Decreased Consumption of Added Fructose Reduces Waist Circumference and Blood Glucose Concentration in Patients with Overweight and Obesity. The DISFRUTE Study: A Randomised Trial in Primary Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives
2.2. Study Design
2.3. Sample Size
2.4. Participants
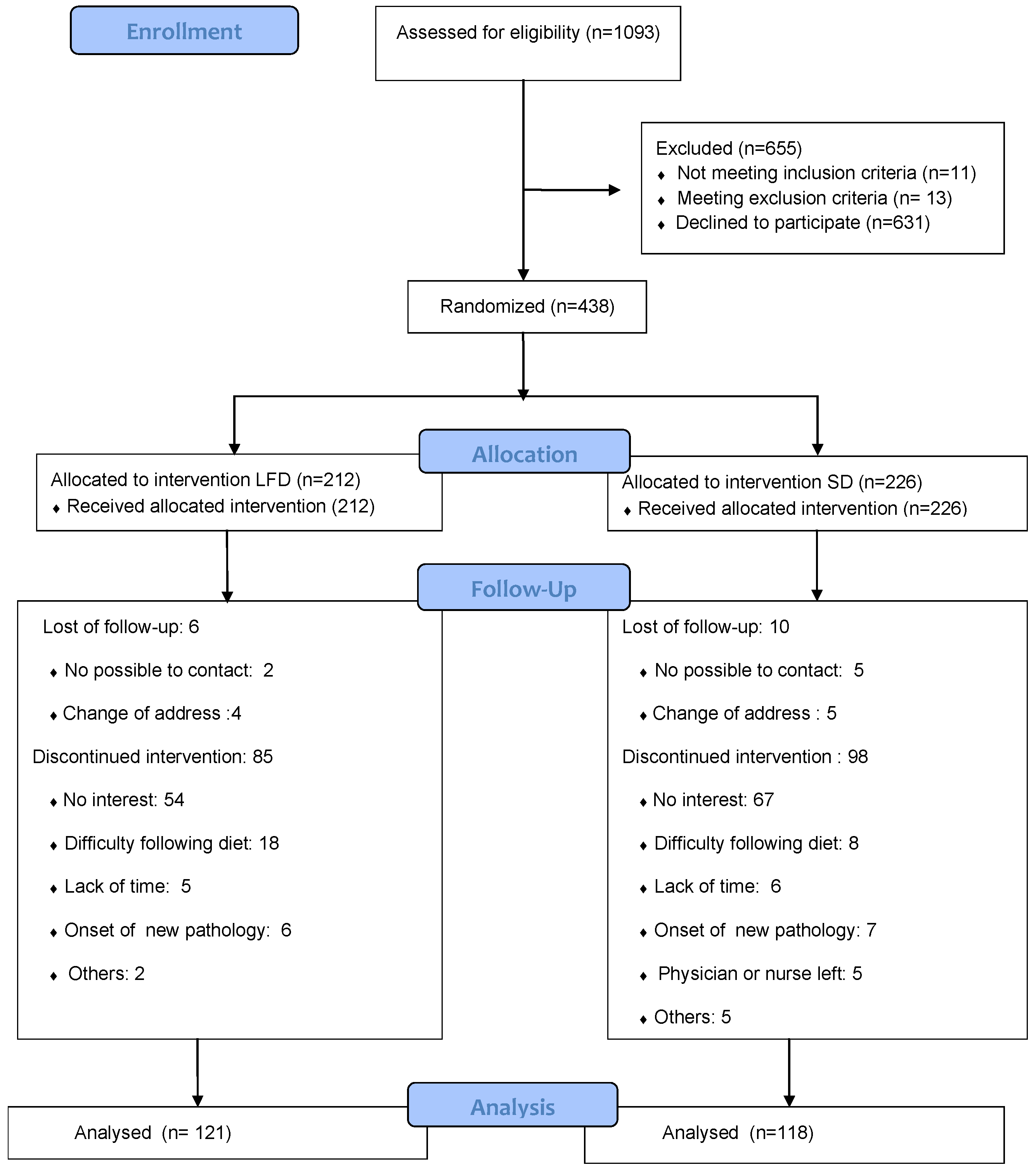
2.5. Randomization and Recruitment
2.6. Intervention
2.6.1. In Both Groups
2.6.2. In LFD Group
2.6.3. General Aspects of Dietetic and Physical Activity Interventions
2.7. Data Recording and Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
4.1. Protein Consumption
4.2. Calorie Intake, Added Glucose and Lipids
4.3. Changes between Week 24 and Week 48
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Meigs, J.B.; Rutter, M.; Sullivan, L.; Fox, C.S.; D’Agostino, R.B.; Wilson, P.W. Impact of Insulin Resistance on Risk of Type 2 Diabetes and Cardiovascular Disease in People With Metabolic Syndrome. Diabetes Care 2007, 30, 1219–1225. [Google Scholar] [CrossRef]
- Gast, K.B.; Tjeerdema, N.; Stijnen, T.; Smit, J.W.A.; Dekkers, O.M. Insulin Resistance and Risk of Incident Cardiovascular Events in Adults without Diabetes: Meta-Analysis. Plos One 2012, 7, e52036. [Google Scholar] [CrossRef]
- Ter Horst, K.; Schene, M.R.; Holman, R.; A Romijn, J.; Serlie, M.J. Effect of fructose consumption on insulin sensitivity in nondiabetic subjects: A systematic review and meta-analysis of diet-intervention trials. Am. J. Clin. Nutr. 2016, 104, 1562–1576. [Google Scholar] [CrossRef]
- Johnson, R.J.; Sanchez-Lozada, L.G.; Andrews, P.; Lanaspa, M.A. Perspective: A Historical and Scientific Perspective of Sugar and Its Relation with Obesity and Diabetes. Adv. Nutr. 2017, 8, 412–422. [Google Scholar] [CrossRef]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2015, 53, 52–67. [Google Scholar] [CrossRef]
- Basaranoglu, M.; Basaranoglu, G.; Sabuncu, T.; Senturk, H. Fructose as a key player in the development of fatty liver disease. World J. Gastroenterol. 2013, 19, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 1–15. [Google Scholar] [CrossRef]
- Domínguez Coello, S.; Carrillo Fernández, L.; Gobierno Hernández, J.; Méndez Abad, M.; Borges Álamo, C.; García Dopico, J.A.; Aguirre Jaime, A.; de León, A.C.; The DISFRUTE Group. Effectiveness of a low-fructose and/or low-sucrose diet in decreasing insulin resistance (DISFRUTE study): Study protocol for a randomized controlled trial. Trials 2017, 18, 369. [Google Scholar] [CrossRef]
- Madero, M.; Arriaga, J.C.; Jalal, D.; Rivard, C.; McFann, K.; Pérez-Méndez, O.; Vázquez, A.; Ruiz, A.; Lanaspa, M.; Jimenez, C.R.; et al. The effect of two energy-restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: A randomized controlled trial. Metabolism 2011, 60, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Weight-Loss Clinical Trials with a Minimum 1-Year Follow-Up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Hojas Informativas y Recomendaciones Para Pacientes. Anexo Dietas. Available online: https://www3.gobiernodecanarias.org/sanidad/scs/contenidoGenerico.jsp?idDocument=74c090c7-6446-11e0-8d0f-2d00982dae83&idCarpeta=61e907e3-d473-11e9-9a19-e5198e027117 (accessed on 5 March 2020).
- Cortazar, A.; Daza, P. Guía de PráCtica Clínica Sobre Diabetes Tipo 2; Guías de práctica clínica en el SNS; Ministerio de Sanidad y Consumo, Ed.; Servicio Central de Publicaciones del Gobierno Vasco: Madrid, Spain, 2008; p. 135.
- Mataix Verdú, J. Tabla de Composición de Alimentos; University of Granada: Granada, Spain, 2011. [Google Scholar]
- Errazuriz, I.; Dube, S.; Slama, M.; Visentin, R.; Nayar, S.; O’Connor, H.; Cobelli, C.; Das, S.K.; Basu, A.; Kremers, W.K.; et al. Randomized Controlled Trial of a MUFA or Fiber-Rich Diet on Hepatic Fat in Prediabetes. J. Clin. Endocrinol. Metab. 2017, 102, 1765–1774. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Strasser, B.; Hoffmann, G. Effects of Monounsaturated Fatty Acids on Glycaemic Control in Patients with Abnormal Glucose Metabolism: A Systematic Review and Meta-Analysis. Ann. Nutr. Metab. 2011, 58, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Roriz, A.K.C.; Passos, L.C.S.; De Oliveira, C.C.; Eickemberg, M.; Moreira, P.D.A.; Sampaio, L.R. Evaluation of the Accuracy of Anthropometric Clinical Indicators of Visceral Fat in Adults and Elderly. PloS ONE 2014, 9, e103499. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.-M.; Noworolski, S.M.; Erkin-Cakmak, A.; Korn, N.J.; Wen, M.J.; Tai, V.W.; Jones, G.M.; Palii, S.P.; Velasco-Alin, M.; Pan, K.; et al. Effects of Dietary Fructose Restriction on Liver Fat, De Novo Lipogenesis, and Insulin Kinetics in Children With Obesity. Gastroenterology 2017, 153, 743–752. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Mehta, V.; Onkaramurthy, N.; Okeefe, J. Fructose-induced inflammation and increased cortisol: A new mechanism for how sugar induces visceral adiposity. Prog. Cardiovasc. Dis. 2017, 61, 3–9. [Google Scholar] [CrossRef]
- Milić, S.; Lulić, D.; Štimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar]
- Sharabi, K.; Tavares, C.D.J.; Rines, A.K.; Puigserver, P. Molecular pathophysiology of hepatic glucose production. Mol. Asp. Med. 2015, 46, 21–33. [Google Scholar] [CrossRef]
- Taskinen, M.-R.; Söderlund, S.; Bogl, L.H.; Hakkarainen, A.; Matikainen, N.; Pietiläinen, K.H.; Räsänen, S.; Lundbom, N.; Björnson, E.; Eliasson, B.; et al. Adverse effects of fructose on cardiometabolic risk factors and hepatic lipid metabolism in subjects with abdominal obesity. J. Intern. Med. 2017, 282, 187–201. [Google Scholar] [CrossRef]
- Layman, D. Dietary Guidelines should reflect new understandings about adult protein needs. Nutr. Metab. 2009, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Teff, K.L.; Elliott, S.S.; Tschöp, M.; Kieffer, T.J.; Rader, D.; Heiman, M.; Townsend, R.R.; Keim, N.L.; D’Alessio, D.; Havel, P.; et al. Dietary Fructose Reduces Circulating Insulin and Leptin, Attenuates Postprandial Suppression of Ghrelin, and Increases Triglycerides in Women. J. Clin. Endocrinol. Metab. 2004, 89, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Davis, H.W.; Gastaldelli, A.; D’Alessio, D. Ghrelin Impairs Prandial Glucose Tolerance and Insulin Secretion in Healthy Humans Despite Increasing GLP-1. J. Clin. Endocrinol. Metab. 2016, 101, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Heppner, K.M.; Tong, J. Mechanisms In Endocrinology: Regulation of glucose metabolism by the ghrelin system: Multiple players and multiple actions. Eur. J. Endocrinol. 2014, 171, R21–R32. [Google Scholar] [CrossRef]
- Page, K.A.; Chan, O.; Arora, J.; Belfort-DeAguiar, R.; Dzuira, J.; Roehmholdt, B.; Cline, G.W.; Naik, S.; Sinha, R.; Constable, R.T.; et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 2013, 309, 63–70. [Google Scholar] [CrossRef]
- Chiavaroli, L.; De Souza, R.J.; Ha, V.; Cozma, A.I.; Mirrahimi, A.; Wang, D.D.; Yu, M.; Carleton, A.J.; Di Buono, M.; Jenkins, A.L.; et al. Effect of Fructose on Established Lipid Targets: A Systematic Review and Meta-Analysis of Controlled Feeding Trials. J. Am. Hear. Assoc. 2015, 4, e001700. [Google Scholar] [CrossRef]
- Aeberli, I.; Hochuli, M.; Berneis, K. Response to Comment on: Aeberli et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: A randomized controlled trial. Diabetes Care 2013, 36, 150–156. [Google Scholar] [CrossRef]
- Sock, E.T.N.; Lê, K.-A.; Ith, M.; Kreis, R.; Boesch, C.; Tappy, L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br. J. Nutr. 2009, 103, 939–943. [Google Scholar] [CrossRef]
- Schwarz, J.-M.; Noworolski, S.M.; Wen, M.J.; Dyachenko, A.; Prior, J.L.; Weinberg, M.E.; Herraiz, L.A.; Tai, V.W.; Bergeron, N.; Bersot, T.P.; et al. Effect of a High-Fructose Weight-Maintaining Diet on Lipogenesis and Liver Fat. J. Clin. Endocrinol. Metab. 2015, 100, 2434–2442. [Google Scholar] [CrossRef]
- Faeh, D.; Minehira, K.; Schwarz, J.-M.; Periasamy, R.; Park, S.; Tappy, L. Effect of Fructose Overfeeding and Fish Oil Administration on Hepatic De Novo Lipogenesis and Insulin Sensitivity in Healthy Men. Diabetes 2005, 54, 1907–1913. [Google Scholar] [CrossRef]
- Lê, K.-A.; Faeh, D.; Stettler, R.; Ith, M.; Kreis, R.; Vermathen, P.; Boesch, C.; Ravussin, E.; Tappy, L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am. J. Clin. Nutr. 2006, 84, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Lê, K.-A.; Ith, M.; Kreis, R.; Faeh, D.; Bortolotti, M.; Tran, C.; Boesch, C.; Tappy, L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Pérez-Rodrigo, C.; López-Sobaler, A.M. Dietary assessment methods: Dietary records. Nutr. Hosp. 2015, 31, 38–45. [Google Scholar] [PubMed]
- Newens, K.J.; Walton, J. A review of sugar consumption from nationally representative dietary surveys across the world. J. Hum. Nutr. Diet. 2015, 29, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef]
- Cruz, K.J.C.; De Oliveira, A.R.S.; Morais, J.B.S.; Severo, J.S.; Mendes, P.M.V.; Melo, S.R.D.S.; De Sousa, G.S.; Marreiro, D.D.N. Zinc and Insulin Resistance: Biochemical and Molecular Aspects. Biol. Trace Elem. Res. 2018, 186, 407–412. [Google Scholar] [CrossRef]
- Kostov, K. Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Severo, M.; Araújo, J.R.; Guimaraes, J.T.; Pestana, D.; Santos, A.R.S.; Ferreira, R.; Ascensão, A.; Magalhães, J.; Azevedo, I.; et al. Relevance of a Hypersaline Sodium-Rich Naturally Sparkling Mineral Water to the Protection against Metabolic Syndrome Induction in Fructose-Fed Sprague-Dawley Rats: A Biochemical, Metabolic, and Redox Approach. Int. J. Endocrinol. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
| Characteristic | Began the Study * | Completed Week 24 ** | ||||
|---|---|---|---|---|---|---|
| All (438) | Low Fructose Diet (212) | Standard Diet (226) | All (239) | Low Fructose Diet (121) | Standard Diet (118) | |
| Age (years) | 47.2 ± 8.6 | 46.3 ± 8.4 | 48.0 ± 8.7 | 47.9 ± 8.6 | 47.5 ± 8.0 | 48.2 ± 9.1 |
| Women (%) | 293 (66.9) | 141 (66.5) | 152 (67.3) | 152 (63.6) | 79 (61.9) | 73 (65.3) |
| Height (cm) | 164.9 ± 9.5 | 165.1 ± 9.5 | 164.8 ± 9.5 | 164.5 ± 9.9 | 164.8 ± 10.2 | 164.3 ± 9.5 |
| Weight (kg) | 94.4 ± 13.5 | 95.0 ± 13.6 | 93.9 ± 13.4 | 92.9 ± 13.1 | 92.8 ± 12.7 | 93.1 ± 13.6 |
| BMI (kg/m2) | 34.6 ± 2.9 | 34.8 ± 3.0 | 34.4 ± 2.8 | 34.2 ± 2.8 | 34.3 ± 2.8 | 34.2 ± 2.7 |
| Waist circumference (cm) | 108.2 ± 9.2 | 108.6 ± 9.1 | 107.7 ± 9.3 | 107.7 ± 8.9 | 107.9 ± 8.7 | 107.5 ± 9.1 |
| Waist circumference/height ratio | 0.656 ± 0.050 | 0.659 ± 0.051 | 0.654 ± 0.050 | 0.656 ± 0.050 | 0.657 ± 0.050 | 0.654 ± 0.050 |
| Hypertension (%) | 163 (37.2) | 75 (35.4) | 88 (38.9) | 90 (37.7) | 45 (37.2) | 45 (38.1) |
| Hypercholesterolemia (%) | 180 (41.1) | 95 (42) | 85 (40.1) | 105 (43.9) | 52 (43.0) | 53 (44.9) |
| Familial antecedent of diabetes (%) | 222 (51.3) | 116(54.7) | 106 (48) | 136 (56.9) | 68 (56.2) | 68 (57.6) |
| Smoking status (%) | ||||||
| -Current smoker | 52 (11.9) | 18 (8.5) | 34 (15.0) | 23 (9.6) | 3 (2.5) | 20 (16.9) |
| -Former smoker | 93 (21.2) | 47 (22.2) | 46 (20.4) | 50 (20.9) | 28 (23.1) | 22 (18.6) |
| -Never smoked | 293 (66.9) | 147 (69.3) | 146 (64.6) | 166 (69.5) | 90 (74.4) | 76 (64.4) |
| Marital status (%) | ||||||
| -Married or cohabitating | 339(77.4) | 166 (78.3) | 173 (76.5) | 189 (79.1) | 96 (79.3) | 93 (78.8) |
| -Divorced/separated | 28 (6.4) | 15 (7.1) | 13 (5.8) | 13(5.4) | 10 (8,3) | 3 (2.5) |
| -Widowed | 13 (3.0) | 7 (3.3) | 6 (2.6) | 9 (3.8) | 6 (5) | 3 (2.5) |
| -Single | 58 (13.2) | 24 (11.3) | 34 (15.1) | 28 (11.7) | 9 (7.4) | 19 (16.1) |
| Social class (%) | ||||||
| -Low | 152 (34.7) | 78 (36.8) | 74 (32.7) | 95 (39.7) | 50 (38.8) | 45 (42.0) |
| -Medium | 153 (34.9) | 78 (36.8) | 75 (33.2) | 76 (31.8) | 37 (31.1) | 39 (33.6) |
| -High | 126 (28.8) | 54(25.5) | 72 (31.9) | 64 (26.8) | 32 (29.6) | 32 (27.6) |
| -Information not provided | 7 (1.6) | 2 (0.9) | 5 (2.2) | 4 (1.7) | 2 (1.5) | 2 (1.6) |
| Blood pressure mmHg | ||||||
| -Systolic | 128.3 ± 15.7 | 129.1 ± 15.2 | 127.6 ± 16.1 | 130.2 ± 15.6 | 130.1 ± 15.9 | 130.3 ± 15.3 |
| -Diastolic | 81.4 ± 9.8 | 82.4 ± 10.4 | 80.5 ± 9.1 | 82.2 ± 9.8 | 82.6 ± 10.7 | 81.7 ± 8.6 |
| Physical activity kcal/day | ||||||
| -Previous 6 months | 287.7 (0–6562.2) | 312.3 (0–6227.6) | 262.6 (0–6562.2) | 285.4 (0–6562.2) | 310.7 (0–6227.6) | 264.4 (0–6562.2) |
| Began the Study * | Completed Week 24 ** | |||||
|---|---|---|---|---|---|---|
| All (438) | Low Fructose Diet (212) | Standard Diet (226) | All (239) | Low Fructose Diet (121) | Standard Diet (118) | |
| Fasting glucose mmol/L | 5.08 ± 0.67 | 4.94 ± 0.64 | 5.22 ± 0.67 | 5.11 ± 0.66 | 4.98 ± 0.64 | 5.24 ± 0.66 |
| Fasting insulin (µU/mL) | 11.7 (2.3–100.6) | 11.8 (3.5–100.6) | 11.5 (2.3–62) | 11.3 (2.3–62.0) | 11.5 (3.5–37.4) | 11.3 (2.3–62.0) |
| HOMA-2IR | 0.3 (0–2.2) | 0.2 (0–2.2) | 0.3 (0–1.5) | 0.2 (0–1.5) | 0.2 (0.1–0.8) | 0.2 (0–1.5) |
| 75 g OGTT Glucose mmol/L | 6.34 ± 2.08 | 6.18 ± 2.10 | 6.49 ± 2.06 | 6.45 ± 2.30 | 6.39 ± 2.40 | 6.58 ± 2.20 |
| 75 g OGTT Insulin (µU/mL) | 63.2 (6.2–300) | 59.5 (8.7–300) | 66.2 (6.2–300) | 65.5 (7.4–300) | 61.3 (13.1–300) | 66.9 (7.4–300) |
| Cholesterol mmol/L | ||||||
| Total | 4.97 ± 0.62 | 4.98 ± 0.91 | 4.97 ± 0.94 | 4.91 ± 0.90 | 4.90 ± 084 | 4.91 ± 0.98 |
| LDL | 2.99 ± 0.78 | 3.03 ± 0.80 | 2.97 ± 0.76 | 2.94 ± 0.77 | 2.90 ± 0.76 | 2.97 ± 0.78 |
| HDL | 1.25 ± 0.31 | 1.24 ± 0.29 | 1.27 ± 0.32 | 1.25 ± 0.28 | 1.28 ± 0.29 | 1.23 ± 0.27 |
| Triglycerides mmol/L | 1.58 ± 0.86 | 1.56 ± 0.82 | 1.61 ± 0.89 | 1.59 ± 0.82 | 1.58 ± 0.82 | 1.60 ± 0.83 |
| Variable | Low Fructose Diet (n = 121) | Standard Diet (n = 118) | Differences between Diets (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 24 | Difference Week 24–Week 0 (95% CI) | Week 0 | Week 24 | Difference Week 24–Week 0 (95% CI) | ||
| Nutrients and Energy Intake a | |||||||
| Kcal/day (% of difference and 95% CI) | 1900.5 ± 515.3 | 1354.6 ± 350.6 | −545.9 (−28.7 %: −36.7, −20.7) | 1841.3 ± 518.2 | 1362.2 ± 316.4 | −479.1 (−26.0%: −33.9, −18.1) | −66.8 (−2.7%: −5.6, 2.0) |
| Protein | 17.3 ± 3.9 | 22.4 ± 4.8 | 5.1 (4.3, 5.8) | 17.8 ± 3.5 | 21.4 ± 5.2 | 3.6 (2.6, 4.6) | 1.4 (0.1,2.7) * |
| Fat | 34.5 ± 8.0 | 29.0 ± 6.4 | −5.5 (−7.2, −3.7) | 33.9 ± 76.7 | 29.8 ± 74.5 | −4.1 (−6.0, −2.2) | −1.4(−3.9, 1.1) |
| SFA | 9.8 ± 2.4 | 7.8 ± 2.3 | −2.0 (−2.4, −1.5) | 9.8 ± 2.9 | 7.9 ± 2.5 | −1.9 (−2.5, −1.3) | −0.1 (−0.8, 0.7) |
| MUFA | 13.2 ± 3.2 | 11.5 ± 3.3 | −1.7 (−2.5, −0.9) | 12.7 ± 3.1 | 12.3 ± 3.8 | −0.4 (−1.2, 0.4) | −1.3 (−2.4, −0.2) * |
| PUFA | 4.8 ± 1.7 | 4.7 ± 1.5 | −0.1 (−0.5, 0.3) | 4.8 ± 1.8 | 4.8 ± 1.9 | 0.0 (−0.4, 0.4) | −0.1 (−0.07, 0.05) |
| Carbohydrates | 49.8 ± 6.8 | 49.3 ± 7.7 | −0.5 (−2.3, 1.2) | 49.2 ± 7.5 | 49.0 ± 8.2 | −0.2 (−1.8, 1.5) | −0.3 (−2.7, 2.1) |
| Starch | 25.5 ± 5.8 | 27.2 ± 7.7 | 1.7 (0.5, 3.3) | 23.9 ± 6.6 | 24.7 ± 7.7 | 0.8 (−0.9, 2.5) | 0.9 (−1.4, 3.2) |
| Lactose | 3.5 ± 2.0 | 4.4 ± 2.2 | 0.9 (0.5, 1.3) | 3.9 ± 2.6 | 4.7 ± 2.3 | 0.8 (0.4, 1.3) | 0.1 (−0.5, 0.7) |
| Galactose | 2.0 ± 1.1 | 2.6 ± 1.3 | 0.6 (0.3, 0.9) | 2.2 ± 1.3 | 2.7 ± 1.3 | 0.5 (0.3, 0.8) | 0.1(−0.2, 0.5) |
| Total sucrose | 11.1 ± 4.2 | 8.2 ± 3.5 | −2.9 (−3.8, −2.1) | 11.0 ± 4.5 | 8.9 ± 4.40 | −2.1 (−3.1, −1.1) | −0.8 (−2.2, 0.4) |
| Sucrose in natural foods | 2.5 ± 1.6 | 3.3 ± 1.9 | 0.7 (0.4, 1.1) | 2.8 ± 1.8 | 3.4 ± 2.1 | 0.6 (0.1, 1.1) | 0.1 (−0.5, 0.7) |
| Added sucrose | 8.6 ± 4.4 | 5.0 ± 3.5 | −3.7 (−4.5, −2.8) | 8.2 ± 4.6 | 5.5 ± 4.1 | −2.7 (−3.7, −2.0) | −1.0 (−2.7, 0.3) |
| Total fructose | 10.2 ± 3.3 | 8.2 ± 3.1 | −2.0 (−2.8, −1.3) | 10.4 ± 4.1 | 9.8 ± 3.7 | −0.6 (−1.6, 0.3) | −1.4 (−2.6, −0.3) * |
| Fructose in natural foods | 4.5 ± 3.4 | 5.2 ± 3.0 | 0.7 (0.0, 1.5) | 5.2 ± 4.1 | 6.5 ± 3.3 | 1.3 (0.0, 2.2) | −0.6 (−1.8, 0.5) |
| Added fructose | 5.7 ± 2.7 | 2.9 ± 2.1 | −2.8 (−3.3, −2.2) | 5.2 ± 2.9 | 3.3 ± 2.3 | −1.9 (−2.5, −1.3) | −0.9 (−1.6, −0.03) * |
| Total glucose | 9.0 ± 2.7 | 7.4 ± 2.6 | −1.6 (−2.2, −0.1) | 8.9 ± 3.1 | 7.8 ± 2.9 | −1.1 (−1.7, −0.4) | −0.5 (−1.4, 0.4) |
| Glucose in natural foods | 3.3 ± 2.3 | 4.1 ± 2.1 | 0.8 (0.4, 1.3) | 3.6 ± 2.4 | 4.3 ± 2.1 | 0.7 (0.2, 1.2) | 0.1 (−0.6, 0.8) |
| Added glucose | 5.7 ± 2.5 | 3.3 ± 2.1 | −2.4 (−2.9, −1.9) | 5.3 ± 2.7 | 3.6 ± 2.3 | −1.7 (−2.3, −1.1) | −0.7 (−0.4, 0.1) |
| Added sugars | 11.4 ± 5.1 | 6.3 ± 4.0 | −5.1 (−6.2, −4.1) | 10.5 ± 5.66 | 6.9 ± 4.5 | −3.6 (−5.0, −2.5) | −1.5(−3.0, 0.1) |
| Fiber# | 11.1 ± 6.5 | 11.7 ± 2.7 | 0.6 (−0.6, 1.8) | 10.6 ± 3.4 | 13.0 ± 12.4 | 2.4 (0.1, 4.7) | −1.8 (−4.4, 0.7) |
| Anthropometric Variables and Blood Pressure | |||||||
| Weight (kg) | 92.8 ± 12.7 | 86.3 ± 13.5 | −6.5 (−7.4, −5.5) | 93.1 ± 13.6 | 87.6 ± 13.3 | −5.5(−6.4, −4.6) | −1.0 (−2.5, 0.2) |
| BMI (kg/m2) | 34.3 ± 2.8 | 31.9 ± 3.3 | −2.4 (−2.8, −2.0) | 34.2 ± 2.7 | 32.2 ± 3.0 | −2.0 (−2.4, −1.7) | −0.4 (−0.9, 0.1) |
| Waist circumference (cm) | 107.9 ± 8.7 | 100.9 ± 10.3 | −7,0 (−8.0, −5.5) | 107.5 ± 9.0 | 102.7 ± 9.3 | −4.8 (−5.9, −3.6) | −2.2 (−3.7, −0.7) * |
| Waist circumference/height ratio | 0.66 ± 0.05 | 0.62 ± 0.06 | −0.04 (−0.049, −0.036) | 0.65 ± 0.05 | 0.62 ± 0.05 | −0.03 (−0.045, −0.023) | −0.01 (−0.021, −0.005) * |
| SBP (mmHg) | 130.1 ± 15.9 | 124.5 ± 13.6 | −5.6 (−8.4, −2.9) | 130.4 ± 15.3 | 126.2 ± 13.9 | −4.2 (−6.5, −1.7) | −1.4 (−5.0, 2.3) |
| DBP (mmHg) | 82.6 ± 10.7 | 80.4 ± 9.1 | −2.2 (−4.0, −0.3) | 81.7 ± 8.6 | 79.2 ± 9.4 | −2.5 (−4.2, −0.8) | 0.3 (−2.3, 2.7) |
| Biochemical Values b | |||||||
| Fasting glucose | 4.98 ± 0.64 | 4,73 ± 0,60 | −0.25 (−0.34, −0.17) | 5,24 ± 0,66 | 5,13 ± 0,63 | −0.11(−0.21,−0.005) | −0.14 (−0.028, −0.02) * |
| Fasting insulin | 12.5 ± 5.9 | 11.0 ± 5.8 | −1.6 (−2.5, −0.7) | 13.3 ± 8.1 | 11.7 ± 7.4 | −1.6 (−2.7, −0.5) | 0.0 (−1.4, 1.4) |
| Fasting HOMA-2IR | 0.27 ± 0.13 | 0.23 ± 0.13 | −0.04 (−0.06, −0.02) | 0.30 ± 0.19 | 0.26 ± 0.17 | −0.04 (−0.06, −0.01) | 0.00 (−0.04, 0.03) |
| Fasting total cholesterol | 4.90 ± 0.84 | 4.85 ± 0.83 | −0.05 (−0.18, 0.08) | 4.91 ± 1.03 | 4.84 ± 0.9 | −0.07 (−0.22, 0.07) | 0.02 (−0.17, 0.22) |
| Fasting HDL | 1.27 ± 0.29 | 1.30 ± 0.29 | 0.03 (−0.01, 0.06) | 1.23 ± 0.27 | 1.26 ± 0.31 | 0.03 (−0.01, 0.06) | 0 (−0.05, 0.05) |
| Fasting LDL | 2.89 ± 0.76 | 2.90 ± 0.76 | 0.01 (−0.11, 0.13) | 2.97 ± 0.78 | 2.91 ± 0.73 | −0.06 (−0.26, 0.24) | 0.07 (−0.09, 0.23) |
| Fasting Triglycerides | 1.58 ± 0.82 | 1.45 ± 0.84 | −0.13 (−0.26, 0.00) | 1.60 ± 0.83 | 1.46 ± 0.79 | −0.14 (−0.28, 0.01) | 0.01 (−0.2, 0.2) |
| 75 g OGTT glucose | 6.39 ± 2.4 | 5.57 ± 1.53 | −0.82 (−1.15, −0.49) | 6.56 ± 2.23 | 5.84 ± 2.03 | −0.72 (−1.07, −0.38) | −0.1 (−0.53, 0.43) |
| 75 g OGTT insulin | 85.1 ± 71.5 | 69.2 ± 67.9 | −15.9 (−25.1, −6.6)) | 96.7 ± 82.3 | 68.8 ± 62.7 | −27.9 (−38.6, −17.1) | 12.0 (−2.0, 26.1) |
| 75 g OGTT triglycerides | 1.54 ± 0.79 | 1.33 ± 0.68 | −0.21 (−0.26, −0.001) | 1.54 ± 0.81 | 1.34 ± 0.68 | −0.20 (−0.34, −0.08) | −0.01 (−0.17, 0.16) |
| Leisure Time Physical Activity c | |||||||
| Previous 6 months | 286 (88, 610) | 322 (145, 606) | 7.3 (–47, 63) | 236 (77, 547) | 343 (93, 638) | –11.2 (–25, 97) | 9 (–51, 90) |
| Caloric Intake and Nutrients | Low Fructose Diet (n = 95) | Standard Diet (n = 97) | Difference between Diets (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Week 0 (4 Diary Registers) | Weeks 8, 12, 16 and 20 (Mean of 2, 3 or 4 24-h Recalls) | Difference (95% CI) | Week 0 (4 Diary Registers) | Weeks 8, 12, 16 and 20 (Mean of 2, 3 or 4 24-h Recalls) | Difference (95% CI) | ||
| Kcal/day (% of difference and 95% CI) | 1904 ± 523 | 1197 ± 317 | −706 (−37.1 %: −46.8, −27.4) | 1825 ± 512 | 1283 ± 328 | −542 (−29.7%: −38.8, −20.6) | −164 (−7.4%: −12.6, −2.2) * |
| Proteins | 17.1 ± 3.9 | 23.5 ± 4.3 | 6.3 (5.4, 7.1) | 17.7 ± 3.4 | 22.3 ± 4.6 | 4.6 (3.6, 5.6) | 1.7 (0.4, 3.0) * |
| Fat | 34.3 ± 8.1 | 28.4 ± 6.8 | −5.9 (−8.0, −3.6) | 34.1 ± 8.2 | 28.8 ± 6.3 | −5.3 (−7.3, −3.3) | −0.6 (−3.6, 2.3) |
| SFA | 9.9 ± 2.4 | 7.2 ± 2.0 | −2.7 (−3.3, 2.0) | 9.7 ± 2.3 | 7.4 ± 2.2 | −2.3 (−2.9, −1.5) | −0.4 (−1.4, 0.5) |
| MUFA | 13.2 ± 3.3 | 11.4 ± 3.3 | −1.9 (−2.8, −0.9) | 12.9 ± 3.9 | 11.9 ± 3.7 | −1.0 (−1.9, −0.1) | −0.9 (−2.2, 0.4) |
| PUFA | 4.7 ± 1.7 | 4.9 ± 1.5 | 0.2 (−0.3, 0.6) | 4.9 ± 1.8 | 4.9 ± 1.7 | 0.0 (−0.4, 0.5) | 0.2 (−0.5, 0.8) |
| Carbohydrates | 50.2 ± 6.7 | 49.0 ± 7.4 | −1.2 (−3.0, 0.7) | 49.0 ± 7.8 | 49.0 ± 7.3 | 0.0 (−1.9, 1.9) | −1.2 (−3.8, 1.5) |
| Starch | 25.8 ± 5.8 | 26.2 ± 8.0 | 0.4 (−1.5, 2.2) | 23.9 ± 6.8 | 24.5 ± 7.6 | 0.6 (−1.3, 2.6) | −0.2 (−2.9, 2.3) |
| Lactose | 3.5 ± 2.0 | 4.9 ± 2.3 | 1.4 (0.8, 1.8) | 3.8 ± 2.6 | 5.2 ± 2.6 | 1.4 (0.8, 1.9) | 0.0 (−0.8, 0.7) |
| Galactose | 2.1 ± 1.1 | 2.7 ± 1.2 | 0.6 (0.3, 0.9) | 2.2 ± 1.3 | 2.9 ± 1.4 | 0.7 (0.4, 1.1) | 0.1 ((−0.6, 0.3) |
| Total sucrose | 11.1 ± 4.3 | 7.7 ± 3.8 | −3.4 (−4.5, −2.1) | 10.9 ± 4.4 | 8.2 ± 3.9 | −2.7 (−3.7, −1.6) | −0.7 (−2.2, 0.9) |
| Sucrose in natural foods | 2.5 ± 1.6 | 3.8 ± 1.9 | 1.3 (0.8, 1.8) | 2.8 ± 1.9 | 3.4 ± 2.0 | 0.6 (0.1, 1.1) | 0.7 (0, 1.3) |
| Added sucrose | 8.5 ± 4.5 | 3.9 ± 3.5 | −4.6 (−5.7, −3.4) | 8.1 ± 4.3 | 48.0 ± 3.6 | −3.3 (−4.3, −2.3) | −1.3 (−2.8, 0.2) |
| Total fructose | 10.3 ± 3.4 | 8.3 ± 3.1 | −2.0 (−2.9, −1.0) | 10.4 ± 3.5 | 9.5 ± 3.3 | −0.9 (−1.9, 0.1) | −1.1 (−2.4, 0.3) |
| Fructose in natural foods | 4.7 ± 3.6 | 6.1 ± 3.2 | 1.4 (0.5, 2.3) | 5.4 ± 4.4 | 6.8 ± 3.4 | 1.4 (0.5, 2.4) | 0.0 (−1.3, 1.3) |
| Added fructose | 5.6 ± 2.6 | 2.2 ± 2.1 | −3.4 (−4.0, −2.7) | 5.0 ± 2.8 | 2.7 ± 2.0 | −2.3 (−2.9, −1.7) | −1.1 (−2.0, −0.2) * |
| Total glucose | 9.1 ± 2.8 | 7.6 ± 2.5 | −1.5 (−2.2, −0.7) | 8.8 ± 3.2 | 7.8 ± 2.5 | −1.0 (−1.8, −0.3) | −0.5 (−1.5, 0.6) |
| Glucose in natural foods | 3.4 ± 2.4 | 4.9 ± 2.3 | 1.5 (0.9, 2.1) | 3.7 ± 2.5 | 4.5 ± 2.2 | 0.8 (2.2, 1.4) | 0.7 (−0.2, 1.5) |
| Added glucose | 5.7 ± 2.5 | 2.7 ± 2.1 | −3.0 (−3.6, −2.3) | 5.1 ± 2.6 | 3.3 ± 2.1 | −1.9 (−2.5, −1.3) | −1.1 (− 2, −0.2) * |
| Added sugars | 11.3 ± 0. 5 | 4.9 ± 4.1 | −6.4 (−7.7, −5.1) | 10.2 ± 5.3 | 6.0 ± 3.9 | −4.2 (−5.4, −3.0) | −2.2 (−4.0, −0.5) * |
| Fiber | 11.3 ± 7.2 | 12.9 ± 3.2 | 1.6 (0, 0.3) | 10.8 ± 3.4 | 12.5 ± 3.4 | 1.7 (0.9, 2.6) | −0.1 (−0.2, 0.2) |
| Outcome | Low Fructose Diet (n = 77) | Standard Diet (n = 77) | Difference between Diets Week 48–Week 24 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 24 | Week 48 | Week 48–Week 24 | Week 0 | Week 24 | Week 48 | Week 48–Week 24 | ||
| Glucose (mmol/L) | 4.98 ± 0.59 | 4.72 ± 0.51 | 5.17 ± 0.56 | 0.45 (0.34, 0.57) | 5.19 ± 0.67 | 5.16 ± 0.68 | 5.24 ± 0.62 | 0.08 (−0.07, 0.23) | 0.37 (0.18, 0.56) |
| Insulin (µU/mL) | 12.2 ± 5.1 | 11.2 ± 6.1 | 10.3 ± 4.8 | −0.9 (−2.0, 0.7) | 12.4 ± 8.2 | 11.3 ± 7.9 | 13.0 ± 10.1 | 1.7 (0.5, 2.9) | −2.6 (−4.2, −1.1) |
| HOMA-2IR | 0.26 ± 0.10 | 0.23 ± 0.14 | 0.22 ± 0.10 | −0.01 (−0.03, 0.02) | 0.28 ± 0.20 | 0.25 ± 0.18 | 0.29 ± 0.231 | 0.04 (0.01, 0.07) | −0.05 (−0.08, −0.01) |
| BMI (kg/m2) | 34.2 ± 2.8 | 31.9 ± 3.2 | 32.1 ± 3.1 | 0.2 (−0.2, 0.6) | 34.3 ± 2.8 | 32.4 ± 3.1 | 33.0 ± 3.4 | 0.6 (0.2, 0.9) | −0.4 (−0.9, 1.1) |
| WC (cm) | 107.2 ± 8.4 | 100.3 ± 10.1 | 100.3 ± 9.5 | 0.0 (0.002, 0.01) | 108.2 ± 9.5 | 103.3 ± 9.4 | 104.5 ± 9.1 | 1.2 (0.2, 2.1) | −1.2 (−2.5, 0.1) |
| WC/H ratio | 0.655 ± 0.05 | 0.614 ± 0.056 | 0.614 ± 0.053 | 0.0(−0.006, 0.006) | 0.658 ± 0.05 | 0.628 ± 0.053 | 0.635 ± 0.053 | 0.007 (0.001, 0.013) | −0.007 (−0.02, 0.001) |
| Weight (kg) | 92.3 ± 12.3 | 85.8 ± 13.4 | 86.2 ± 12.8 | 0.4 (−0.5, 1.3) | 93.5 ± 14.1 | 88.4 ± 13.5 | 89.9 ± 14.1 | 1.5 (0.6, 2.5) | −1.1 (−2.5, 0.2), |
| Total cholesterol (mmol/L) | 4.93 ± 0.79 | 4.86 ± 0.86 | 5.17 ± 0.88 | 0.31 (0.16, 0.47) | 4.77 ± 0.88 | 4.78 ± 0.83 | 5.04 ± 0.94 | 0.26 (0.1, 0.43) | 0.05 (−0.18, 0.27) |
| HDL (mmol/L) | 1.25 ± 0.28 | 1.28 ± 0.30 | 1.36 ± 0.34 | 0.08 (0.03, 0.13) | 1.22 ± 0.28 | 1.25 ± 0.30 | 1.32 ± 0.33 | 0.07 (0.02, 0.11) | 0.01(−0.06, 0.08) |
| LDL (mmol/L) | 2.92 ± 0.75 | 2.88 ± 0.79 | 3.08 ± 0.82 | 0.20 (0.06, 0.33) | 2.87 ± 0.66 | 2.86 ± 0.58 | 3.02 ± 0.82 | 0.16 (0.02, 0.29) | 0.04 (−0.15, 0.23) |
| Triglycerides (mmol/L) | 1.66 ± 0.92 | 1.55 ± 0.94 | 1.48 ± 0.80 | −0.07 (−0.25, 0.11) | 1.58 ± 0.84 | 1.42 ± 0.67 | 1.48 ± 0.88 | 0.06 (−0.07, 0.19) | −0.13 (−0.35, 0.38) |
| SBP (mmHg) | 129.8 ± 17.0 | 124.0 ± 14.2 | 123.0 ± 14.1 | −1.0 (−4.1, 2.1) | 128.2 ± 14.6 | 125.2 ± 14.7 | 126.3 ± 14.7 | 1.1 (−1.7, 3.9) | −2.1 (−6.2, 2.1) |
| DBP (mmHg) | 82.3 ± 11.6 | 80.3 ± 9.9 | 77.8 ± 9.5 | −2.5 (−4.3, −0.7) | 80.7 ± 8.5 | 79.1 ± 9.3 | 81.2 ± 13.4 | 2.1 (−0.8, 5.0) | −4.6 (−8.0, −1.2) |
| Outcomes a | Group (SD = 0, LFD = 1). | |
|---|---|---|
| Primary | B (95% CI) | p |
| HOMA2-IR | −0.004 (−0.040, 0.032) | 0.822 |
| Fasting glucose (mmol/L) | −0.27 (−0.39, −0.14) | <0.001 |
| Fasting insulin (µU/mL) | −0.03 (−1.6, 1.5) | 0.971 |
| 75 g OGTT glucose (mmol/L) | −0.22 (−0.72, 0.28) | 0.382 |
| 75 g OGTT insulin (µU/mL) | 14.9 (−0.3, 30.1) | 0.054 |
| Secondary | B (95% CI) | p |
| Weight (kg) | −0.1 (−1.6, 1.3) | 0.665 |
| BMI (kg/m2) | −0.08 (−0.61, 0.45) | 0.772 |
| WC (cm) | −1.7 (−3.3, −0.062) | 0.043 |
| WC/H ratio | −0.01 (−0.020, −0.001) | 0.035 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Coello, S.; Carrillo-Fernández, L.; Gobierno-Hernández, J.; Méndez-Abad, M.; Borges-Álamo, C.; García-Dopico, J.A.; Aguirre-Jaime, A.; Cabrera-de León, A. Decreased Consumption of Added Fructose Reduces Waist Circumference and Blood Glucose Concentration in Patients with Overweight and Obesity. The DISFRUTE Study: A Randomised Trial in Primary Care. Nutrients 2020, 12, 1149. https://doi.org/10.3390/nu12041149
Domínguez-Coello S, Carrillo-Fernández L, Gobierno-Hernández J, Méndez-Abad M, Borges-Álamo C, García-Dopico JA, Aguirre-Jaime A, Cabrera-de León A. Decreased Consumption of Added Fructose Reduces Waist Circumference and Blood Glucose Concentration in Patients with Overweight and Obesity. The DISFRUTE Study: A Randomised Trial in Primary Care. Nutrients. 2020; 12(4):1149. https://doi.org/10.3390/nu12041149
Chicago/Turabian StyleDomínguez-Coello, Santiago, Lourdes Carrillo-Fernández, Jesús Gobierno-Hernández, Manuel Méndez-Abad, Carlos Borges-Álamo, José Antonio García-Dopico, Armando Aguirre-Jaime, and Antonio Cabrera-de León. 2020. "Decreased Consumption of Added Fructose Reduces Waist Circumference and Blood Glucose Concentration in Patients with Overweight and Obesity. The DISFRUTE Study: A Randomised Trial in Primary Care" Nutrients 12, no. 4: 1149. https://doi.org/10.3390/nu12041149
APA StyleDomínguez-Coello, S., Carrillo-Fernández, L., Gobierno-Hernández, J., Méndez-Abad, M., Borges-Álamo, C., García-Dopico, J. A., Aguirre-Jaime, A., & Cabrera-de León, A. (2020). Decreased Consumption of Added Fructose Reduces Waist Circumference and Blood Glucose Concentration in Patients with Overweight and Obesity. The DISFRUTE Study: A Randomised Trial in Primary Care. Nutrients, 12(4), 1149. https://doi.org/10.3390/nu12041149





