Estradiol Replacement Improves High-Fat Diet-Induced Obesity by Suppressing the Action of Ghrelin in Ovariectomized Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Experimental Protocols
2.3. IP Injection of GHRP-6
2.4. Immunohistochemistry
2.5. Sampling of Gastric Mucosae and Measurement of Adipose Tissues
2.6. Immunoblotting
2.7. RNA Isolation and RT-qPCR
2.8. Assessment of Plasma Acyl Ghrelin Level
2.9. Statistical Analyses
3. Results
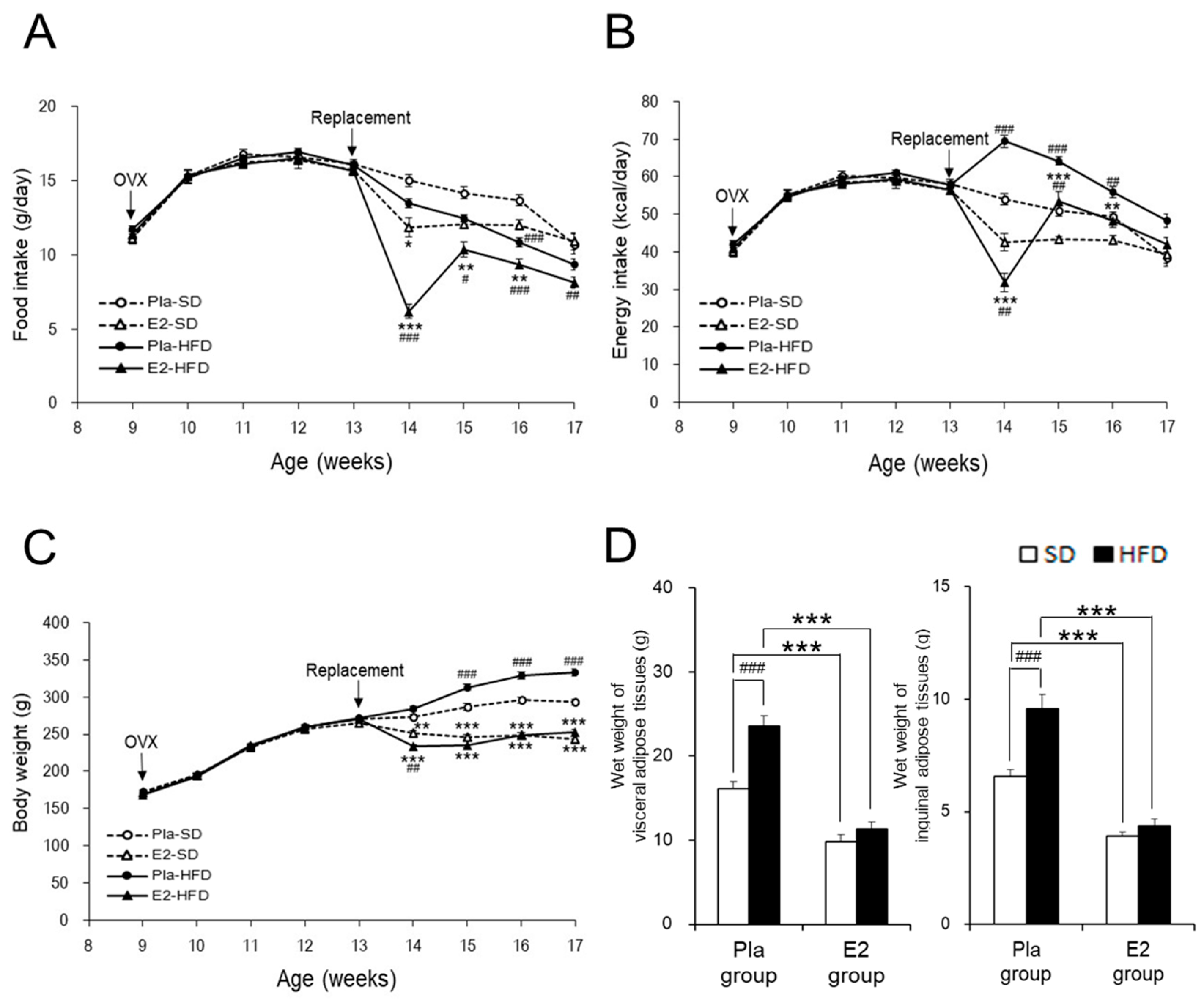
3.1. Characterization of the Studied Rats
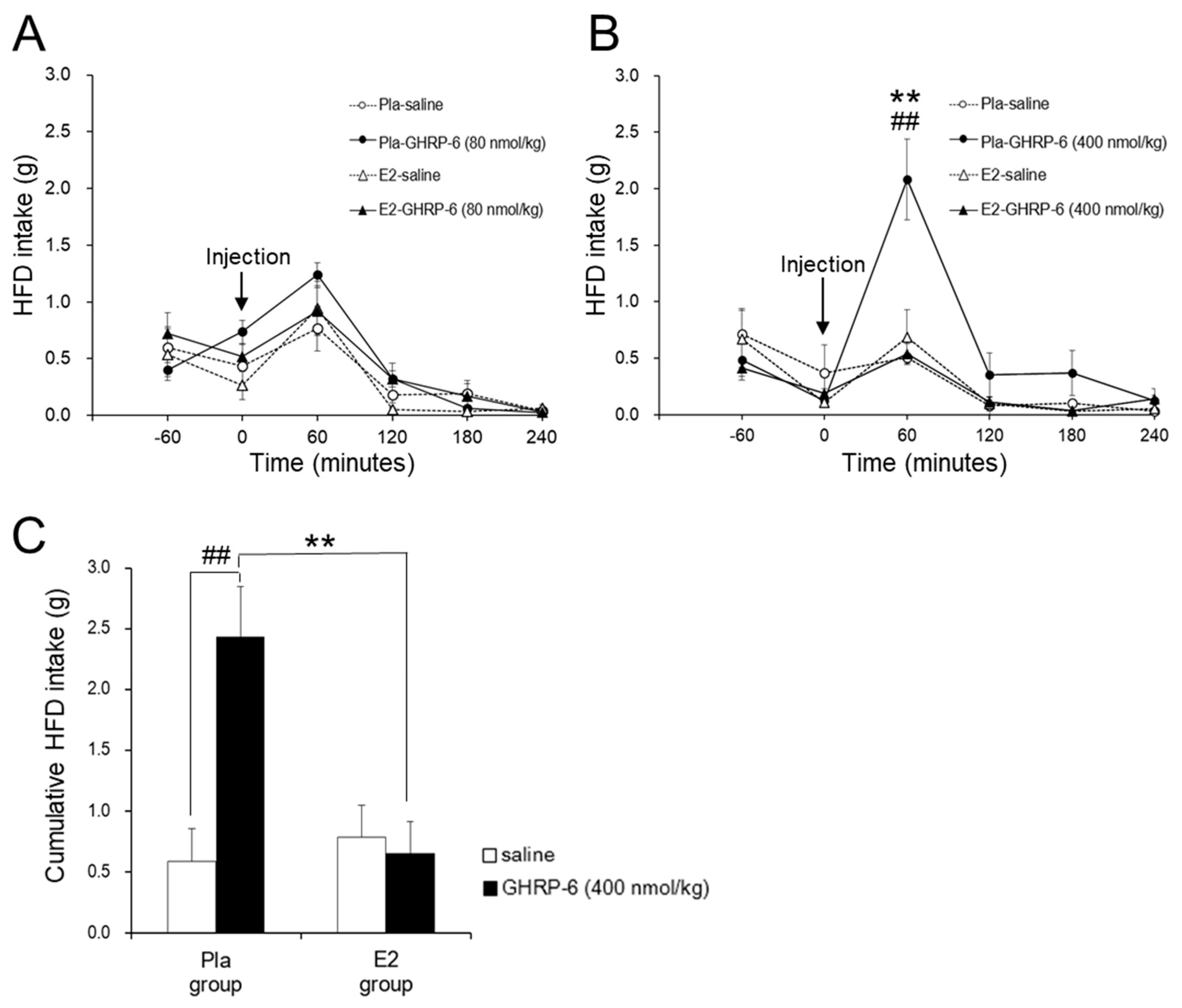
3.2. Effect of the IP GHRP-6 Injection on HFD Intake
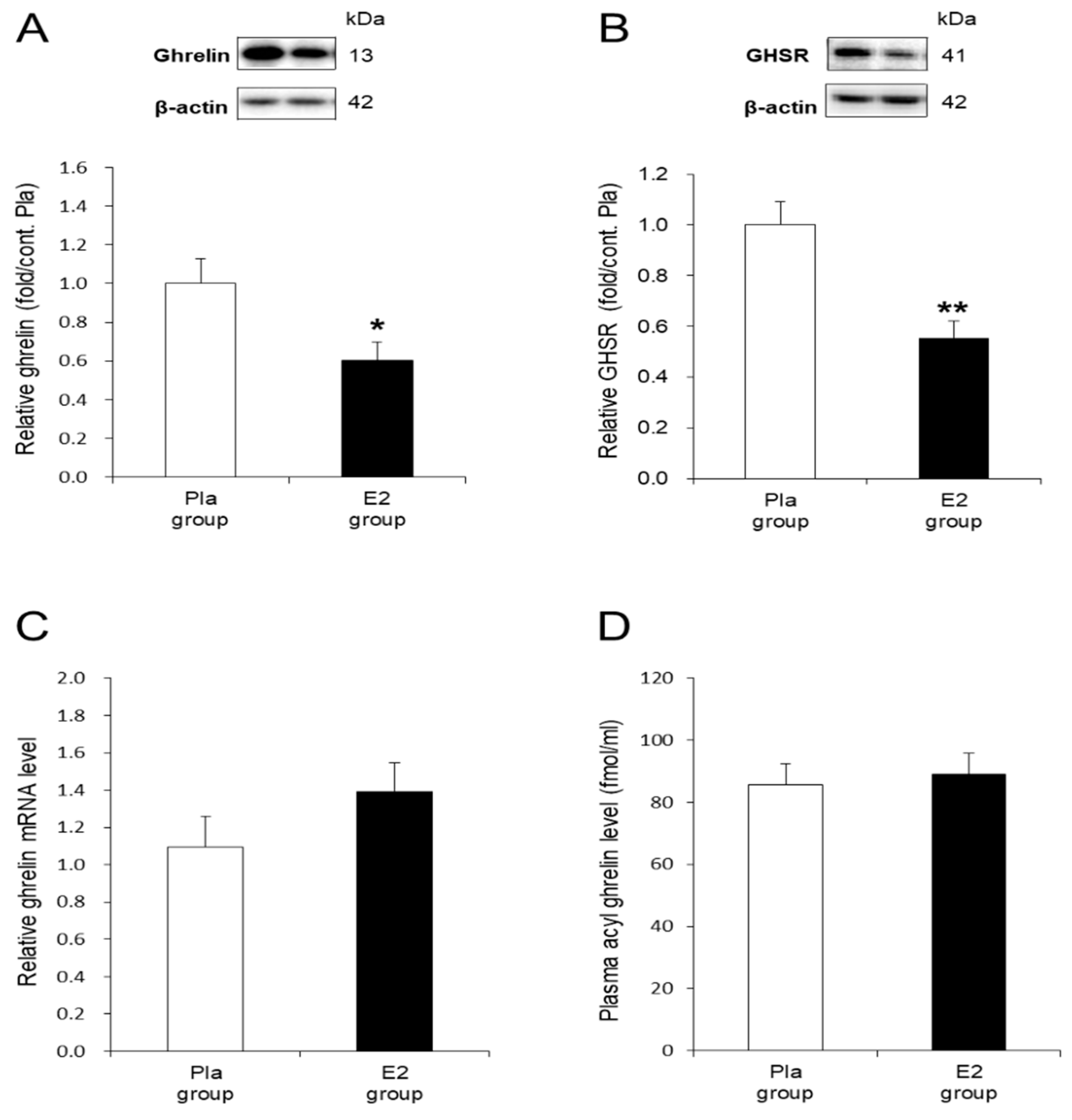
3.3. In Vivo Effects of E2 Replacement on Ghrelin and GHSR in the Stomach
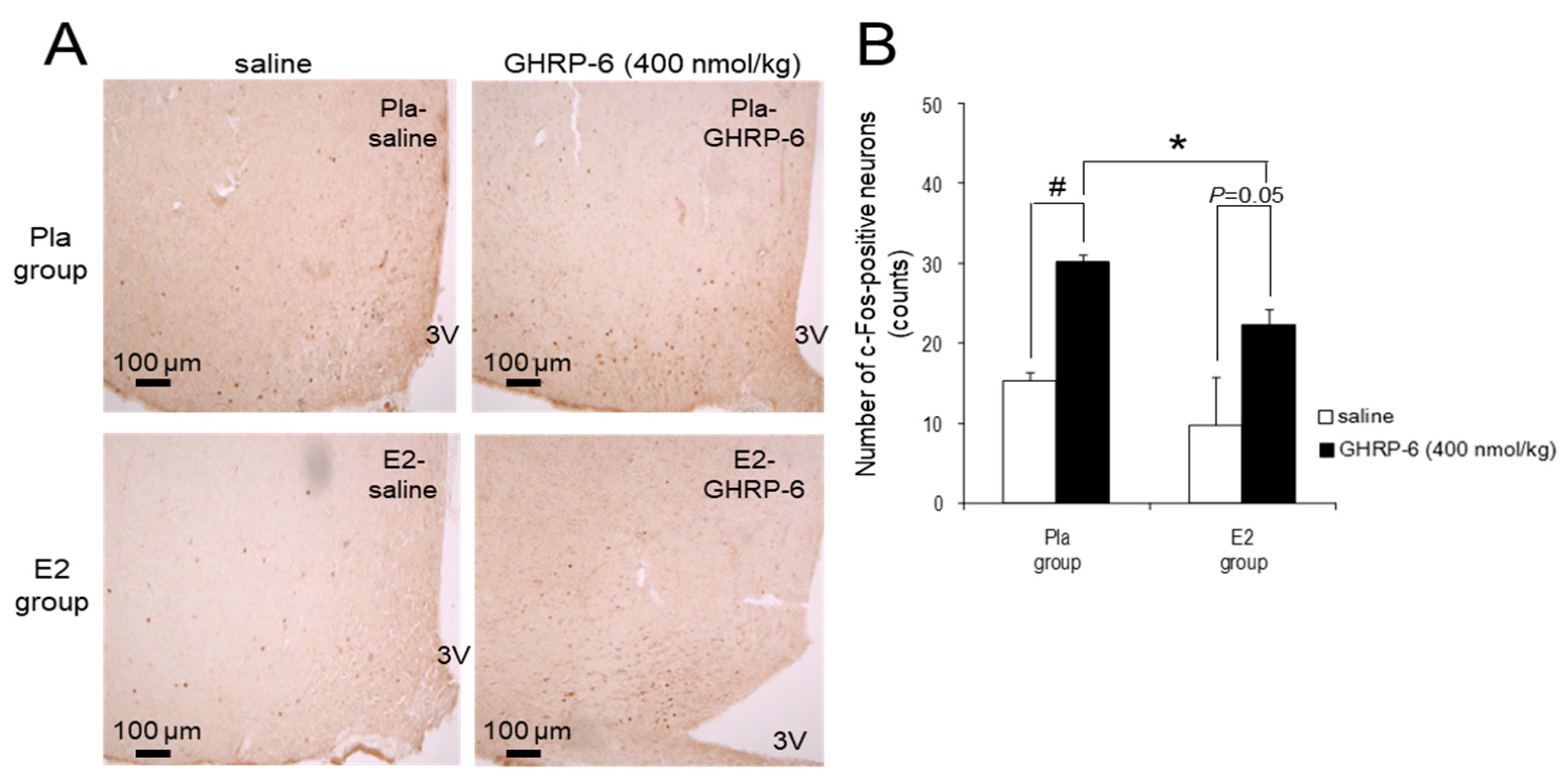
3.4. c-Fos-Positive Neurons in the ARC Induced by IP Injection of GHRP-6
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caballero, B. The global epidemic of obesity: An overview. Epidemiol. Rev. 2007, 29, 1–5. [Google Scholar] [CrossRef]
- Bray, G.A. Medical consequences of obesity. J. Clin. Endocrinol. Metab. 2004, 89, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Popkin, B.M. Dietary fat intake does affect obesity! Am. J. Clin. Nutr. 1998, 68, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, F.; Alexiu, A.; Lemonnier, D. Dietary-induced obesity: Effect of dietary fats on adipose tissue cellularity in mice. Br. J. Nutr. 1983, 49, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Asarian, L.; Geary, N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm. Behav. 2002, 42, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1215–R1267. [Google Scholar] [CrossRef]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Prud’homme, D.; Rabasa-Lhoret, R.; Strychar, I.; Brochu, M.; Lavoie, J.M.; Doucet, E. Effects of the menopausal transition on dietary intake and appetite: A MONET group study. Eur. J. Clin. Nutr. 2014, 68, 271–276. [Google Scholar] [CrossRef]
- Kristensen, K.; Pedersen, S.B.; Vestergaard, P.; Mosekilde, L.; Richelsen, B. Hormone replacement therapy affects body composition and leptin differently in obese and non-obese postmenopausal women. J. Endocrinol. 1999, 163, 55–62. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Walsh, J.M.; Ormiston, T.M.; Greyber, E.; Buckley, N.S.; Salpeter, E.E. Meta-analysis: Effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab. 2006, 8, 538–554. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Bruun, J.M.; Kristensen, K.; Richelsen, B. Regulation of UCP1, UCP2, and UCP3 mRNA expression in brown adipose tissue, white adipose tissue, and skeletal muscle in rats by estrogen. Biochem. Biophys. Res. Commun. 2001, 288, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Yokota-Nakagi, N.; Uji, M.; Yoshida, K.I.; Tazumi, S.; Takamata, A.; Uchida, Y.; Morimoto, K. Estrogen replacement enhances insulin-induced AS160 activation and improves insulin sensitivity in ovariectomized rats. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E1296–E1304. [Google Scholar] [CrossRef] [PubMed]
- Weigt, C.; Hertrampf, T.; Zoth, N.; Fritzemeier, K.H.; Diel, P. Impact of estradiol, ER subtype specific agonists and genistein on energy homeostasis in a rat model of nutrition induced obesity. Mol. Cell. Endocrinol. 2012, 351, 227–238. [Google Scholar] [CrossRef]
- Asarian, L.; Geary, N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Clegg, D.J. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid Biochem. Mol. Biol. 2010, 122, 65–73. [Google Scholar] [CrossRef]
- Clegg, D.J.; Brown, L.M.; Zigman, J.M.; Kemp, C.J.; Strader, A.D.; Benoit, S.C.; Woods, S.C.; Mangiaracina, M.; Geary, N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 2007, 56, 1051–1058. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matauo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Date, Y.; Kojima, M.; Hosoda, H.; Sawaguchi, A.; Mondal, M.S.; Suganuma, T.; Matsukura, S.; Kangawa, K.; Nakazato, M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141, 4255–4261. [Google Scholar] [CrossRef]
- Date, Y.; Murakami, N.; Toshinai, K.; Matsukura, S.; Niijima, A.; Matsuo, H.; Kangawa, K.; Nakazato, M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002, 123, 1120–1128. [Google Scholar] [CrossRef]
- Sakata, I.; Yamazaki, M.; Inoue, K.; Hayashi, Y.; Kangawa, K.; Sakai, T. Growth hormone secretagogue receptor expression in the cells of the stomach-projected afferent nerve in the rat nodose ganglion. Neurosci. Lett. 2003, 342, 183–186. [Google Scholar] [CrossRef]
- Yanagi, S.; Sato, T.; Kangawa, K.; Nakazato, M. The homeostatic force of ghrelin. Cell Metab. 2018, 27, 786–804. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.P.; Dube, M.G.; Pu, S.; Xu, B.; Horvath, T.L.; Kalra, P.S. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 1999, 20, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Hewson, A.K.; Dickson, S.L. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J. Neuroendocrinol. 2000, 12, 1047–1049. [Google Scholar] [CrossRef]
- Wang, L.; Saint-Pierre, D.H.; Taché, Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y—Synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci. Lett. 2002, 325, 47–51. [Google Scholar] [CrossRef]
- Dragunow, M.; Faull, R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods 1989, 29, 261–265. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Smith, M.S.; Verbalis, J.G. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front. Neuroendocrinol. 1993, 14, 173–213. [Google Scholar] [CrossRef]
- Zigman, J.M.; Nakano, Y.; Coppari, R.; Balthasar, N.; Marcus, J.N.; Lee, C.E.; Jones, J.E.; Deysher, A.E.; Waxman, A.R.; White, R.D.; et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Investig. 2005, 115, 3564–3572. [Google Scholar] [CrossRef]
- Maletínská, L.; Matyšková, R.; Maixnerová, J.; Sýkora, D.; Pýchová, M.; Spolcová, A.; Blechová, M.; Drápalová, J.; Lacinová, Z.; Haluzík, M.; et al. The Peptidic GHS-R antagonist [D-Lys3]GHRP-6 markedly improves adiposity and related metabolic abnormalities in a mouse model of postmenopausal obesity. Mol. Cell. Endocrinol. 2011, 343, 55–62. [Google Scholar] [CrossRef]
- Sakurazawa, N.; Mano-Otagiri, A.; Nemoto, T.; Shibasaki, T. Effects of intracerebroventricular ghrelin on food intake and Fos expression in the arcuate nucleus of the hypothalamus in female rats vary with estrous cycle phase. Neurosci. Lett. 2013, 541, 204–208. [Google Scholar] [CrossRef]
- Morimoto, K.; Kurahashi, Y.; Shintani-Ishida, K.; Kawamura, N.; Miyashita, M.; Uji, M.; Tan, N.; Yoshida, K. Estrogen replacement suppresses stress-induced cardiovascular responses in ovariectomized rats. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1950–H1956. [Google Scholar] [CrossRef] [PubMed]
- Takamata, A.; Torii, K.; Miyake, K.; Morimoto, K. Chronic oestrogen replacement in ovariectomised rats attenuates food intake and augments c-Fos expression in the suprachiasmatic nucleus specifically during the light phase. Br. J. Nutr. 2011, 106, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Tazumi, S.; Yokota, N.; Kawakami, M.; Omoto, S.; Takamata, A.; Morimoto, K. Effects of estrogen replacement on stress-induced cardiovascular responses via renin-angiotensin system in ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R898–R905. [Google Scholar] [CrossRef] [PubMed]
- Camporez, J.P.; Jornayvaz, F.R.; Lee, H.Y.; Kanda, S.; Guigni, B.A.; Kahn, M.; Samuel, V.T.; Carvalho, C.R.; Petersen, K.F.; Jurczak, M.J.; et al. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology 2013, 154, 1021–1028. [Google Scholar] [CrossRef]
- Riant, E.; Waget, A.; Cogo, H.; Arnal, J.F.; Burcelin, R.; Gourdy, P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 2009, 150, 2109–2117. [Google Scholar] [CrossRef]
- Witte, M.M.; Resuehr, D.; Chandler, A.R.; Mehle, A.K.; Overton, J.M. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen. Comp. Endocrinol. 2010, 166, 520–528. [Google Scholar] [CrossRef]
- Sato, T.; Nakashima, Y.; Nakamura, Y.; Ida, T.; Kojima, M. Continuous antagonism of the ghrelin receptor results in early induction of salt-sensitive hypertension. J. Mol. Neurosci. 2011, 43, 193–199. [Google Scholar] [CrossRef]
- Wren, A.M.; Small, C.J.; Abbott, C.R.; Dhillo, W.S.; Seal, L.J.; Cohen, M.A.; Batterham, R.L.; Taheri, S.; Stanley, S.A.; Ghatei, M.A.; et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001, 50, 2540–2547. [Google Scholar] [CrossRef]
- Kobelt, P.; Wisser, A.S.; Stengel, A.; Goebel, M.; Inhoff, T.; Noetzel, S.; Veh, R.W.; Bannert, N.; van der Voort, I.; Wiedenmann, B.; et al. Peripheral injection of ghrelin induces Fos expression in the dorsomedial hypothalamic nucleus in rats. Brain Res. 2008, 1204, 77–86. [Google Scholar] [CrossRef]
- Okada, T.; Waise, T.M.Z.; Toshinai, K.; Mita, Y.; Sakoda, H.; Nakazato, M. Analysis of peripheral ghrelin signaling via the vagus nerve in ghrelin receptor-restored GHSR-null mice. Neurosci. Lett. 2018, 681, 50–55. [Google Scholar] [CrossRef]
- Papka, R.E.; Srinivasan, B.; Miller, K.E.; Hayashi, S. Localization of estrogen receptor protein and estrogen receptor messenger RNA in peripheral autonomic and sensory neurons. Neuroscience 1997, 79, 1153–1163. [Google Scholar] [CrossRef]
- Date, Y.; Shimbara, T.; Koda, S.; Toshinai, K.; Ida, T.; Murakami, N.; Miyazato, M.; Kokame, K.; Ishizuka, Y.; Ishida, Y.; et al. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 2006, 4, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef]
- Pirnik, Z.; Majercikova, Z.; Holubova, M.; Pirnik, R.; Zelezna, B.; Maletinska, L.; Kiss, A. Effect of ghrelin receptor agonist and antagonist on the activity of arcuate nucleus tyrosine hydroxylase containing neurons in C57BL/6 male mice exposed to normal or high fat diet. J. Physiol. Pharmacol. 2014, 65, 477–486. [Google Scholar] [PubMed]
- Matsubara, M.; Sakata, I.; Wada, R.; Yamazaki, M.; Inoue, K.; Sakai, T. Estrogen modulates ghrelin expression in the female rat stomach. Peptides 2004, 25, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Gualillo, O.; Caminos, J.E.; Kojima, M.; Kangawa, K.; Arvat, E.; Ghigo, E.; Casanueva, F.F.; Diéguez, C. Gender and gonadal influences on ghrelin mRNA levels in rat stomach. Eur. J. Endocrinol. 2001, 144, 687–690. [Google Scholar] [CrossRef]
- Al-Massadi, O.; Crujeiras, A.B.; González, R.C.; Pardo, M.; Diéguez, C.; Casanueva, F.F.; Seoane, L.M. Age, sex, and lactating status regulate ghrelin secretion and GOAT mRNA levels from isolated rat stomach. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E341–E350. [Google Scholar] [CrossRef]
- Nishi, Y.; Hiejima, H.; Mifune, H.; Sato, T.; Kangawa, K.; Kojima, M. Developmental changes in the pattern of ghrelin’s acyl modification and the levels of acyl-modified ghrelins in murine stomach. Endocrinology 2005, 146, 2709–2715. [Google Scholar] [CrossRef]
- Sato, T.; Fukue, Y.; Teranishi, H.; Yoshida, Y.; Kojima, M. Molecular forms of hypothalamic ghrelin and its regulation by fasting and 2-deoxy-d-glucose administration. Endocrinology 2005, 146, 2510–2516. [Google Scholar] [CrossRef]
- Huang, K.P.; Ronveaux, C.C.; de Lartigue, G.; Geary, N.; Asarian, L.; Raybould, H.E. Deletion of leptin receptors in vagal afferent neurons disrupts estrogen signaling, body weight, food intake and hormonal controls of feeding in female mice. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E568–E577. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokota-Nakagi, N.; Takahashi, H.; Kawakami, M.; Takamata, A.; Uchida, Y.; Morimoto, K. Estradiol Replacement Improves High-Fat Diet-Induced Obesity by Suppressing the Action of Ghrelin in Ovariectomized Rats. Nutrients 2020, 12, 907. https://doi.org/10.3390/nu12040907
Yokota-Nakagi N, Takahashi H, Kawakami M, Takamata A, Uchida Y, Morimoto K. Estradiol Replacement Improves High-Fat Diet-Induced Obesity by Suppressing the Action of Ghrelin in Ovariectomized Rats. Nutrients. 2020; 12(4):907. https://doi.org/10.3390/nu12040907
Chicago/Turabian StyleYokota-Nakagi, Naoko, Haruka Takahashi, Mizuho Kawakami, Akira Takamata, Yuki Uchida, and Keiko Morimoto. 2020. "Estradiol Replacement Improves High-Fat Diet-Induced Obesity by Suppressing the Action of Ghrelin in Ovariectomized Rats" Nutrients 12, no. 4: 907. https://doi.org/10.3390/nu12040907
APA StyleYokota-Nakagi, N., Takahashi, H., Kawakami, M., Takamata, A., Uchida, Y., & Morimoto, K. (2020). Estradiol Replacement Improves High-Fat Diet-Induced Obesity by Suppressing the Action of Ghrelin in Ovariectomized Rats. Nutrients, 12(4), 907. https://doi.org/10.3390/nu12040907




