Google Scholar] [CrossRef]
- Tint, M.-T.; Ward, L.C.; Soh, S.E.; Aris, I.M.; Chinnadurai, A.; Saw, S.M.; Gluckman, P.D.; Godfrey, K.M.; Chong, Y.-S.; Kramer, M.S.; et al. Estimation of fat-free mass in Asian neonates using bioelectrical impedance analysis. Br. J. Nutr. 2016, 115, 1033–1042. [Google Scholar] [CrossRef]
- Gridneva, Z.; Hepworth, A.R.; Ward, L.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Determinants of body composition in breastfed infants using bioimpedance spectroscopy and ultrasound skinfolds-methods comparison. Pediatric Res. 2017, 81, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Kushner, R.F.; Schoeller, D.A.; Fjeld, C.R.; Danford, L. Is the impedance index (ht2/R) significant in predicting total body water? Am. J. Clin. Nutr. 1992, 56, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.; Mantzioris, E.; Lingwood, B.; Couper, J.; Makrides, M.; Gibson, R.A.; Muhlhausler, B.S. The effect of maternal DHA supplementation on body fat mass in children at 7 years: Follow-up of the DOMInO randomized controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2018, 139, 49–54. [Google Scholar] [CrossRef]
- Wall, C.R.; Hill, R.J.; Lovell, A.L.; Matsuyama, M.; Milne, T.; Grant, C.C.; Jiang, Y.; Chen, R.X.; Wouldes, T.A.; Davies, P.S.W. A multicenter, double-blind, randomized, placebo-controlled trial to evaluate the effect of consuming Growing Up Milk “Lite” on body composition in children aged 12-23 mo. Am. J. Clin. Nutr. 2019, 109, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Toffano, R.B.D.; Hillesheim, E.; Margutti, A.V.B.; Camelo Junior, J.S.; Ferraz, I.S.; Del Ciampo, L.A.; Monteiro, J.P. Bioelectrical Impedance Vector Analysis in Healthy Term Infants in the First Three Months of Life in Brazil. J. Am. Coll. Nutr. 2018, 37, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Brantlov, S.; Jodal, L.; Lange, A.; Rittig, S.; Ward, L.C. Standardisation of bioelectrical impedance analysis for the estimation of body composition in healthy paediatric populations: A systematic review. J. Med. Eng. Technol. 2017, 41, 460–479. [Google Scholar] [CrossRef]
- Brantlov, S.; Ward, L.C.; Jodal, L.; Rittig, S.; Lange, A. Critical factors and their impact on bioelectrical impedance analysis in children: A review. J. Med. Eng. Technol. 2017, 41, 22–35. [Google Scholar] [CrossRef]
- National Institutes of Health. Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am. J. Clin. Nutr. 1996, 64, 524S–532S. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Piccoli, A.; Fanos, V.; Peruzzi, L.; Schena, S.; Pizzini, C.; Borgione, S.; Bertino, E.; Chiaffoni, G.; Coppo, R.; Tatò, L. Reference values of the bioelectrical impedance vector in neonates in the first week after birth. Nutrition 2002, 18, 383–387. [Google Scholar] [CrossRef]
- Raghavan, C.V.; Super, D.M.; Chatburn, R.L.; Savin, S.M.; Fanaroff, A.A.; Kalhan, S.C. Estimation of total body water in very-low-birth-weight infants by using anthropometry with and without bioelectrical impedance and H2[(18)O]. Am. J. Clin. Nutr. 1998, 68, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Ridout, D.; Modi, N. Assessment of total body water using bioelectrical impedance analysis in neonates receiving intensive care. Arch. Dis. Child. -Fetal Neonatal Ed. 1997, 77, F123. [Google Scholar] [CrossRef] [PubMed]
- ImpediMed. SFB7 Brochure. Available online: https://www.impedimed.com/wp-content/products/SFB7/SFB7_CA_Brochure.pdf (accessed on 7 July 2019).
- Bodystat. FAQ’S. Available online: https://www.bodystat.com/support/ (accessed on 30 October 2019).
- Svensson, B.J.; Dylke, E.S.; Ward, L.C.; Kilbreath, S.L. Electrode Equivalence for Use in Bioimpedance Spectroscopy Assessment of Lymphedema. Lymphat. Res. Biol. 2019, 17, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.S.; Triggs, E.J.; Charles, B.G.; Tudehope, D.I. Electrode placement in neonatal bioelectrical impedance analysis. Med. Biol. Eng. Comput. 1994, 32, 456–459. [Google Scholar] [CrossRef]
- Sesmero, M.A.; Mazariegos, M.; Pedrón, C.; Jones, J.; Solomons, N.W. Bioimpedance electrical spectroscopy in the first six months of life: Some methodologic considerations. Nutrition 2005, 21, 567–573. [Google Scholar] [CrossRef]
- Nescolarde, L.; Lukaski, H.; De Lorenzo, A.; de-Mateo-Silleras, B.; Redondo-Del-Rio, M.P.; Camina-Martin, M.A. Different displacement of bioimpedance vector due to Ag/AgCl electrode effect. Eur. J. Clin. Nutr. 2016, 70, 1401–1407. [Google Scholar] [CrossRef]
- Bogónez-Franco, P.; Nescolarde, L.; Bragós, R.; Rosell-Ferrer, J.; Yandiola, I. Measurement errors in multifrequency bioelectrical impedance analyzers with and without impedance electrode mismatch. Physiol. Meas. 2009, 30, 573–587. [Google Scholar] [CrossRef]
- Buendía, R.; Bogónez-Franco, P.; Nescolarde, L.; Seoane, F. Influence of electrode mismatch on Cole parameter estimation from Total Right Side Electrical Bioimpedance Spectroscopy measurements. Med. Eng. Phys. 2012, 34, 1024–1028. [Google Scholar] [CrossRef]
- Caicedo-Eraso, J.C.; González-Correa, C.H.; González-Correa, C.A. Use of electrocardiogram (ECG) electrodes for Bioelectrical Impedance Analysis (BIA). J. Phys. Conf. Ser. 2012, 407, 012008. [Google Scholar] [CrossRef]
- González-Correa, C.H.; Caicedo-Eraso, J.C. Looking for optimum ECG electrodes for bioelectrical impedance analysis (BIA). The need for evaluation. Nutr. Hosp. 2018, 35, 110–116. [Google Scholar]
- Gridneva, Z.; Hepworth, A.R.; Ward, L.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Bioimpedance spectroscopy in the infant: Effect of milk intake and extracellular fluid reservoirs on resistance measurements in term breastfed infants. Eur. J. Clin. Nutr. 2016, 70, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Evans, J.A.; Smye, S.W.; Holland, P. Variables affecting BIA measurement of body water. Med. Biol. Eng. Comput. 1999, 37, 106–107. [Google Scholar]
- Benjamin-Neelon, S.E.; Bai, J.; Ostbye, T.; Neelon, B.; Pate, R.R.; Crainiceanu, C. Physical Activity and Adiposity in a Racially Diverse Cohort of US Infants. Obesity 2020, 28, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Kushner, R.F.; Gudivaka, R.; Schoeller, D.A. Clinical characteristics influencing bioelectrical impedance analysis measurements. Am. J. Clin. Nutr. 1996, 64, 423S–427S. [Google Scholar] [CrossRef]
- Gibson, A.; Beam, J.; Alencar, M.; Zuhl, M.; Mermier, C. Time course of supine and standing shifts in total body, intracellular and extracellular water for a sample of healthy adults. Eur. J. Clin. Nutr. 2014, 69, 14–19. [Google Scholar] [CrossRef]
- Segal, K.R.; Van Loan, M.; Fitzgerald, P.I.; Hodgdon, J.A.; Van Itallie, T.B. Lean body mass estimation by bioelectrical impedance analysis: A four-site cross-validation study. Am. J. Clin. Nutr. 1988, 47, 7–14. [Google Scholar] [CrossRef]
- Ellis, K.J.; Yao, M.; Shypailo, R.J.; Urlando, A.; Wong, W.W.; Heird, W.C. Body-composition assessment in infancy: Air-displacement plethysmography compared with a reference 4-compartment model. Am. J. Clin. Nutr. 2007, 85, 90–95. [Google Scholar] [CrossRef]
- Van Der Ploeg, G.E.; Withers, R.T.; Laforgia, J. Percent body fat via DEXA: Comparison with a four-compartment model. J. Appl. Physiol. (1985) 2003, 94, 499–506. [Google Scholar] [CrossRef]
- Sopher, A.B.; Thornton, J.C.; Wang, J.; Pierson, R.N., Jr.; Heymsfield, S.B.; Horlick, M. Measurement of percentage of body fat in 411 children and adolescents: A comparison of dual-energy X-ray absorptiometry with a four-compartment model. Pediatrics 2004, 113, 1285–1290. [Google Scholar] [CrossRef]
- Wells, J.C.K.; Haroun, D.; Williams, J.E.; Wilson, C.; Darch, T.; Viner, R.M.; Eaton, S.; Fewtrell, M.S. Evaluation of DXA against the four-component model of body composition in obese children and adolescents aged 5-21 years. Int. J. Obes. 2010, 34, 649–655. [Google Scholar] [CrossRef][Green Version]
- Wong, W.W.; Hergenroeder, A.C.; Stuff, J.E.; Butte, N.F.; Smith, E.O.; Ellis, K.J. Evaluating body fat in girls and female adolescents: Advantages and disadvantages of dual-energy X-ray absorptiometry. Am. J. Clin. Nutr. 2002, 76, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Gately, P.J.; Radley, D.; Cooke, C.B.; Carroll, S.; Oldroyd, B.; Truscott, J.G.; Coward, W.A.; Wright, A. Comparison of body composition methods in overweight and obese children. J. Appl. Physiol. (1985) 2003, 95, 2039–2046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andersen, G.S.; Girma, T.; Wells, J.C.; Kæstel, P.; Leventi, M.; Hother, A.-L.; Michaelsen, K.F.; Friis, H. Body composition from birth to 6 mo of age in Ethiopian infants: Reference data obtained by air-displacement plethysmography. Am. J. Clin. Nutr. 2013, 98, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Carberry, A.E.; Colditz, P.B.; Lingwood, B.E. Body Composition From Birth to 4.5 Months in Infants Born to Non-Obese Women. Pediatric Res. 2010, 68, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.; Löf, M.; Forsum, E. Body composition in full-term healthy infants measured with air displacement plethysmography at 1 and 12 weeks of age. Acta Paediatr. 2010, 99, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.A.; Gilchrist, J.M.; Catalano, P.M.; Giannì, M.L.; Roggero, P.M.; Mosca, F. Longitudinal body composition data in exclusively breast-fed infants: A multicenter study. Obesity 2011, 19, 1887–1891. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, H.; Eriksson, B.; Forsum, E.; Flinke, E.; Henriksson, P.; Löf, M. Longitudinal assessment of body composition in healthy Swedish children from 1 week until 4 years of age. Eur. J. Clin. Nutr. 2017, 71, 1345–1352. [Google Scholar] [CrossRef]
- Roggero, P.; Giannì, M.L.; Orsi, A.; Piemontese, P.; Amato, O.; Liotto, N.; Morlacchi, L.; Taroni, F.; Fields, D.A.; Catalano, P.M.; et al. Quality of Growth in Exclusively Breast-Fed Infants in the First Six Months of Life: An Italian Study. Pediatric Res. 2010, 68, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.; Löf, M.; Eriksson, O.; Hannestad, U.; Forsum, E. Fat-free mass hydration in newborns: Assessment and implications for body composition studies. Acta Paediatr. 2011, 100, 680–686. [Google Scholar] [CrossRef]
| Critical Factors | Recommendations | Achievable? |
|---|---|---|
| Choice of BIA device | Use the same device consistently throughout the study. | Y |
| Anthropometry | Weight and height should be measured at ±0.1 kg and ±0.5 cm, respectively, and measured at the time of the BIA test. | Y |
| Weight should be measured naked. | Y | |
| Length should be measured with an infant measuring board, measuring mat or measuring rod. | Y | |
| Self-reported measurements should not be used. | Y | |
| Fasting | Perform measurement after a fast of at least 4 h. | N |
| Hydration | Subjects should be normally hydrated. | N |
| Voiding | Voiding should be done prior to measurement. | N |
| Exercise | Intense physical activity should be limited for a minimum of four hours prior to measurement. | N |
| Clothing | Ensure that no metals are in the clothing. | Y |
| Electrodes | Use BIA electrodes supplied by the manufacturer. | Y |
| Use electrodes with a surface area ≥4 cm2. | N | |
| Skin preparation | Clean with alcohol before placement of the electrodes. | Y |
| Positioning of electrodes | Place at the dorsal surfaces of the wrist and ankle. | Y |
| Apply voltage electrodes at the midline between the prominent bone ends of the wrist and the ankle. | Y | |
| Place current electrodes 5 cm distal to these positions. | N | |
| Specify on which side of the body measurements are made. | Y | |
| Body position | Subjects should be supine for at least 4 – 10 min before measurements are taken. | N |
| Movement | Subjects should be relaxed during measurements. | N |
| Electrical interference | Arms and legs should be abducted within a 30–45° angle from the trunk. | Y |
| Measurements should be made on non-conductive surfaces. | Y | |
| Ensure that the device cables are not touching the ground, subjects, metal objects, routed near high voltage equipment, are used in a neutral environment and are not intertwined. | Y | |
| Temperature | Measurements should be taken at an ambient temperature. | Y |
| Time of measurement | For longitudinal follow-up studies, measurements should be performed at the same time of day. | Y |
| Calibration | Calibrate BIA device regularly. | Y |
| Operator | Ensure proper training in order to get valid and reproducible measurements. | Y |
| Data quality | Test of data quality should be performed by inspection of impedance values in BIA devices, and by inspection of Cole plots in BIS devices. | Y |
| Number of measurements | Measurements should be continued until stable values are achieved, measured to the nearest Ohm. | Y |
| The average of a minimum of three repeated measurements should be calculated. | Y |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyons-Reid, J.; Ward, L.C.; Kenealy, T.; Cutfield, W. Bioelectrical Impedance Analysis—An Easy Tool for Quantifying Body Composition in Infancy? Nutrients 2020, 12, 920. https://doi.org/10.3390/nu12040920
Lyons-Reid J, Ward LC, Kenealy T, Cutfield W. Bioelectrical Impedance Analysis—An Easy Tool for Quantifying Body Composition in Infancy? Nutrients. 2020; 12(4):920. https://doi.org/10.3390/nu12040920
Chicago/Turabian StyleLyons-Reid, Jaz, Leigh C. Ward, Timothy Kenealy, and Wayne Cutfield. 2020. "Bioelectrical Impedance Analysis—An Easy Tool for Quantifying Body Composition in Infancy?" Nutrients 12, no. 4: 920. https://doi.org/10.3390/nu12040920
APA StyleLyons-Reid, J., Ward, L. C., Kenealy, T., & Cutfield, W. (2020). Bioelectrical Impedance Analysis—An Easy Tool for Quantifying Body Composition in Infancy? Nutrients, 12(4), 920. https://doi.org/10.3390/nu12040920





