Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications
Abstract
1. Introduction
2. Methods
3. Cereals and Pseudocereals Used for Food Production by LAB Fermentation
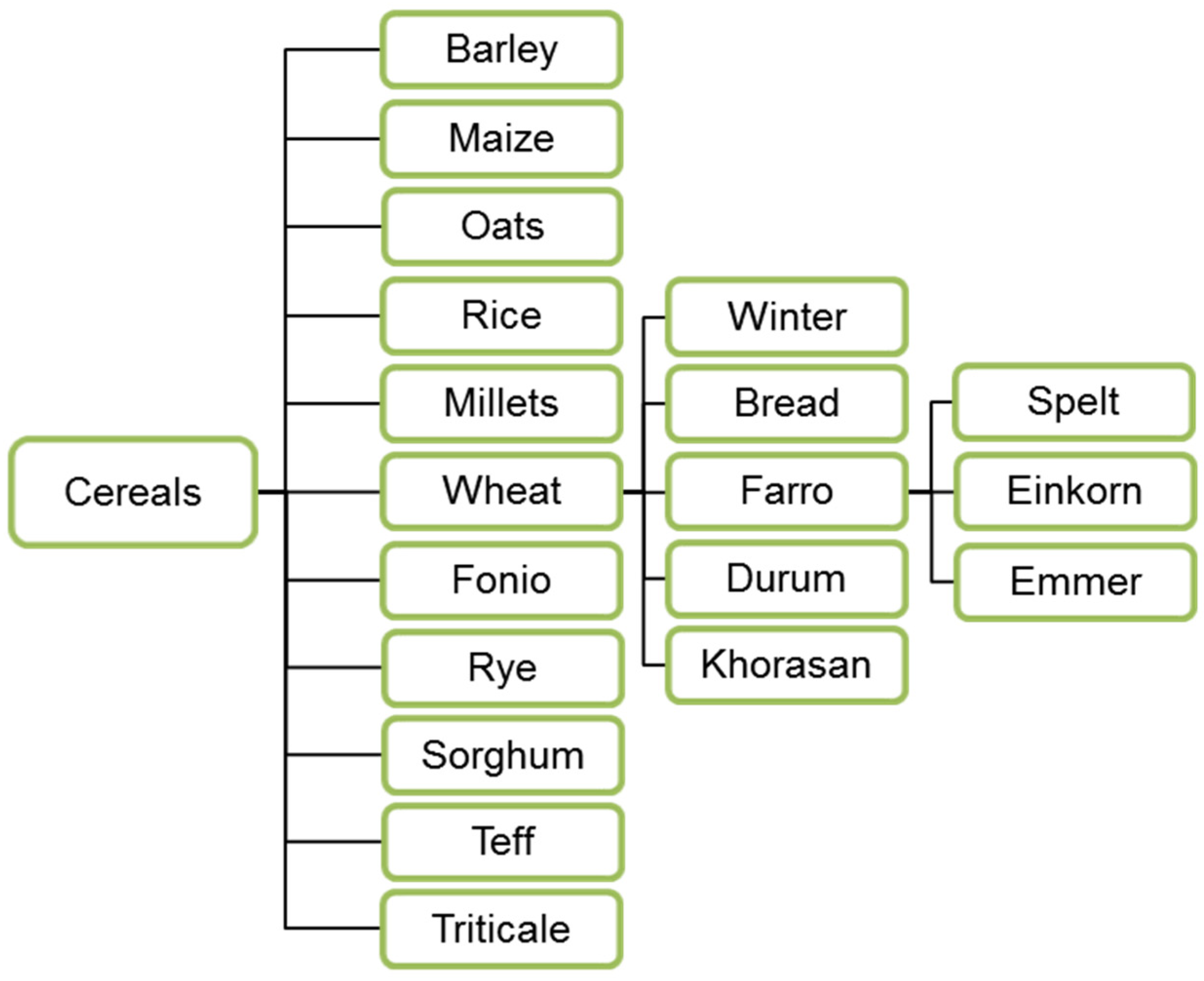
3.1. Cereals—Modern and Ancient Plant Types
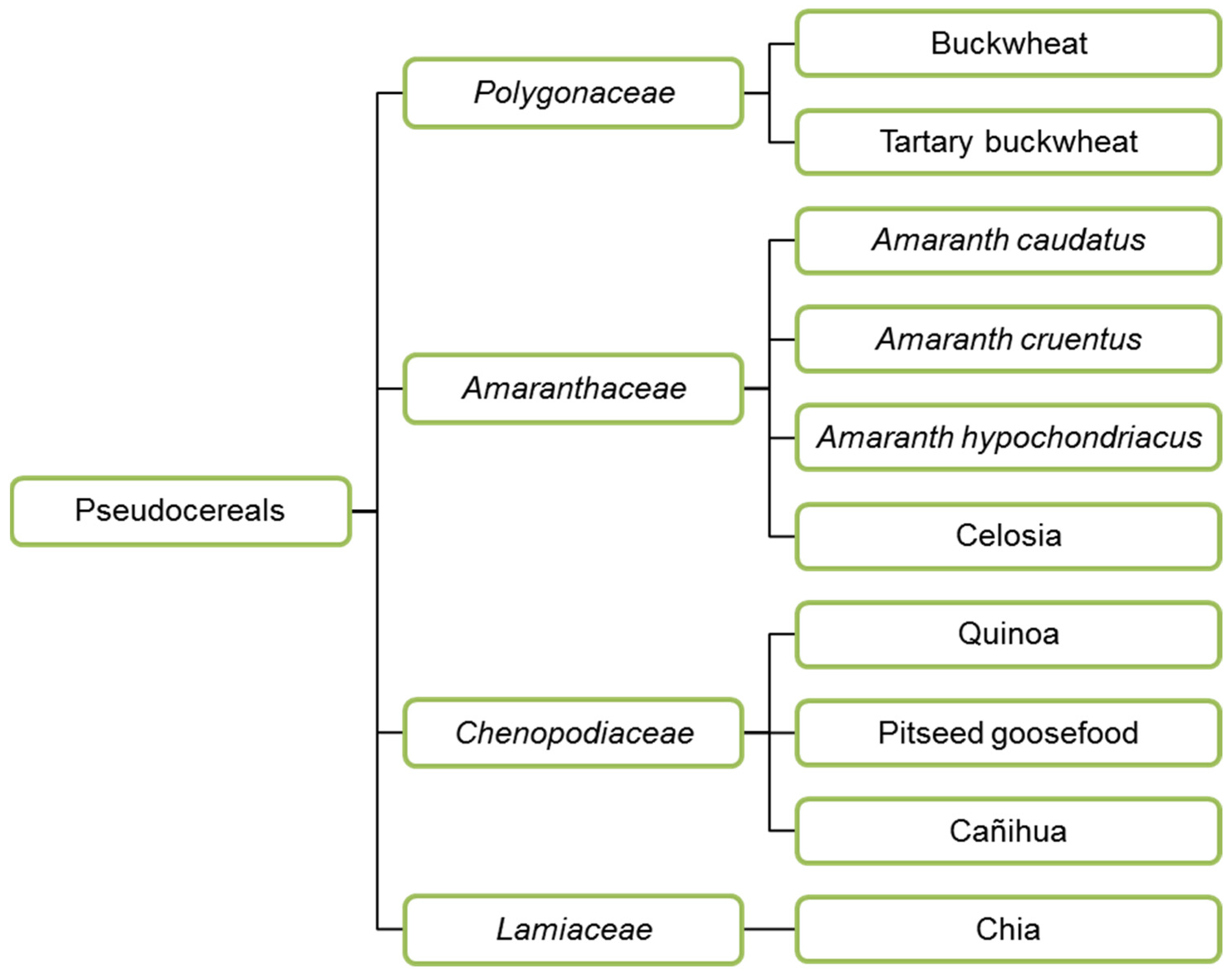
3.2. Pseudocereals
4. LAB Cereal Fermentation: Microbiology and Biochemistry of the Process
4.1. The Beneficial Impact of Cereal-Fermenting LAB on Human Health
4.2. LAB Taxonomy
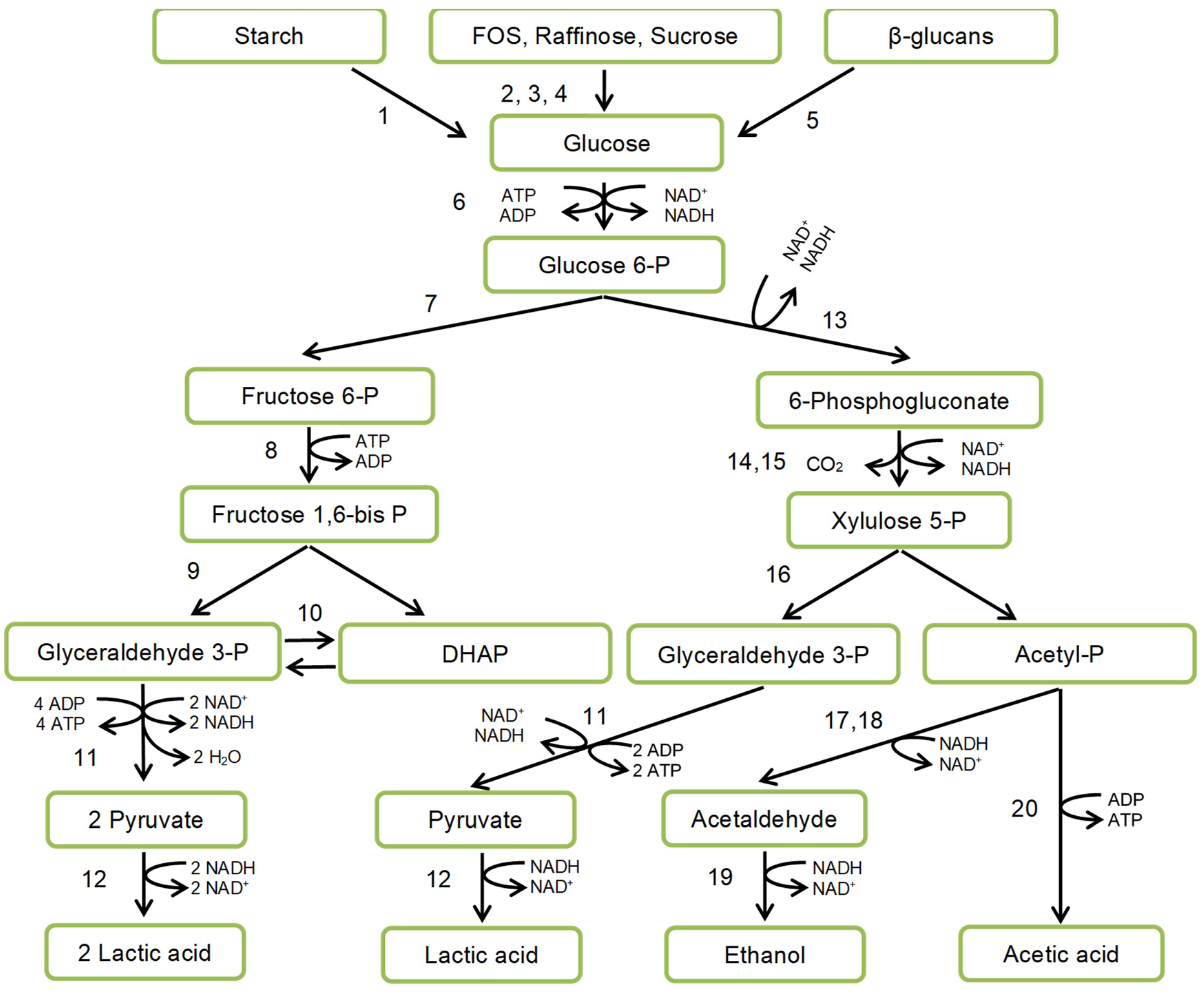
4.3. Starch Fermentation by Amylolytic Lactic Acid Bacteria (ALAB)
4.4. Fibres Fermentation by LAB: The Prebiotic Effect
5. LAB Fermented Cereal Foods and Beverages around the World
6. Traditional Microbial Processes with Modern Applications: LAB Fermented Rye Bread, Pasta and Cereal Beverages
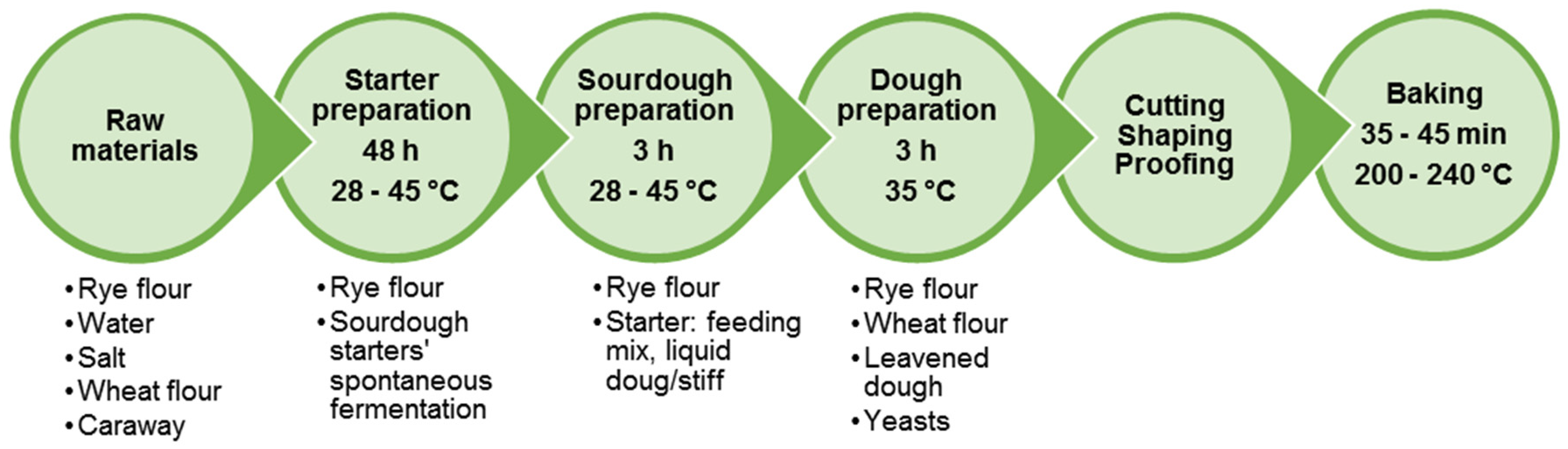
6.1. Rye Bread Production Technology
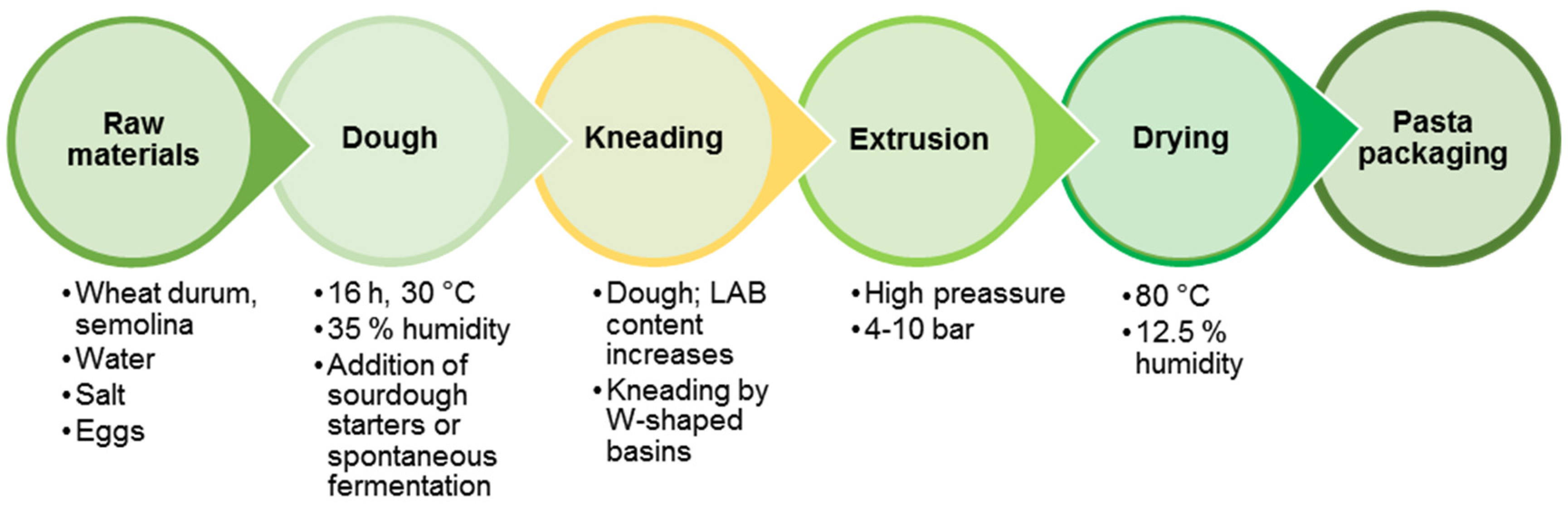
6.2. Fermented Pasta Production Technology
6.3. Production Technology of LAB Fermented Cereal Beverages
7. LAB Pseudocereals Fermentation for the Development of Foods for Consumers with Health Problems
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALAB | Amylolytic Lactic acid bacteria |
| GIT | Gastro-Intestinal Tract |
| GOS | Galactooligosaccharides |
| FAO | Food Agricultural Organization of the United Nations |
| FOS | Fructooligosaccharides |
| LA | Lactic acid |
| LAB | Lactic acid bacteria |
| p.a. | per annum |
| SBD | Starch-Binding Domain |
| SCFA | Short Chain Fatty Acids |
References
- Pollard, E.; Rosenberg, C.; Tigor, R. Worlds Together, Worlds Apart, 1st ed.; W. W. Norton & Company: New York, NY, USA, 2015. [Google Scholar]
- Weiss, E.; Zohary, D. The neolithic southwest asian founder crops: Their biology and archaeobotany. Curr. Anthropol. 2011, 52, S239–S240. [Google Scholar] [CrossRef]
- Cohen, D.J. The beginnings of agriculture in China: A multiregional view. Curr. Anthropol. 2011, 52, S273–S293. [Google Scholar] [CrossRef]
- Rosentrater, K.A.; Evers, A.D. Introduction to cereals and pseudocereals and their production. In Kent’s Technology of Cereals: An Introduction for Students of Food Science and Agriculture, 5th ed.; Woodhead Publishing: Duxford, UK, 2018; pp. 1–76. [Google Scholar]
- FAO Homepage. Available online: http://www.fao.org/faostat (accessed on 23 January 2020).
- Anal, A.K. Quality ingredients and safety concerns for traditional fermented foods and beverages from Asia: A review. Fermentation 2019, 5, 8. [Google Scholar] [CrossRef]
- Gille, D.; Schmid, A.; Walther, B.; Vergères, G. Fermented food and non-communicable chronic diseases: A review. Nutrients 2018, 10, 448. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Traditional cereal beverage Boza—Fermentation technology; microbial content and healthy effects. In Fermented Food—Part II: Technological Interventions, 1st ed.; Ray, R., Montet, D., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 284–305. [Google Scholar]
- Panda, S.; Ray, R. Amylolytic lactic acid bacteria—Technological interventions in food fermentations. In Fermented Foods; Part I: Biochemistry and Biotechnology, 1st ed.; Montet, D., Ray, R., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 133–150. [Google Scholar]
- Wacoo, A.P.; Mukisa, I.M.; Meeme, R.; Byakika, S.; Wendiro, D.; Sybesma, W.; Kort, R. Probiotic enrichment and reduction of Aflatoxins in a traditional African maize-based fermented food. Nutrients 2019, 11, 265. [Google Scholar] [CrossRef]
- Rollan, G.C.; Gerez, C.L.; Leblanc, J.G. Lactic fermentation as a strategy to improve the nutritional and functional values of pseudocereals. Front. Nutr. 2019, 6, 98. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef]
- Tamene, A.; Baye, K.; Kariluoto, S.; Edelmann, M.; Bationo, F.; Leconte, N.; Humblot, C. Lactobacillus plantarum P2R3FA isolated from traditional cereal-based fermented food increase folate status in deficient rats. Nutrients 2019, 11, 2819. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Loiseau, G.; Icard-Verniere, C.; Rochette, I.; Treche, S.; Guyot, J.-P. Effect of fermentation by amylolytic lactic acid bacteria; in process combinations; on characteristics of rice/soybean slurries: A new method for preparing high energy density complementary foods for young children. Food Chem. 2007, 100, 623–631. [Google Scholar] [CrossRef]
- Cabello-Olmo, M.; Oneca, M.; Torre, P.; Sainz, N.; Moreno-Aliaga, M.J.; Guruceaga, E.; Díaz, J.V.; Encio, I.J.; Barajas, M.; Araña, M. A fermented food product containing lactic acid bacteria protects ZDF rats from the development of type 2 diabetes. Nutrients 2019, 11, 2530. [Google Scholar] [CrossRef] [PubMed]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Achi, O.; Asamudo, N. Cereal-based fermented foods of Africa as functional foods. In Bioactive Molecules in Food; Reference Series in Phytochemistry, 1st ed.; Mérillon, J., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2019; pp. 1527–1558. [Google Scholar]
- Turpin, W.; Humblot, C.; Guyot, J.-P. Genetic screening of functional properties of lactic acid bacteria in a fermented pearl millet slurry and in the metagenome of fermented starchy foods. Appl. Environ. Microbiol. 2011, 77, 8722–8734. [Google Scholar] [CrossRef] [PubMed]
- Guyot, J.-P. Cereal-based fermented foods in developing countries: Ancient foods for modern research. Int. J. Food Sci. Tech. 2012, 47, 1109–1114. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K.; Stoyancheva, G. Starch-modifying enzymes of lactic acid bacteria—Structures; properties; and applications. Starch-Stärke 2013, 65, 34–47. [Google Scholar] [CrossRef]
- Velikova, P.; Stoyanov, A.; Blagoeva, G.; Popova, L.; Petrov, K.; Gotcheva, V.; Angelov, A.; Petrova, P. Starch utilization routes in lactic acid bacteria: New insight by gene expression assay. Starch-Stärke 2016, 68, 953–960. [Google Scholar] [CrossRef]
- Løje, H.; Møller, B.; Laustsen, A.M.; Hansen, A. Chemical composition, functional properties and sensory profiling of einkorn (Triticum monococcum L.). J. Cereal Sci. 2003, 37, 231–240. [Google Scholar] [CrossRef]
- McKevith, B. Nutritional aspects of cereals. Nutrition Bulletin. Br. Nutr. Found. 2004, 29, 111–142. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Parker, M.L. Quinoa. In Pseudocereals and Less Common Cereals, 1st ed.; Belton, P.S., Taylor, J.R.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 93–122. [Google Scholar]
- La Vieille, S.; Pulido, O.M.; Abott, M.; Koerner, T.B.; Godefroy, S. Celiac disease and gluten-free oats: A Canadian position based on a literature review. Can. J. Gastroenterol. Hepatol. 2016, 2016, 1870305. [Google Scholar] [CrossRef]
- El Khoury, D.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta glucan: Health benefits in obesity and metabolic syndrome. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3236515/ (accessed on 12 January 2020).
- Angelov, A.; Gotcheva, V.; Kuncheva, R.; Hristozova, T. Development of a new oat-based probiotic drink. Int. J. Food Microbiol. 2006, 112, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Ponsa, I.; Aurab, M.A.; Vuorelab, S.; Kolehmainena, M.; Mykkanena, H.; Poutanen, K. Rye phenolics in nutrition and health. J. Cereal Sci. 2009, 49, 323–336. [Google Scholar] [CrossRef]
- Pontonio, E.; Rizzello, C.G. Minor and ancient cereals: Exploitation of the nutritional potential through the use of selected starters and sourdough fermentation. In Flour and Breads and Their Fortification in Health and Disease Prevention, 1st ed.; Preedy, V., Watson, R., Patel, V., Eds.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: London, UK, 2019; pp. 443–452. [Google Scholar]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. Sorghum grain: From genotype, nutrition, and phenolic profile to its health benefits and food applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2025–2046. [Google Scholar] [CrossRef]
- Čurna, V.; Lacko-Bartošová, M. Chemical composition and nutritional value of Emmer wheat (Triticum dicoccon Schrank): A review. J. Cent. Eur. Agric. 2017, 18, 117–134. [Google Scholar]
- Hawkesford, M.J.; Zhao, F.-J. Strategies for increasing the selenium content of wheat. J. Cereal Sci. 2017, 46, 282–292. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food; pharmaceutical ingredients; and the potential health benefits. J. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Gebremariam, M.M.; Zarnkow, M.; Becker, T. Teff (Eragrostis tef) as a raw material for malting, brewing and manufacturing of gluten-free foods and beverages: A review. J. Food Sci. Technol. 2014, 51, 2881–2895. [Google Scholar] [CrossRef]
- Koubová, E.; Sumczynski, D.; Šenkárová, L.; Orsavová, J.; Fišera, M. Dietary intakes of minerals, essential and toxic trace elements for adults from Eragrostis tef L.: A nutritional assessment. Nutrients 2018, 10, 479. [Google Scholar] [CrossRef]
- Mohammed, S.H.; Taye, H.; Sissay, T.A.; Larijani, B.; Esmaillzadeh, A. Teff consumption and anemia in pregnant Ethiopian women: A case-control study. Eur. J. Nutr. 2019, 58, 2011–2018. [Google Scholar] [CrossRef]
- Nardi, E.P.; Evangelista, F.S.; Tormen, L.; SaińtPierre, T.D.; Curtius, A.J.; de Souza, S.; Barbosa, F., Jr. The use of inductively coupled plasma mass spectrometry (ICP-MS) for the determination of toxic and essential elements in different types of food samples. Food Chem. 2009, 112, 727–732. [Google Scholar] [CrossRef]
- Spaenij-Dekking, L.; Kooy-Winkelaar, Y.; Koning, F. The ethiopian cereal tef in celiac disease. N. Engl. J. Med. 2005, 353, 1748–1749. [Google Scholar] [CrossRef] [PubMed]
- Hopman, G.D.; Dekking, E.H.A.; Blokland, M.L.J.; Wuisman, M.C.; Zuijderduin, W.; Koning, F.; Schweizer, J.J. Tef in the diet of celiac patients in the Netherlands. Scand. J. Gastroenterol. 2008, 43, 277–282. [Google Scholar] [CrossRef]
- Edema, M.O.; Emmambux, M.N.; Taylor, J.R.N. Improvement of fonio dough properties through starch modification by sourdough fermentation. Starch-Stärke 2013, 65, 730–737. [Google Scholar] [CrossRef]
- Amézqueta, S.; Galán, E.; Vila-Fernández, I.; Pumarola, S.; Carrascal, M.; Abian, J.; RibasBarba, L.; Serra-Majem, L.; Torres, J.L. The presence of D-fagomine in the human diet from buckwheat-based foodstuffs. Food Chem. 2013, 136, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Zieliński, H. Buckwheat as a functional food and its effects on health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef]
- Dodok, L.; Modhir, A.A.; Buchtova, V.; Halasova, G.; Polaček, I. Importance and utilization of amaranth in food industry. Part 2. Composition of amino acids and fatty acids. Food/Nahr. 1997, 41, 108–110. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef]
- Velikova, P.; Petrov, K.; Lozanov, V.; Tsvetanova, F.; Stoyanov, A.; Wu, Z.; Liu, Z.; Petrova, P. Microbial diversity and health-promoting properties of the traditional Bulgarian yogurt. Biotechnol. Biotechnol. Eq. 2018, 32, 1205–1217. [Google Scholar] [CrossRef]
- Carrizo, S.L.; de Montes Oca, C.E.; Hébert, M.E.; Saavedra, L.; Vignolo, G.; LeBlanc, J.G.; Rollán, G.C. Lactic acid bacteria from Andean grain Amaranth: A source of vitamins and functional value enzymes. J. Mol. Microb. Biotech. 2017, 27, 289–298. [Google Scholar] [CrossRef]
- Ruiz Rodrıguez, L.; Vera Pingitore, E.; Rollan, G.; Martos, G.; Saavedra, L.; Fontana, C.; Hebert, E.M.; Vignolo, G. Biodiversity and technological potential of lactic acid bacteria isolated from spontaneously fermented amaranth sourdough. Lett. Appl. Microbiol. 2016, 63, 147–154. [Google Scholar] [CrossRef]
- Ruiz Rodriguez, L.; Vera Pingitore, E.; Rollan, G.; Cocconcelli, P.; Fontana, C.; Saavedra, L.; Vignolo, G.; Hébert, E. Biodiversity and technological-functional potential of lactic acid bacteria isolated from spontaneously fermented quinoa sourdoughs. J. Appl. Microbiol. 2016, 120, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Bustos, A.Y.; Gerez, C.L.; Mohtar, L.G.; Paz Zanini, V.I.; Nazareno, M.A.; Taranto, M.P.; Iturriaga, L.B. Lactic acid fermentation improved textural behaviour; phenolic compounds and antioxidant activity of chia (Salvia hispanica L.) dough. Food Technol. Biotechnol. 2017, 55, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Maidana, S.D.; Ficoseco, C.A.; Bassi, D.; Cocconcelli, P.S.; Puglisi, E.; Savoy, G.; Vignolo, G.; Fontana, C. Biodiversity and technological-functional potential of lactic acid bacteria isolated from spontaneously fermented chia sourdough. Int. J. Food Microbiol. 2020, 316, 108425. [Google Scholar] [CrossRef] [PubMed]
- Mantziari, A.; Tölkkö, S.; Ouwehand, A.C.; Löyttyniemi, E.; Isolauri, E.; Salminen, S.; Rautava, S. The effect of donor human milk fortification on the adhesion of probiotics in vitro. Nutrients 2020, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Chaves-López, C.; Rossi, C.; Maggio, F.; Paparella, A.; Serio, A. Changes occurring in spontaneous maize fermentation: An overview. Fermentation 2020, 6, 36. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, T.; Tang, H.; Li, X.; Chen, Y.; Zhang, L.; Zhang, J. Probiotic potential and amylolytic properties of lactic acid bacteria isolated from Chinese fermented cereal foods. Food Control 2020, 111, 107057. [Google Scholar] [CrossRef]
- Laurent-Babot, C.; Guyot, J.-P. Should research on the nutritional potential and health benefits of fermented cereals focus more on the general health status of populations in developing countries? Microorganisms 2017, 5, 40. [Google Scholar] [CrossRef]
- Ayodeji Adebo, O.; Medina-Meza, I.G. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Dordevic, T.M.; Siler-Marinkovic, S.S.; Dimitrijevic-Brankovic, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Synthesis of angiotensin-I converting enzyme (ACE)-inhibitory peptides and γ-aminobutyric acid (GABA). J. Agric. Food Chem. 2008, 56, 6936–6943. [Google Scholar] [CrossRef] [PubMed]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Peñas, E.; Diana, M.; Frias, J.; Quílez, J.; Martínez-Villaluenga, C. A multistrategic approach in the development of sourdough bread targeted towards blood pressure reduction. Plant. Foods Hum. Nutr. 2015, 70, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; Gobbetti, M. Synthesis of the cancer preventive peptide lunasin by lactic acid bacteria during sourdough fermentation. Nutr. Cancer 2012, 64, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Morlon-Guyot, J.; Guyot, J.P.; Pot, B.; de Haut, I.J.; Raimbault, M. Lactobacillus manihotivorans sp. nov.; a new starch-hydrolyzing lactic acid bacterium isolated from cassava sour starch fermentation. Int. J. Syst. Bacteriol. 1998, 48, 1101–1109. [Google Scholar] [CrossRef]
- Nakamura, L.K. Lactobacillus amylovorus; a new starch hydrolyzing species from cattle waste-corn fermentation. Int. J. Syst. Bacteriol. 1981, 31, 56–63. [Google Scholar] [CrossRef]
- Cardarelli, H.R.; Martinez, R.C.; Albrecht, S.; Schols, H.; Franco, B.D.; Saad, S.M.; Smidt, H. In vitro fermentation of prebiotic carbohydrates by intestinal microbiota in the presence of Lactobacillus amylovorus DSM 16998. Benef. Microbes 2016, 7, 119–133. [Google Scholar] [CrossRef]
- Champ, M.; Szylit, O.; Raibaud, P.; Aïut-Abdelkader, N. Amylase production by three Lactobacillus strains isolated from chicken crop. J. Appl. Bacteriol. 1983, 55, 487–493. [Google Scholar] [CrossRef]
- Lee, H.; Se, G.; Carter, S. Amylolytic cultures of Lactobacillus acidophilus: Potential probiotics to improve dietary starch utilization. J. Food Sci. 2001, 66, 338–344. [Google Scholar] [CrossRef]
- Sen, S.; Chakrabarty, S.L. Amylase from Lactobacillus cellobiosus isolated from vegetable wastes. J. Ferment. Technol. 1984, 62, 407–413. [Google Scholar]
- Agati, V.; Guyot, J.P.; Morlon-Guyot, J.; Talamond, P.; Hounhouigan, D.J. Isolation and characterization of new amylolytic strains of Lactobacillus fermentum from fermented maize doughs (mave and ogi) from Benin. J. Appl. Microbiol. 1998, 85, 512–520. [Google Scholar] [CrossRef]
- Giraud, E.; Champailler, A.; Raimbault, M. Degradation of starch by a wild amylolytic strain of Lactobacillus plantarum. Appl. Environ. Microbiol. 1994, 60, 4319–4323. [Google Scholar] [CrossRef] [PubMed]
- Olympia, M.; Fukuda, H.; Ono, H.; Kaneko, Y.; Takano, M. Characterization of starch-hydrolyzing lactic acid bacteria isolated from a fermented fish and rice food “burong isda” and its amylolytic enzyme. J. Ferment. Bioeng. 1995, 80, 124–130. [Google Scholar] [CrossRef]
- Sanni, A.; Morlon-Guyot, J.; Guyot, J.P. New efficient amylase-producing strains of Lactobacillus plantarum and L. fermentum isolated from different Nigerian traditional fermented foods. Int. J. Food Microbiol. 2002, 72, 53–62. [Google Scholar] [CrossRef]
- Hamad, S.H.; Dieng, M.C.; Ehrmann, M.A.; Vogel, R.F. Characterization of the bacterial flora of the Sudanese sorghum flour and sorghum sourdough. J. Appl. Microbiol. 1997, 83, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Bohak, I.; Back, W.; Richter, L.; Ehrmann, M.; Ludwig, W.; Schleifer, K.-H. Lactobacillus amylolyticus sp. nov.; isolated from beer malt and beer wort. Syst. Appl. Microbiol. 1998, 21, 360–364. [Google Scholar] [CrossRef]
- Naser, S.M.; Vancanneyt, M.; Snauwaert, C.; Vrancken, G.; Hoste, B.; De Vuyst, L.; Swings, J. Reclassification of Lactobacillus amylophilus LMG 11400 and NRRL B-4435 as Lactobacillus amylotrophicus sp. nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 2523–2527. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Direct starch conversion into L (+) lactic acid by a novel amylolytic strain of Lactobacillus paracasei B41. Starch-Stärke 2012, 64, 10–17. [Google Scholar] [CrossRef]
- Rodrıguez-Sanoja, R.; Ruiz, B.; Guyot, J.P.; Sanchez, S. Starch-binding domain affects catalysis in two Lactobacillus α-amylases. Appl. Environ. Microbiol. 2005, 71, 297–302. [Google Scholar] [CrossRef]
- Rocha, J.M.; Malcata, F.X. On the microbiological profile of traditional Portuguese sourdough. J. Food Prot. 1999, 62, 1416–1429. [Google Scholar] [CrossRef]
- Diaz-Ruiz, G.; Guyot, J.P.; Ruiz-Teran, F.; Morlon-Guyot, J.; Wacher, C. Microbial and physiological characterization of weakly amylolytic but fast-growing lactic acid bacteria: A functional role in supporting microbial diversity in pozol; a Mexican fermented maize beverage. Appl. Environ. Microbiol. 2003, 69, 4367–4373. [Google Scholar] [CrossRef] [PubMed]
- Petrov, K.; Urshev, Z.; Petrova, P. L(+)-Lactic acid production from starch by a novel amylolytic Lactococcus lactis subsp. lactis B84. Food Microbiol. 2008, 25, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, A.; Embarek, P.K.B.; Wedell-Neergaard, C.; Huss, H.H.; Gram, L. Characterization of anti-listerial lactic acid bacteria isolated from Thai fermented fish products. Food Microbiol. 1998, 15, 223–233. [Google Scholar] [CrossRef]
- Doman-Pytka, M.; Renault, P.; Bardowski, J. Gene-cassette for adaptation of Lactococcus lactis to a plant environment. Lait 2004, 84, 33–37. [Google Scholar] [CrossRef]
- Satoh, E.; Ito, Y.; Sasaki, Y.; Sasaki, T. Application of the extracellular alpha-amylase gene from Streptococcus bovis 148 to construction of a secretion vector for yogurt starter strains. Appl. Environ. Microbiol. 1997, 63, 4593–4596. [Google Scholar] [CrossRef]
- Sun, Z.; Harris, H.M.B.; McCann, A.; Guo, C.; Agrimon, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F.; et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains ang associated genera. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Shibata, K.; Flores, D.M.; Kobayashi, G.; Sonomoto, K. Direct lactic acid fermentation with sago starch by a novel amylolytic lactic acid bacterium Enterococcus faecium. Enzym. Microb. Technol. 2007, 41, 149–155. [Google Scholar] [CrossRef]
- Muyanja, C.M.; Narvhus, J.A.; Treimo, J.; Langsrud, T. Isolation; characterisation and identification of lactic acid bacteria from bushera: A Ugandan traditional fermented beverage. Int. J. Food Microbiol. 2003, 80, 201–210. [Google Scholar] [CrossRef]
- Amari, M.; Laguerre, S.; Vuillemin, M.; Robert, H.; Loux, V.; Klopp, C.; Morel, S.; Gabriel, B.; Remaud-Siméon, M.; Gabriel, V.; et al. Genome sequence of Weissella confusa LBAE C39-2; isolated from a wheat sourdough. J. Bacteriol. 2012, 194, 1608–1609. [Google Scholar] [CrossRef][Green Version]
- Van Laere, K.M.; Hartemink, R.; Bosveld, M.; Schols, H.A.; Voragen, A.G. Fermentation of plant cell wall derived polysaccharides and their corresponding oligosaccharides by intestinal bacteria. J. Agric. Food Chem. 2000, 48, 1644–1652. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate; short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Fincher, G.B.; Stone, B.A. Cell walls and their components in cereal grain technology. In Advances in Cereal Science and Technology, 1st ed.; Pomeraz, Y., Ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 1986; pp. 207–295. [Google Scholar]
- Williams, B.A.; Mikkelsen, D.; Flanagan, B.M.; Gidley, M.J. “Dietary fibre”: Moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J. Anim. Sci. Biotechnol. 2019, 10, 45. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Z.; Shu, X.L.; Zhang, L.L.; Wang, X.Y.; Zhao, H.J.; Ma, C.X.; Wu, D.X. Starch properties of mutant rice high in resistant starch. J. Agric. Food Chem. 2006, 54, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Jiao, G.; Fitzgerald, M.A.; Yang, C.; Shu, Q.; Wu, D. Starch structure and digestibility of rice high in resistant starch. Starch-Stärke 2006, 58, 411–417. [Google Scholar] [CrossRef]
- Asp, N.-G. Resistant starch. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S1. [Google Scholar] [CrossRef]
- Zhang, G.; Hamaker, B.R. Cereal carbohydrates and colon health. Cereal Chem. 2010, 87, 331–341. [Google Scholar] [CrossRef]
- Asp, N. Nutritional classification and analysis of food carbohydrates. Am. J. Clin. Nutr. 1994, 59, 6795–6815. [Google Scholar] [CrossRef]
- Wolever, T.; Mchling, C. High-carbohydrate-low-glycemic-index dietary advice improves glucose disposition index in subjects with impaired glucose tolerance. Br. J. Nutr. 2002, 87, 477–487. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Wesson, V.; Wolever, T.M.S.; Jenkins, A.L.; Kalmusky, J.; Guidici, S.; Csima, A.; Josse, R.G.; Wong, G.S. Wholemeal versus wholegrain breads—Proportion of whole or cracked grain and the glycemic response. BMJ 1988, 297, 958–960. [Google Scholar] [CrossRef]
- Granfeldt, Y.; Bjorck, I.; Hagander, B. On the importance of processing conditions, product thickness and egg addition for the glycemic and hormonal responses to pasta—A comparison with bread made from pasta ingredients. Eur. J. Clin. Nutr. 1991, 45, 489–499. [Google Scholar]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33–S50. [Google Scholar]
- Seneviratne, H.D.; Biliaderis, C.G. Action of a-amylases on amylose-lipid complex superstructures. J. Cereal Sci. 1991, 13, 129–143. [Google Scholar] [CrossRef]
- Carvalho, A.F.A.; de Oliva Neto, P.; da Silva, D.F.; Pastore, G.M. Xylooligosaccharides from lignocellulosic materials: Chemical structure; health benefits and production by chemical and enzymatic hydrolysis. Food Res. Int. 2013, 51, 75–85. [Google Scholar] [CrossRef]
- Grasten, S.; Liukkonen, K.H.; Chrevatidis, A.; El-Nezami, H.; Poutanen, K.; Mykkanen, H. Effects of wheat pentosan and inulin on the metabolic activity of fecal microbiota and on bowel function in healthy humans. Nutr. Res. 2003, 23, 1503–1514. [Google Scholar] [CrossRef]
- Ellegard, L.; Andersson, H.; Bosaeus, I. Inulin and oligofructose do not influence the absorption of cholesterol; Ca; Mg; Zn; Fe; or bile acids but increase energy excretion in ileostomy subjects. Eur. J. Clin. Nutr. 1997, 51, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.; Delattre, C.; Urdaci, M.; Schmitter, J.M.; Bressollier, P. An overview of the last advances in probiotic and prebiotic field. LWT—Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef]
- Arena, M.P.; Caggianiello, G.; Fiocco, D.; Russo, P.; Torelli, M.; Spano, G.; Capozzi, V. Barley β-glucans-containing food enhances probiotic performances of beneficial bacteria. Int. J. Mol. Sci. 2014, 15, 3025–3039. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.; Petrov, K. Prebiotic–probiotic relationship: The genetic fundamentals of polysaccharides conversion by Bifidobacterium and Lactobacillus genera. In Handbook of Food Bioengineering, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: San Diego, CA, USA, 2017; Volume 2, pp. 237–278. [Google Scholar]
- Tosi, E.A.; Ciappini, M.C.; Masciarelli, R. Utilisation of whole amaranthus (Amaranthus cruentus) flour in the manufacture of biscuits for coeliacs. Alimentaria 1996, 34, 49–51. [Google Scholar]
- Schoenlechner, R.; Linsberger, G.; Kaczyc, L.; Berghofer, E. Production of short dough biscuits from the pseudocereals amaranth; quinoa and buckwheat with common bean. Ernährung 2006, 30, 101–107. [Google Scholar]
- Jeske, S.; Zannini, E.; Cronin, M.F.; Arendt, E.K. Impact of protease and amylase treatment on proteins and the product quality of a quinoa-based milk substitute. Food Funct. 2018, 9, 3500–3508. [Google Scholar] [CrossRef] [PubMed]
- Carrizo, S.L.; de Moreno de LeBlanc, A.; LeBlanc, J.G.; Rollán, G.C. Quinoa pasta fermented with lactic acid bacteria prevents nutritional deficiencies in mice. Food Res. Intern. 2020, 127, 108735. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.; Altaf, M.; Naveena, B.J.; Venkateshwar, M.; Kumar, E.V. Amylolitic bacterial lactic acid fermentation—A rewiew. Biotehnol. Adv. 2008, 26, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, A.; Lavermicocca, P.; Morea, M.; Baruzzi, F.; Tosti, N.; Gobbetti, M. Phenotypic and molecular identification and clustering of lactic acid bacteria and yeasts from wheat (species Triticum durum and Triticum aestivum) sourdoughs of Southern Italy. Int. J. Food Microbiol. 2001, 64, 95–104. [Google Scholar] [CrossRef]
- De Vuyst, L.; Neysens, P. The sourdough microflora: Biodiversity and metabolic interactions. Trends Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Vermeulen, N.; Vogel, R.F. Carbohydrate; peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol. 2007, 24, 128–138. [Google Scholar] [CrossRef]
- De Vuyst, L.; van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.-M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef]
- Gotcheva, V.; Pandiella, S.S.; Angelov, A.; Roshkova, Z.G.; Webb, C. Microflora identification of the Bulgarian cereal-based fermented beverage boza. Proc. Biochem. 2000, 36, 127–130. [Google Scholar] [CrossRef]
- Petrova, P.; Emanuilova, M.; Petrov, K. Amylolytic Lactobacillus strains from Bulgarian fermented beverage Boza. Z. Nat. C 2010, 65c, 218–224. [Google Scholar] [CrossRef]
- Settanni, L. Sourdough and cereal-based foods. In Starter Cultures in Food Production, 1st ed.; Speranza, B., Bevilacqua, A., Corbo, M., Sinigaglia, M., Eds.; John Wiley & Sons: Chichester, UK, 2017; pp. 199–230. [Google Scholar]
- Thakkar, P.; Modi, H.A.; Prajapati, J.B. Isolation; characterization and safety assessment of lactic acid bacterial isolates from fermented food products. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 713–725. [Google Scholar]
- Chavan, J.K.; Kadam, S.S. Critical reviews in food science and nutrition. Food Sci. 1989, 28, 348–400. [Google Scholar]
- Antognoni, F.; Mandrioli, R.; Bordoni, A.; Di Nunzio, M.; Viadel, B.; Gallego, E.; Villalba, M.P.; Tomás-Cobos, L.; Taneyo Saa, D.L.; Gianotti, A. Integrated evaluation of the potential health benefits of einkorn-based breads. Nutrients 2017, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Rizzello, C.G.; Trani, A.; Gobbetti, M. Manufacture and characterization of functional emmer beverages fermented by selected lactic acid bacteria. Food Microbiol. 2011, 28, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Nionelli, L.; Rizzello, C.G.; De Angelis, M.; Tossut, P.; Gobbetti, M. Spelt and emmer flours: Characterization of the lactic acid bacteria microbiota and selection of mixed starters for bread making. J. Appl. Microbiol. 2010, 108, 925–935. [Google Scholar] [CrossRef]
- Ferri, M.; Serrazanetti, D.I.; Tassoni, A.; Baldissarri, M.; Gianotti, A. Improving the functional and sensorial profile of cereal-based fermented foods by selecting Lactobacillus plantarum strains via a metabolomics approach. Food Res. Int. 2016, 89, 1095–1105. [Google Scholar] [CrossRef]
- Väkeväinen, K.; Valderrama, A.; Espinosa, J.; Centurión, D.; Rizo, J.; Reyes-Duarte, D.; Díaz-Ruiz, G.; von Wright, A.; Elizaquível, P.; Esquivel, K.; et al. Characterization of lactic acid bacteria recovered from atole agrio; a traditional Mexican fermented beverage. LWT—Food Sci. Technol. 2018, 88, 109–118. [Google Scholar] [CrossRef]
- Pérez-Cataluña, A.; Elizaquível, P.; Carrasco, P.; Espinosa, J.; Reyes, D.; Wacher, C.; Aznar, R. Diversity and dynamics of lactic acid bacteria in Atole agrio; a traditional maize-based fermented beverage from South-Eastern Mexico; analysed by high throughput sequencing and culturing. Antonie Leeuwenhoek 2018, 111, 385–399. [Google Scholar] [CrossRef]
- Chelule, P.K.; Mokgatle, M.M.; Zungu, L.I. South African rural community understanding of fermented foods preparation and usage. J. Edu. Health Promot. 2015, 4, 82. [Google Scholar]
- Pswarayi, F.; Gänzle, M. Composition and origin of the fermentation microbiota of Mahewu; a Zimbabwean fermented cereal beverage. Appl. Environ. Microbiol. 2019, 85, e03130-18. [Google Scholar] [CrossRef]
- Sawadogo-Lingani, H.; Lei, V.; Diawara, B.; Nielsen, D.S.; Møller, P.L.; Traore, A.S.; Jakobsen, M. The biodiversity of predominant lactic acid bacteria in dolo and pito wort for the production of sorghum beer. J. Appl. Microbiol. 2007, 103, 765–777. [Google Scholar] [CrossRef]
- Mugula, J.K.; Nnko, S.A.; Narvhus, J.A.; Sørhaug, T. Microbiological and fermentation characteristics of togwa; a Tanzanian fermented food. Int. J. Food Microbiol. 2003, 80, 187–199. [Google Scholar] [CrossRef]
- Das, A.; Raychaudhuri, U.; Chakraborty, R. Cereal based functional food of Indian subcontinent: A review. J. Food Sci. Technol. 2012, 49, 665–672. [Google Scholar] [CrossRef]
- Lee, M.; Regu, M.; Seleshe, S. Uniqueness of Ethiopian traditional alcoholic beverage of plant origin, tella. J. Ethn. Foods 2015, 2, 110–114. [Google Scholar] [CrossRef]
- Efiuvwevwere, B.; Akoma, O. The microbiology of ‘kunun-zaki’; a cereal beverage from Northern Nigeria; during the fermentation (production) process. World J. Microbiol. Biotechnol. 1995, 11, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Souane, M.; Kim, Y.B.; Lee, C.H. Microbial characterization of Gajami-sikhae fermentation. Korean J. Appl. Microbiol. Bioeng. 1987, 15, 150–157. [Google Scholar]
- Senapati, A.K.; Ann, A.; Raj, A.; Gupta, A.; Sharma, A.; Neopany, B.; Panmei, C.; Diwedi, D.H.; Raj, D.; Angchok, D.; et al. Diversity of indigenous fermented foods and beverages of South Asia. In Indigenous Fermented Foods of South Asia, 1st ed.; Joshi, V., Ed.; CRC Press, Taylor&Francis Group: Boca Raton, FL, USA, 2016; pp. 69–106. [Google Scholar]
- Sujaya, I.N.; Amachi, S.; Yokota, A.; Asano, K.; Tomit, F. Identification and characterization of lactic acid bacteria in ragitape. World J. Microbiol. Biotechnol. 2001, 17, 349–357. [Google Scholar] [CrossRef]
- Haard, N.F.; Odunfa, S.A.; Lee, C.H.; Quintero-Ramírez, R.; Lorence-Quiñones, A.; Wacher-Radarte, C. Fermented Cereals. A Global Perspective. Available online: http://www.fao.org/3/x2184e/x2184e00.htm (accessed on 10 January 2020).
- Ghosh, D.; Chattopadhyay, P. Preparation of idli batter; its properties and nutritional improvement during fermentation. J. Food Sci. Technol. 2011, 48, 610–615. [Google Scholar] [CrossRef]
- Lim, S.B.; Tingirikari, J.; Seo, J.S.; Li, L.; Shim, S.; Seo, J.H.; Han, N.S. Isolation of lactic acid bacteria starters from Jeung-pyun for sourdough fermentation. Food Sci. Biotechnol. 2018, 27, 73–78. [Google Scholar] [CrossRef]
- Pisitkul, C.; Rengpipat, S. Isolation and characterization of lactic acid bacteria starter for preparation of fermented Khanom-jeen. In Proceedings of the 26th Annual Meeting of the Thai Society for Biotechnology and International Conference, Chiang Rai, Thailand, 26–29 November 2014; pp. 3533–3559. [Google Scholar]
- Rhee, S.J.; Lee, J.E.; Lee, C.H. Importance of lactic acid bacteria in Asian fermented foods. Microb. Cell Factories 2011, 10, S5. [Google Scholar] [CrossRef]
- Kopermsub, P.; Yunchalard, S. Identification of lactic acid bacteria associated with the production of plaa-som; a traditional fermented fish product of Thailand. Int. J. Food Microbiol. 2010, 138, 200–204. [Google Scholar] [CrossRef]
- Yonzan, H.; Tamang, J.P. Microbiology and nutritional value of selroti; an ethnic fermented cereal food of the Himalayas. Food Biotechnol. 2010, 24, 227–247. [Google Scholar] [CrossRef]
- Savić, D.; Savić, T.; Škrinjar, M.M.; Joković, N. Profile of lactic acid bacteria in rye flour and sourdough. J. Cult. Collect. 2007, 5, 384–385. [Google Scholar]
- Ganchev, I.; Koleva, Z.; Kizheva, Y.; Moncheva, P.; Hristova, P. Lactic acid bacteria from spontaneously fermented rye sourdough. Bulg. J. Agric. Sci. 2014, 20 (Suppl. 1), 69–73. [Google Scholar]
- Kariluoto, S.; Vahteristo, L.; Salovaara, H.; Katina, K.; Liukkonen, K.H.; Piironen, V. Effect of baking method and fermentation on folate content of rye and wheat breads. Cereal Chem. 2004, 81, 134–139. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Mattila, O.; Katina, K.; Poutanen, K.; Aura, A.M.; Hanhineva, K. Metabolic profling of sourdough fermented wheat and rye bread. Sci. Rep. 2018, 8, 5684. [Google Scholar] [CrossRef]
- Ashagrie, Z.; Abate, D. Improvement of injera shelf life through the use of chemical preservatives. Afr. J. Food Agric. Nutr. Dev. 2012, 12, 6409–6423. [Google Scholar]
- Tafere, G. A review on traditional fermented beverages of Ethiopian. J. Nat. Sci. Res. 2015, 5, 94–102. [Google Scholar]
- Onda, T.; Yanagida, F.; Uchimura, T.; Tsuji, M.; Ogino, S.; Shinohara, T.; Yokotsuka, K. Analysis of lactic acid bacterial flora during Miso fermentation. Food Sci. Technol. Res. 2003, 9, 17–24. [Google Scholar] [CrossRef]
- Fischer, M.M.; Egli, I.M.; Aeberli, I.; Hurrell, R.F.; Meile, L. Phytic acid degrading lactic acid bacteria in tef-injera fermentation. Int. J. Food Microbiol. 2014, 190, 54–60. [Google Scholar] [CrossRef]
- Olasupo, N.A.; Odunfa, S.A.; Obayori, O.S. Ethnic African fermented foods. In Fermented Foods and Beverages of the World, 1st ed.; Tamang, J., Kailasapathy, K., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2010; pp. 3233–3252. [Google Scholar]
- Wronkowska, M.; Jeliński, T.; Majkowska, A.; Zieliński, H. Physical properties of buckwheat water biscuits formulated from fermented flours by selected lactic acid bacteria. Pol. J. Food Nutr. Sci. 2018, 68, 25–31. [Google Scholar] [CrossRef]
- Hammes, W.P.; Brandt, M.J.; Francis, K.L.; Rosenheim, J.; Seitter, M.F.H.; Vogelmann, S.A. Microbial ecology of cereal fermentations. Trends Food Sci. Technol. 2005, 16, 4–11. [Google Scholar] [CrossRef]
- Minervini, F.; De Angelis, M.; Di Cagno, R.; Gobbetti, M. Ecological parameters influencing microbial diversity and stability of traditional sourdough. Int. J. Food Microbiol. 2014, 171, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, J.; Seleshi, S.; Nega, F.; Lee, M. Revisit to Ethiopian traditional barley-based food. J. Ethn. Foods 2016, 3, 135–141. [Google Scholar] [CrossRef]
- Shobana, S.; Krishnaswamy, K.; Sudha, V.; Malleshi, N.G.; Anjana, R.M.; Palaniappan, L.; Mohan, V. Finger millet (Ragi, Eleusine coracana L.). Review of its nutritional properties, processing and plausible health benefits. Adv. Food Nutr. Res. 2013, 69, 1–39. [Google Scholar] [PubMed]
- Lei, V.; Friis, H.; Michealsen, K.F. Spontaneously fermented finger millet product as a natural probiotic treatment for diarrhea in young children: An intervention study in Northern Ghana. Int. J. Food Microbiol. 2006, 110, 246–253. [Google Scholar] [CrossRef]
- Murtaza, N.; Baboota, R.; Jagtap, S.; Singh, D.; Khare, P.; Sarma, S.; Podili, K.; Alagesan, S.; Chandra, T.S.; Bhutani, K.K.; et al. Finger millet bran supplementation alleviates obesity-induced oxidative stress, inflammation and gut microbial derangements in high-fat diet-fed mice. Br. J. Nutr. 2014, 112, 1447–1458. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, H.J. Food biotechnology. In Food Science and Technology, 2nd ed.; Campbell-Platt, G., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017; pp. 105–136. [Google Scholar]
- Rosenquist, H.; Hansen, A. The microbial stability of two bakery sourdoughs made from conventionally and organically grown rye. Food Microbiol. 2000, 17, 241–250. [Google Scholar] [CrossRef]
- Katina, K.; Arendt, E.; Liukkonen, K.H.; Autio, K.; Flander, L.; Poutanen, K. Potential of sourdough for healthier cereal products. Trends Food Sci. Technol. 2005, 16, 104–112. [Google Scholar] [CrossRef]
- Juodeikiene, G. Traditional rye sourdough bread in the Baltic region. In Traditional Foods, Integrating the Food Science and Engineering Knowledge Into the Food Chain, 1st ed.; Kristbergsson, K., Oliveira, J., Eds.; Springer Science+Business Media: New York, NY, USA, 2016; pp. 173–187. [Google Scholar]
- Serventi, S.; Sabban, F.; Shugaar, A. Pasta: The story of a universal food. In Arts and Traditions of the Table: Perspectives on Culinary History; Columbia University Press: New York, NY, USA, 2002; pp. 44–49. [Google Scholar]
- Russo, P.; Beleggia, R.; Ferrer, S.; Pardo, I.; Spano, G. A polyphasic approach in order to identify dominant lactic acid bacteria during pasta manufacturing. LWT-Food Sci. Technol. 2010, 43, 982–986. [Google Scholar] [CrossRef]
- Di Cagno, R.; de Angelis, M.; Alfonsi, G.; de Vincenzi, M.; Silano, M.; Vincentini, O.; Gobbetti, M. Pasta made from durum wheat semolina fermented with selected lactobacilli as a tool for apotential decrease of the gluten intolerance. J. Agric. Food Chem. 2005, 53, 4393–4402. [Google Scholar] [CrossRef]
- Capozzi, V.; Menga, V.; Digesu, A.M.; De Vita, P.; van Sinderen, D.; Cattivelli, L.; Fares, C.; Spano, G. Biotechnological production of vitamin B2-enriched bread and pasta. J. Agric. Food Chem. 2011, 59, 8013–8020. [Google Scholar] [CrossRef] [PubMed]
- Schoenlechner, R.; Jurackova, K.; Berghofer, E. Pasta production from the pseudocereals amaranth; quinoa and buckwheat. In Using Cereal Science and Technology for the Benefit of Consumers, Proceedings of the 12th ICC Cereal and Bread Congress, Harrogate, UK, 23–26 May 2004; Cauvain, S.P., Salmon, S.S., Young, L.S., Eds.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Caperuto, L.; Amaya-Farfan, J.; Camargo, C. Performance of quinoa (Chenopodium quinoa Willd) flour in the manufacture of gluten-free spaghetti. J. Sci. Food Agric. 2000, 81, 95–101. [Google Scholar] [CrossRef]
- Botes, A.; Todorov, S.D.; von Mollendorff, J.W.; Botha, A.; Dicks, L.M.T. Identification of lactic acid bacteria and yeast from boza. Proc. Biochem. 2007, 42, 267–270. [Google Scholar] [CrossRef]
- Vasudha, S.; Mishra, H.N. Non-dairy probiotic beverages. Int. Food Res. J. 2013, 20, 7–15. [Google Scholar]
- Capuani, A.; Behr, J.; Vogel, R.F. Influence of lactic acid bacteria on redox status and on proteolytic activity of buckwheat (Fagopyrum esculentum Moench) sourdoughs. Int. J. Food Microbiol. 2013, 165, 148–155. [Google Scholar] [CrossRef]
- Zieliński, H.; Ciesarová, Z.; Kukurová, K.; Zielinska, D.; Szawara-Nowak, D.; Starowicz, M.; Wronkowska, M. Effect of fermented and unfermented buckwheat flour on functional properties of gluten-free muffins. J. Food Sci. Technol. 2017, 54, 1425–1432. [Google Scholar] [CrossRef]
- Zieliński, H.; Honke, J.; Bączek, N.; Majkowska, A.; Wronkowska, M. Bioaccessibility of D-chiro-inositol from water biscuits formulated from buckwheat flours fermented by lactic acid bacteria and fungi. LWT—Food Sci. Technol. 2019, 106, 37–43. [Google Scholar] [CrossRef]
- Rocchetti, G.; Miragoli, F.; Zacconi, C.; Lucini, L.; Rebecchi, A. Impact of cooking and fermentation by lactic acid bacteria on phenolic profile of quinoa and buckwheat seeds. Food Res. Int. 2019, 119, 886–894. [Google Scholar] [CrossRef]
- Kiskini, A.; Argiri, K.; Kalogeropoulos, M.; Komaitis, M.; Kostaropoulos, A.; Mandala, I.; Kapsokefalou, M. Sensory characteristics and iron dialyzability of gluten-free bread fortified with iron. Food Chem. 2007, 102, 309–316. [Google Scholar] [CrossRef]
- Feng, L.; Xie, Y.; Peng, C.; Liu, Y.; Wang, H. A novel antidiabetic food produced via solid-state fermentation of Tartary buckwheat by L. plantarum TK9 and L. paracasei TK1501. Food Technol. Biotechnol. 2018, 56, 373–380. [Google Scholar] [CrossRef]

| Grain/Seed | Carbohydrates | Protein | Fat | Fibres |
|---|---|---|---|---|
| Wheat (bread) | 71.2 | 12.6 | 1.5 | 12.2 |
| Einkorn | 65.5 | 15.8 | 4.2 | 8.7 |
| Emmer | 65.0 | 14.0 | 1.8 | 2.7 |
| Spelt | 53.9 | 14.6 | 2.4 | 10.7 |
| Khorasan | 52.4 | 14.7 | 2.2 | 9.1 |
| Maize † | 74.0 | 9.4 | 4.7 | 7.3 |
| Rice (white) † | 80.0 | 7.1 | 0.7 | 1.3 |
| Barley | 77.7 | 9.9 | 1.2 | 15.6 |
| Proso Millet † | 72.8 | 11.0 | 4.2 | 8.5 |
| Sorghum Millet † | 75.0 | 11.3 | 3.3 | 6.3 |
| Oat † | 66.3 | 16.9 | 6.9 | 11.6 |
| Rye | 60.7 | 8.8 | 1.7 | 13.2 |
| Teff (cooked) | 19.9 | 3.9 | 0.7 | 2.8 |
| Buckwheat † | 58.9 | 12.5 | 2.1 | 29.5 |
| Amaranth † | 61.4 | 16.5 | 5.7 | 20.6 |
| Quinoa † | 64.2 | 14.5 | 5.2 | 14.2 |
| Chia † | 42.1 | 16.5 | 30.7 | 34.4 |
| Cereal | Product | Use | LAB Species | Country of Main Use | Reference |
|---|---|---|---|---|---|
| Wheat and varieties | |||||
| Wheat | Sourdough | Bread | L. brevis, L. fermentum, L. buchneri, L. reuteri, L. frumenti, L. pontis, L. fructivorans, L. sanfranciscensis, L. panis, W. confusa, W. cibaria, L. casei, L. alimentarius, L. plantarum, P. pentosaceus, L. amylovorus, L. paralimentarius, L. acidophilus, L. acidophilus, L. delbrueckii, L. farciminis, L. mindensis, L. johnsonii | Italy, Belgium, France, Portugal, Germany, Central Europe countries | [115,116,117,118] |
| Boza | Thick beverage | L. coryniformis, L. fermentum L. plantarum, L. pentosus, L. paracasei, Leuc. paramesenteroides, Leuc. sanfrancisco, Leuc. mesenteroides | Bulgaria, Albania, Turkey | [8,119,120] | |
| Hamanato | Snack | Streptococcus, Pediococcus | Japan | [121] | |
| Jalebies | Pretzel | L. plantarum, E. faecium, L. fermentum, L. paracasei | India | [122] | |
| Kishk | Meal | L. plantarum, L. brevis, L. casei | Egypt, Syria | [123] | |
| Einkorn | Sourdough | Bread | L. plantarum, L. sanfranciscensis, L. brevis | Italy | [124] |
| Emmer | Functional beverage | Drink | L. plantarum, W. confusa | Italy | [125] |
| Spelt | Bread | Bread | L. brevis, W. confusa, L. plantarum | Italy | [126] |
| Khorasan | Sourdough | Bread | L. plantarum | Italy | [127] |
| Farro | Acha, Iburu | Porridge Couscous | P. pentosaceus, L. curvatus, L. plantarum | Nigeria | [42] |
| Maize | Atole agrio | Porridge | Weissella, Pediococcus, Lactococcus, Lactobacillus | Mexico | [128,129] |
| Broa | Bread | Leuconostoc, L. brevis, L. curvatus, Lc. lactis ssp. lactis, E. durans, Ent. casseliflavus, E. faecium, S. equinus, Str. constellantus | Portugal | [79] | |
| Busaa | Alcoholic drink | L. helveticus, L. salivarius, L. casei, L. brevis, L. plantarum, L. buchneri | Nigeria, Ghana | [17] | |
| Kenkey | Mush | L. fermentum, L. reuteri | Ghana | [17,87] | |
| Koko | Porridge | L. plantarum, L. brevis | Ghana | [17,87] | |
| Kwete | Beverage | L. rhamnosus, Str. thermophilus | Uganda | [15] | |
| Magu | Porridge | L. delbrueckii | RSA | [130] | |
| Mahewu | Beverage | L. plantarum, L. fermentum | RSA | [131] | |
| Pito | Drink | Lactobacillus | Nigeria, Ghana | [132] | |
| Pozol | Beverage | E. faecium, Str. bovis, L. fermentum | Mexico | [80] | |
| Togwa | Gruel | L. plantarum, L. brevis, L. fermentum, W. confusa, P. pentosaceus | Tanzania | [133] | |
| Millet | Ambali | Porridge | Leuc. mesenteroides, L. fermentum, Str. faecalis | India | [134] |
| Bagni | Alcoholic drink | Lactobacillus spp. | Russia | [135] | |
| Kunun-zaki | Thick beverage | Lactobacillus spp., L. fermentum | Nigeria | [136] | |
| Merrisa | Drink | Lactobacillus spp. | Sudan | [135] | |
| Sikhae | Fish meal | Leuc. mesenteroides, L. plantarum | Korea | [137] | |
| Sorghum | Bantu | Beer | L. delbrueckii | RSA | [87] |
| Kishra | Bread | E. faecium | Sudan | [86] | |
| Nasha | Porridge | Lactobacillus, Streptococcus | Sudan | [16] | |
| Ogi | Paste | L. plantarum | Nigeria | [70] | |
| Uji | Porridge | Leuc. mesenteroides, L. plantarum | Kenya | [17] | |
| Rice | Adai/Vada | Snack | Leuconostoc, LA cocci | India | [138] |
| Brem | Cake | P. pentosaceus, E. faecium, L. curvatus, W. confusa, W. paramesenteroides | Indonesia | [139] | |
| Dhokla | Cake | Leuc. mesenteroides, Ent. faecalis | India | [140] | |
| Idli | Cake | Leuc. mesenteroides, Ent. faecalis | India | [141] | |
| Jeung-pyun | Cake | Leuc. lactis, Leuc. citreum, L. brevis.L. crustorum, L. fermentum, L. harbinensis | Korea | [142] | |
| Khanom-jeen | Noodle | Lactobacillus, Streptococcus | Thailand | [143,144] | |
| Puto | Paste | Leuc. mesenteroides | Philippines | [140] | |
| Plaa-som | Fish/Rice | Lc. garvieae, S. bovis, W. cibaria, P. pentosaceus, L. plantarum, L. fermentum | Thailand | [145] | |
| Selroti | Bread | Leuc. mesenteroides, E. faecium, P. pentosaceus, L. curvatus | Nepal | [146] | |
| Tapuy | Beverage | Leuconostoc, Lactobacillus | Philippines | [17] | |
| Rye | Sourdough | Bread | P. pentosaceus, Streptococcus, Lactobacillus, W. paramesenteroides | Germany, Serbia | [147] |
| Sourdough | Bread | Lc. lactis, L. paralimentarius, L. kimchii, L. sanfranciscensis | Bulgaria | [81,148] | |
| Sourdough | Bread | L. plantarum, L. brevis, L. plantarum | Finland, Denmark, Norway, Sweden | [149,150] | |
| Barley | Injera | Bread | L. buchneri, P. pentosaceus | Ethiopia | [151] |
| Keribo | Beverage | Lactobacillus spp. | Ethiopia | [152] | |
| Miso/Soy | Paste | E. faecium, E. durans, E. faecalis, P. acidilactici, P. pentosaceus, L. plantarum, W. confusa | Japan, China | [153] | |
| Oat | Probiotic drink | Beverage | L. plantarum | Bulgaria | [29] |
| Teff | Injera | Soft pancake | L. buchneri, P. pentosaceus, Lb. pontis, Lb. plantarum, Leuc. mesenteroides | Ethiopia | [154,155] |
| Tella | Alcoholic drink | L. pastorianumi, Leuc. mesenteroides | Ethiopia | [135] | |
| Buckwheat | Sourdough | Bread | L. acidophilus, L. casei, L. plantarum, L. rhamnosus, L. salivarius, L. delbrueckii subsp. bulgaricus | Poland | [156] |
| Amaranth | Sourdough | Bread | L. plantarum, L. rhamnosus, E. mundtii, E. hermanniensis, E. durans, Leuc. mesenteroides | Argentina | [48,49] |
| Quinoa | Sourdough | Bread | E. hermanniensis, E. casseliflavus, E. mundtii, Lc. lactis, Leuc. citreum, L. plantarum, L. brevis, Leuc. mesenteroides | Argentina | [50] |
| Chia | Sourdough | Bread | L. plantarum, E. faecium, E. mundtii, W. cibaria, L. rhamnosus, Lc. lactis | Argentina | [51,52] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, P.; Petrov, K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients 2020, 12, 1118. https://doi.org/10.3390/nu12041118
Petrova P, Petrov K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients. 2020; 12(4):1118. https://doi.org/10.3390/nu12041118
Chicago/Turabian StylePetrova, Penka, and Kaloyan Petrov. 2020. "Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications" Nutrients 12, no. 4: 1118. https://doi.org/10.3390/nu12041118
APA StylePetrova, P., & Petrov, K. (2020). Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients, 12(4), 1118. https://doi.org/10.3390/nu12041118






