Abstract
Alzheimer’s disease (AD) is the most common form of senile dementia, accounting for up to 70% of dementia cases. AD is a slowly progressive disease, which causes global mental deterioration by affecting various cognitive areas. A growing body of evidence has demonstrated that lifestyle habits and nutritional patterns could delay the natural course of the neurodegeneration process. There is no single dietary pattern unequivocally proven to prevent AD. Nevertheless, epidemiological data suggest that by adopting several dietary habits, especially if accompanied with a healthy lifestyle, the negative consequences of AD could potentially be delayed. Alongside with others, two specific eating patterns have been well investigated concerning their potential beneficial effect on cognitive status: the Mediterranean diet (MedDi) and the Ketogenic Diet (KD). Despite the different underlying mechanisms, both of them have demonstrated a fairly profitable role in reducing or delaying cognitive impairment. The aim of the present narrative review is to overview the existing research on the efficacy of MedDi and KD against AD-related cognitive decline, focusing on the proposed protective mechanisms of action. Although the current knowledge on this complex topic does not allow us, at this point, to make exhaustive conclusions, this information could be of help in order to better characterize the possible role of MedDi and KD as nonpharmacological therapies in the treatment of AD and, more generically, of neurodegenerative disorders.
1. Introduction
Neurological diseases represent the largest cause of disability and the second leading cause of death worldwide [1]. A consistent number of these conditions are represented by neurodegenerative disorders, which determine a progressive and global deterioration of the cognitive functions such as memory, language, learning, comprehension, orientation, and judgment, thereby affecting people’s everyday life [2]. The prevalence of neurodegenerative disorders is growing at an alarming rate all over the world, largely due to the aging of population [3]. It has in fact been estimated that dementia rate will triplicate within 2050, reaching 135 million people suffering from these conditions globally [4,5].
Alzheimer’s disease (AD), the most common cause of dementia [6], is characterized by the accumulation of amyloid β (Aβ) and hyper-phosphorylation of the Tau protein, with additional reduced acetylcholine levels and cerebral blood flow [2,7]. Furthermore, modifications in the blood-brain barrier (BBB), oxidative stress, mitochondrial impairment, neuroinflammation, and alterations in the insulin signaling have been observed in this condition [8].
Treatment for AD can be divided into pharmacological and nonpharmacological strategies. Pharmacological treatments comprise the symptomatic therapies, e.g., acetylcholinesterase inhibitors or N-methyl-D-aspartate (NMDA) receptor antagonists [9] and “disease-modifying” therapies, which are directed towards amyloid accumulation or Tau protein hyper-phosphorylation [10,11,12]. Despite the multifaceted pharmacological approach, to date the effectiveness of pharmacologic treatments is still inconsistent and the curative treatment towards AD does not exist [13,14].
Nonpharmacological treatments are directed to prevent the neurodegeneration and additionally to modify the risk factors for AD [15,16]. There is, in fact, a growing body of evidence showing that healthy lifestyle habits play an important role in preventing or delaying cognitive impairment [17,18]. Thus, nutrition offers an interesting opportunity for investigation. Data from recent studies show that reducing the intake of trans-fat and saturated fats, (e.g., red and processed meats); decreasing the consumption of dairy products; and increasing intake of fruits, vegetables, legumes, and whole grains could be associated with the improvement of cognitive function and prevention of AD [19,20]. Moreover, it has been suggested that different eating patterns, including Mediterranean diet (MedDi), Ketogenic Diet (KD), caloric restriction, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH diet Intervention for Neurological Delay (MIND), could possibly reduce the pathophysiological hallmarks of AD [19,21,22,23].
Among others, in the last decade, the MedDi and KD have been the two most studied eating patterns for their possible beneficial effect on cognitive functions. Both of them have, in fact, demonstrated to be effective in reducing or delaying cognitive impairment in older people by acting through different pathophysiological mechanisms [22,23,24]. Table 1 synthetizes the most important features of these two dietary patterns. The main characteristics of the MedDi is the high consumption of olive oil, legumes, whole grains, fruit, vegetables, nuts, poultry, fish, low-fat milk derivatives, moderate consumption of wine, and a reduced consumption of red meat, sausages, and refined cereal products [20]. Instead, KD is a very high-fat and low-carbohydrate diet, which forces the system to shift from glucose metabolism toward the metabolism of fatty acids with the production of ketone bodies [25].

Table 1.
Main characteristics of the Mediterranean and ketogenic diet eating patterns.
Despite the increasing availability of preclinical and clinical scientific evidence regarding the nonpharmacological approach in the prevention of neurodegeneration, a review paper describing the current knowledge, differences, and similarities of underlying mechanisms and the exerted effects of these two nutritional approaches on cognitive function is still missing [22]. Another open issue regards the impact of these dietary patterns on specific populations characterized by clinical frailty, which have been demonstrated to be at higher risk to develop AD-related cognitive impairment, such as those individuals with diabetes [26], chronic kidney disease [27], and other chronic-degenerative disorders associated with the aging process [28,29]. These populations could, in fact, easily develop malnutrition and/or sarcopenia, which must be accurately avoided in order to prevent further cognition deterioration.
The aim of this narrative bibliographic review is to overview the existing literature on the efficacy and pathways through which MedDi and KD associate with age-related cognitive decline and, therefore, to evaluate their possible role in the prevention and/or treatment of neurodegenerative disorders.
2. Methodology
We analyzed several studies of the last 20 years (2000–2020) about the relationship between the MedDi/KD and the prevalence of AD-related cognitive impairment.
The revision started with a research on PubMed/MEDLINE of a series of keywords related to both the studied dietary patterns (either MedDi or KD) and their influence on cognitive functions (“Mediterranean diet”, “Ketogenic diet”, “Dietary patterns”, “Nutritional patterns”, “Alzheimer’s disease”, “Mild cognitive impairment”, “Cognition disorders”, “Cognitive decline”, “Neurodegeneration”). The key words for each component of the research were linked using “or” as a Boolean function, and the results of the two sections were combined by using the “and” Boolean for further search. Titles and abstracts of retrieved studies were screened to select potentially relevant articles. Full texts were analyzed independently to determine whether they met the established inclusion criteria. Moreover, references of eligible articles were searched manually for additional papers that could have been missed by the electronic search only.
With respect to article types, prospective cohort studies, retrospective and cross-sectional studies, randomized controlled trials, meta-analyses, and systematic and narrative reviews were included in the present work. Case reports and case series were also considered in the analysis when it was appropriate for completeness purposes. Finally, existing editorials and commentaries to the most relevant articles were comprised.
Afterwards, we included in the examination both the papers describing a significant direct or inverse relationship between the studied dietary pattern and the cognitive effects and those papers without statistically significant findings. We marked out the most important results for each study, focusing on the supposed underlying mechanisms through which the reported effect was determined.
Conversely, nonoriginal articles and studies in languages other than English were excluded.
The last update of our dataset was performed in March 2020. Considering the nature of the study, Ethics committee approval was not required.
3. Mediterranean Diet
The traditional MedDi is a nutritional pattern inspired by the food traditions of the populations that live in countries bathed by the Mediterranean Sea [30]. Among other benefits, MedDi has been linked to a lower risk of various chronic diseases [31], and it is assumed that its healthful effects are due to the high intake of monounsaturated fats (MUFAs) and polyphenols from olive oil, the high consumption of polyunsaturated fats (PUFAs) from fish, and the antioxidant properties of vegetables, fruits, and wine [32].
Several randomized controlled trials (RCTs) have examined the association between MedDi and cognitive function [33,34,35]. The Spanish PREDIMED (Prevención con Dieta Mediterranea) study was the cornerstone trial showing that patients at high cardiovascular risk consuming MedDi (supplemented with either extra-virgin olive oil or nuts) had a reduced incidence of cardiovascular events compared to those following a low-fat diet after a follow-up period of five years [36,37]. In a subcohort of 447 subjects (aged 55–80 years) participating in the PREDIMED study, an improved cognitive function was observed in the MedDi group compared to the group with low-fat dietary pattern, after a follow-up of 6.5 years [38,39].
Another RCT, the MedLey study, was conducted among 137 non-Mediterranean patients with a mean age of 72 years, who were randomly assigned to either a MedDi or a control diet (their usual diet). No significant beneficial effects during six months of study period were observed on cognitive function in the MedDi group compared to the control group. The limited number of participants, the short duration of follow-up (6 months)—especially for detecting modification of cognitive functions—and the milder modification of the nutritional habits of the patients in respect of their usual diet (intervention diet was essentially based on Australian foods rather than the conventional MedDi) could have effected on the nonobserved effects on cognitive function of MedDiet intervention group [34].
A third RCT regarding MedDi and cognitive performance was recently conducted among 65–79 year-old patients from Mediterranean and non-Mediterranean regions (the NU-AGE trial) [35]. In this study, the intervention group followed a MedDi-like diet, tailored on the cultural habits and dietary recommendations of the different regions involved in the study, while the participants of the control group followed their habitual diet. After one year of follow-up, individuals of the intervention group showed improvements in their cognitive performance compared to those of the control group, even if these differences were not statistically significant. Nevertheless, a higher adherence to the MedDi-like diet was related to a significant improvement of episodic memory, which is associated to neurodegeneration [35,40].
Moreover, a recent meta-analysis of RCTs showed an association of MedDi with an improved global cognition among healthy older adults when compared with controls [41]. Several other meta-analyses have been published regarding the role of MedDi on the process of neurodegeneration, suggesting an inverse association between adherence to MedDi and the risk of developing cognitive impairment [42,43,44].
A number of cross-sectional and longitudinal studies performed both in Mediterranean and non-Mediterranean countries and using different MedDi adherence scores have demonstrated that higher adherence to the MedDi is associated with a significantly lower risk for developing mild cognitive impairment (MCI) and AD [45,46,47,48,49]. Better cognitive functions and lower rates of cognitive decline have been also demonstrated in systematic reviews of RCTs and observational studies [32,50], concluding that a higher level of adherence to the MedDi nutritional pattern is associated with overall better cognitive performance. Additionally, adherence to MedDi has been associated with reduced mortality rates in AD patients [51].
Beside these observed positive associations of MedDi with the cognitive function, there are also some prospective studies that have not found association of MedDi with the improvement of cognitive status [52]. Nonobserved association was also seen in a Swedish longitudinal cohort study, with 12 years of follow-up, where the MedDi was not associated with the prevention of AD [53].
Several factors should be taken into account when interpreting these nonobserved associations. Firstly, the use of different score-based methods for evaluating the adherence to the studied nutritional pattern (e.g., the Trichopoulou’s or the Panagiotakos’s scores) [54,55] does not allow a homogeneous interpretation of the clinical findings. These scores have been mainly tested on Mediterranean cohorts and, thus, are not fully applicable to people living in non-Mediterranean countries and, therefore, having different dietary/lifestyle habits [47,56]. Secondly, it is important to consider that the abovementioned studies imply profound differences in terms of study design, average age of the study population, neuropsychological tests used, as well as the duration of the follow-up period. Nevertheless, the majority of the evidence suggests that MedDi has been shown to associate with improved cognitive function among elderly participants (Table 2).

Table 2.
Evidence on association between Mediterranean diet and cognitive decline.
As it has been stated by several authors, the MedDi cannot be merely considered only as a nutritional pattern. Rather, it represents a cultural heritage and a lifestyle, which is often associated with factors that are, per se, related to a better cognitive function, such as moderate physical activity and behaviors of social and familiar aggregation—that is, for example, eating meals together with friends or family members [30,69].
3.1. Mediterranean Diet and Cognitive Impairment in Type 2 Diabetic Patients
It is known that type 2 diabetes (T2D) is a risk factor for cognitive disorders, given that more than 30% of patients with T2D have MCI [70]. Diabetes has been shown to significantly deteriorate the cognitive function, not only inducing acute and chronic vascular disorders, but also increasing the risk for neurodegenerative diseases such as AD [71]. To date, there is a lack of scientific evidence regarding the role of MedDi on cognitive status and decline in T2D patients. However, one recent study investigated the correlation of two-year change in global cognitive status z-score, executive cognitive functions, and several cognitive tests to the adherence to different eating patterns (e.g., MedDi, DASH diet, Healthy Eating and Alternate Healthy Eating indices). Among others, adherence to MedDi was significantly associated with higher two-year change in global cognitive function in adults with T2D, thus demonstrating a positive effect of this eating pattern in people with impaired glucose metabolism [72]. Moreover, the study demonstrated that good glycemic control further sustained these benefits. Better cognitive scores were, in fact, confirmed in those patients under glycemic control at baseline and stable/improved over the two years of follow-up, but not for individuals with uncontrolled or poor/declined glycemic control at the end of the observation period. Therefore, the treatment of diabetes should be strictly targeted and effective in order to preserve optimal cognitive function. Further studies are needed to confirm these findings.
3.2. Suggested Mechanisms between Mediterranean Diet and Alzheimer’s Disease
To date, the pathways underlying the proposed neuroprotective effects of the MedDi are not fully understood. There are several possible mechanisms that could explain the effect of the nutritional pattern of MedDi on cognitive health. Neurodegenerative diseases such as AD are displayed with typical features of cognitive dysfunction, which may be partially corrected by adhering to a balanced nutritional pattern. Besides the histopathological hallmarks (e.g., Aβ accumulation, Tau hyper-phosphorylation), the neuronal loss seen in AD has been additionally associated with other mechanisms as reduced blood flow (due to the atherosclerotic process), mitochondrial dysfunction, oxidative stress, neuroinflammation, and alteration of the gut microbiota [32].
There is evidence that adherence to the MedDi may modify some of the major cardiovascular risk factors such as atherosclerotic dyslipidemia [36]. Furthermore, a meta-analysis of clinical trials, which compared the effects of the MedDi with low-fat diet on cardiovascular risk factors among 2650 study participants with and without T2D, confirmed that MedDi is more effective in reducing body weight, systolic and diastolic blood pressure, fasting blood glucose, and C-reactive protein during a two-year follow-up [73].
The benefits of the MedDi diet on the cognitive status are probably mediated by reduced oxidative stress and inflammation [20]. Evidence from epidemiological and clinical studies showed, in fact, that some of the main components of MedDi (e.g., fruit and vegetables) are able to reduce the brain damage induced by reactive oxygen species (ROS), by improving antioxidant defenses and by lowering lipid oxidation and platelet aggregation [74,75]. MUFAs, the most abundant types of fat in the MedDi, are able to increase antioxidant pathways, whereas saturated fatty acids (SFA) display a harmful effect. Higher plasma levels of lipoproteins rich in SFA rather than in MUFA could affect the responsiveness of low-density lipoprotein (LDL)-receptors in the liver, inducing an increase in LDL levels and making them more predisposed to oxidation, a condition that increases the formation of atherosclerotic plaques at vascular level [75,76].
Furthermore, some authors have proposed that MedDi could also influence Tau metabolism and Aβ plaque formation [77], however nonobserved associations have been also reported [78].
Additionally, mechanisms considering the association of MedDi with the modification of gut microbiota should be discussed. Significant reduction of several anti-inflammatory gut bacteria (e.g., Bacteroides fragilis) and increase of proinflammatory bacteria (e.g., Escherichia coli) have been, in fact, detected in patients with cognitive impairment and AD [79]. Several authors have demonstrated a positive correlation between MedDi and a beneficial modification of gut microbiota, with an increased amount of Bifidobacteria and, thereafter, increased anti-inflammatory and hypocholesterolemic activity [80].
The proposed protective pathways of MedDi on brain health are summarized in Figure 1. Although the scientific evidence on the role of the nutritional pattern of MedDi in the prevention and/or delay of cognitive impairment is continuously growing, the precise identification and description of these mechanisms requires even further preclinical and clinical studies to be fully elucidated.
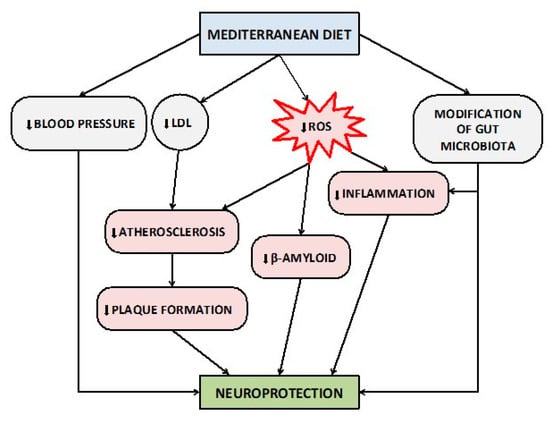
Figure 1.
Proposed protective mechanisms of Mediterranean diet on brain health and cognitive functions. Abbreviations: LDL, Low-Density Lipoprotein; ROS, Reactive Oxygen Species.
4. Ketogenic Diet
The KD represents a particular dietary pattern capable of inducing and maintaining a chronic state of ketosis, that is, a metabolic condition where the body mainly uses ketone bodies (KBs) instead of glucose as the major fuel to produce energy [81]. The most powerful condition that can induce ketosis is fasting [82]. In fact, after less than 24 h of fasting, the body initiates the utilization of the majority of liver glycogen reserves and depends mainly on the endogenous synthesis of glucose and fatty acid oxidation to produce Adenosine Triphosphate (ATP) [82]. A similar process can be achieved with very low-carbohydrate diet and dietary patterns that imply a high percentage of energy intake derived from fatty acids [83].
In case of unavailability of glucose, the liver of an adult is able to release into the bloodstream per day up to 150–300 g of KBs [84], most of which are utilized by the tissues of high energy requirement (e.g., skeletal muscle, heart, and brain) [85]. In particular, in the brain, the mitochondria of the neuronal cells are able to oxidize the acetoacetic acid and the β-hydroxybutyric acid producing acetyl-CoA by supporting, in this way, the production of ATP via the Krebs cycle. This process becomes essential in the case of glucose deficiency, because the neurons are not able to carry out gluconeogenesis [86].
The neuroprotective role of ketosis is known since the 1920s, when it was observed among epileptic patients as a way to reduce the frequency of seizures, followed by prolonged fasting [86,87].
In 1921, Wilder et al. [88] proposed a protocol to utilize KD for the treatment of drug resistant epilepsy. In its original version, KD was able to provide a caloric intake reduced to about 75% of the energy requirement and included a limitation in the assumption of liquids. The induction of ketosis was implemented with a fasting period of 12–48 h followed by the gradual consumption of ketogenic meals [88].
The dietary restrictions for the induction and maintenance of ketosis have resulted in the search for alternative protocols. Some of these include the traditional induction of ketosis but without initial fasting, or a progressive reduction of carbohydrate content, which corresponds to a gradual increase in the lipid/carbohydrate ratio. The latter possibility of induction makes the start of the diet possible even in an outpatient setting. The replacement of a proportion of long-chain triglycerides, at the base of the classic protocol, with medium-chain triglycerides (MCTs), is a KD alternative introduced in 1971 by Huttenlocher et al. [89]. This alternative allows to increase the proportion of carbohydrates in the diet, since the MCTs are more ketogenic compared to long-chain triglycerides [90]. Another form of KD used for the treatment of drug-resistant epilepsy is a modified version called Atkins diet. It is based on an initial drastic reduction of carbohydrate intake, while the protein-containing foods can be consumed without restrictions. Subsequently, in the course of treatment, the carbohydrates are partially included. Although the Atkins diet needs less dietary control compared to the classical KD, it induces a low and fluctuating level of ketosis due to the neoglucogenic effect of the proteins [88,91,92,93].
The beneficial effects of the KBs on the human cognitive function have been observed in several studies. Two separate double-blinded clinical trials compared the influence of MCTs vs. placebo on cognitive capacity [94,95]. Both studies clearly showed that an increase in serum levels of β-hydroxybutyric acid was associated with an improvement of cognitive function and memory. Additionally, the blood KB levels were also positively associated with the improvement in verbal memory in patients with MCI even in the absence of differences in other cognitive functions [96]. In addition, some evidence received from Positron Emission Tomography imaging studies have shown an increase in brain uptake of KBs following administration of MCTs, suggesting that KBs could compensate for the glucose uptake deficiency that is present in the brain tissue of patients with AD [97,98]. The benefits of KDs have been demonstrated not only in adults but also in children, particularly in those with epilepsy, in which adherence to KD was associated with a better cognitive performance [99,100]. Table 3 summarizes both the animal and human studies, which aimed to characterize the role of KD on cognitive functions.

Table 3.
Evidence on association between ketogenic diet and cognitive decline.
Evidence from preclinical studies reported positive effects of ketones on cognitive function [101,102] but also on the regulation of Aβ, which represents the main hallmark of AD. In a study using a mouse model of AD, Van der Auwera et al. [103] demonstrated for the first time a correlation between KD treatment and the reduction of Aβ expression and, consequently, of senile plaque formation and cerebral oxidative stress. This result was replicated in a subsequent study, where the authors showed that prolonged treatment with ketone esters were associated not only with a reduction of Aβ and Tau protein deposition, but also with the improvement of performance on learning and memory tests [104].
Furthermore, it has been demonstrated that ketones—blocking Aβ entry into neurons—reduce intracellular amyloid aggregation and improve mitochondrial function and memory ability [105].
In disagreement with these results, a study reported that KD improved motor performance but did not reduce the deposition of Aβ or Tau protein in a transgenic mouse model [106].
Zhao et al. [107] reported detrimental effects of KD on cognitive functions. In particular, the authors found that KD impaired spatial learning, memory, and brain growth in rats. It should be noted that the KD used in this trial had a fat-to-protein plus carbohydrate ratio that was more than 2-fold higher than standard KD diet and that protein represented only 8% of the diet, which is less than half of the protein content of a regular diet. Severe protein-energy restriction could be, therefore, the factor responsible for the reported negative effects on cognitive function and brain development [108].
In humans, some studies showed that administration of MCTs supplements, which raises beta-hydroxybutyrate (B-OHB) levels, improves cognition and memory in AD subjects in the short-term [109,110,111]. However, some of these studies suggest that neurological effects depend on the apolipoprotein E4 (ApoE4) genotype, the most prevalent genetic risk factor for AD, because cognitive benefits have been demonstrated only among subjects who were ApoE4-negative [109,110]. Nevertheless, a recent case study reported cognition improvements after KD in a patient who was heterozygous for ApoE4 and with a family history of AD and diagnosis of mild AD [112].
Administration of MCTs in patients with mild-to-moderate AD induces significant improvement in immediate and delayed logical memory tests after 8 weeks and in the digit-symbol coding test and immediate logical memory tests after 12 weeks [113]. A positive effect on memory function after KD has also been demonstrated in older adults with MCI [96,114].
The Ketogenic Diet Retention and Feasibility Trial conducted on 15 patients with AD showed that during ketosis, obtained with MCTs-supplemented KD, the mean of the Alzheimer’s disease Assessment Scale–cognitive subscale score (ADAS–cog) significantly improved but returned to baseline after 1-month washout period [115].
A recent pilot study evaluated neurological effects of a modified Mediterranean–ketogenic diet (MMKD) in older adults at risk for AD [116]. MMKD differed from KD because it provided slightly higher consumption of carbohydrate such as vegetables and fruits, proteins derived from fish, and fats derived from olive oil. In this trial, an increased cerebral perfusion and cerebral ketone body uptake following MMKD has been reported [116].
Despite abovementioned beneficial effects of KD on patients with MCI or AD, evidence from a randomized controlled study suggested that KD has no effect on the cognitive performance of 11 healthy, normal-weight individuals [117].
4.1. Suggested Mechanisms between Ketogenic Diet and Alzheimer’s Disease
In recent years, several hypotheses have been proposed to explain the neuroprotective role of KBs [23]. Some studies have shown that during a long period of starvation, KBs may provide up to 70% of cerebral energy requirements, representing a more efficient energy source than glucose due to the fact that KBs can enter directly into the Krebs cycle to produce ATP, while glucose needs to be first metabolized through glycolysis [118]. This effect seems to play a particularly important role in individuals with AD, because in this condition, there is an alteration of the BBB that results in altered expression of some membrane transporters and, in particular, a reduction of the expression of the glucose transporters. Under these conditions, the brain is more dependent from the catabolism of KBs that, unlike glucose, cross the BBB hanks to specific carriers that are not downregulated in AD [91]. This effect may explain why, in fasting patients suffering from MCI or AD, after ingestion of a dose of MCTs able to increase the plasma levels of β-hydroxybutyric acid, a significant improvement in the cognitive abilities was shown compared to placebo [109].
KBs improve mitochondrial function, by enhancing nicotinamide adenine dinucleotide (NADH) oxidation in the mitochondrial respiratory chain and by inhibiting mitochondrial permeability transition [119].
As already mentioned, oxidative stress and chronic inflammation represent two of the most important neurotoxic mechanisms underlying the development and progression of MCI and AD, as they cause neuronal death in the brain areas responsible for memory and cognitive processes [120].
The metabolism of KBs has been demonstrated to associate with a lower production of ROS, and thus with a reduction in oxidative stress, as compared to glycolysis, assuming an additional neuroprotective mechanism carried out by these compounds [23]. In particular, B-OHB reduces mitochondrial ROS production and inhibits histone deacetylases, upregulating the transcription of some genes that are protective against oxidative stress [121,122]. Moreover, KBs contribute to the reduction of ROS production through the expression of mitochondrial uncoupling protein (UCP), thereby decreasing mitochondrial membrane potential [123]. In addition, the activation of peroxisome proliferator-activated receptor gamma (PPARγ) after KD seems to inhibit neuroinflammation in mouse hippocampus [124]. In addition, it has been reported that KD has the capability to induce antioxidant glutathione peroxidase activity in the hippocampus with a potential protective effect against neurodegeneration of this structure [125,126].
Other neuroprotective mechanisms that have been highlighted against the development of AD are the reduction of the Amyloid Precursor Protein (APP) production [91] and of Aβ deposition [103]. Moreover, a recent study has shown that in vitro KBs are able to induce Aβ clearance from the brain to blood [127]. According to some evidence, treatment with KD has been also associated with an increase of angiogenesis and capillary density, suggesting that the KBs may support the cognitive processes through an improvement of the cerebral microvascularization [94].
Finally, as is widely known, the KD improves the ability to secrete insulin by the β-cell as well as enhances insulin-sensitivity [128]. Insulin resistance is a major risk factor for the development and progression of cognitive impairment and AD, because it is associated with increased cerebral atherosclerosis, smaller volume of the hippocampus, formation of advanced glycation end-products (AGEs), increased oxidative stress, production of proinflammatory cytokines, and deposition of beta-amyloid [97]. Insulin resistance has been also demonstrated in the brain in patients with AD, even in the absence of diabetes mellitus [129]. In the brain, insulin exerts a neuroprotective effect by modulating synaptic plasticity, memory functions, and learning [130]. In particular, the hippocampal neurons express the glucose transporter type 4 (GLUT–4) which, unlike the GLUT–1 and GLUT–3 (that are ubiquitously expressed at the neuronal level), needs the presence of insulin in order to allow the entry of glucose in the cell [131]. Figure 2 summarizes the underlined protective mechanisms of KD against cognitive impairment.
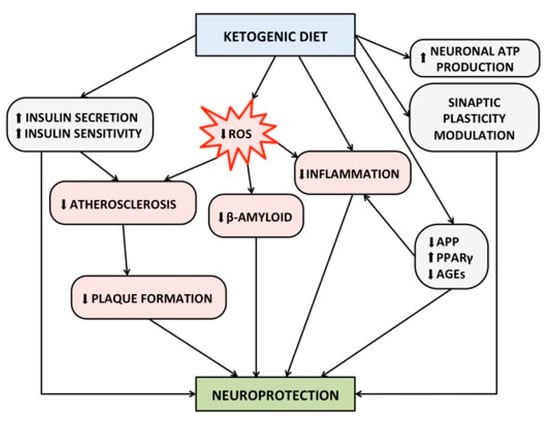
Figure 2.
Proposed protective mechanism of ketogenic diet on brain health and cognitive functions. Abbreviations: ATP, Adenosine Triphosphate; AGEs, Advanced Glycosylated End-products; APP, Amyloid Precursor Protein; PPAR-γ, Peroxisome Proliferator-Activated Receptor gamma; ROS, Reactive Oxygen Species.
For the abovementioned characteristics, KD appears to be a promising option for slowing the progression of cognitive impairment in patients with MCI and AD. However, the best results on memory and learning ability can be achieved when the ketosis status is induced at the earliest stages of cognitive impairment.
4.2. Ketogenic Diet in the Elderly Patients, Possible Concerns
Even if the effects of KD on cognition function seem encouraging, there are several issues raised by the utilization of this nutritional pattern in people who suffer from neurological disorders. Furthermore, among frail elderly individuals, the comorbidity is common, and they are at high risk for developing malnutrition.
One of the characteristics of KD is the reduction of appetite, which may be additionally associated with gastrointestinal side effects [132]. This anorectic effect could further reduce dietary portions and overall food intake in elderly patients following a KD eating pattern with the subsequent increased risk of malnutrition and deterioration of neurological symptoms [25].
Moreover, since the risk of sarcopenia is high in the elderly population, we have to consider that, although the percentage of energy intake belonging from proteins is often normal or even higher than recommended, KD may lead to an insufficient supply of protein, leading to catabolism of muscular proteins [133].
Lastly, because of the concerns regarding use of KD in people with T2D, chronic kidney disease, and disordered eating patterns, further research is needed before recommendations can be made for these subgroups of individuals. Adopting a KD eating plan can, in fact, increase diuresis and rapidly reduce plasma glucose-inducing hypoglycemia.
Therefore, consultation with a specialist is imperative prior to the consumption of KD to avoid dehydration, and to modify the dosages of antihyperglycemic medications and insulin, if necessary, in order to prevent hypoglycemia in people with T2D [134].
5. Discussion
Acceleration of aging worldwide, and thus increasing incidence of neurodegenerative diseases such as AD, in combination with the fact that no effective drugs for the treatment of dementia has been discovered so far, have increased interest in the identification of possible modifiable factors that could prevent, or at least delay, the development of cognitive impairment in the elderly. In this regard, during the last two decades, a growing body of evidence has been produced in order to elucidate the role of healthy lifestyle in counteracting the negative consequences of the aging process on cognitive health. In this regard, proper nutrition has been demonstrated to be an important factor for the maintenance of good cognitive function among healthy individuals and for the prevention of further mental deterioration in patients already affected by cognitive impairment. Furthermore, for a person with AD or dementia, poor nutrition may worsen behavioral symptoms and cause excessive weight loss.
With this review, we aimed to overview the current knowledge on the links between two of the most used eating patterns (e.g., MedDi and KD) and the risk of developing mild-to-severe cognitive damage, focusing specifically on the supposed underlying pathophysiological mechanisms. Our effort was directed to comprehensively show, in an orderly and equable fashion, a large amount of studies which are, so far, unbalanced between the two studied nutritional patterns—the evidence on MedDi being much wider.
The strength of the present narrative review consists in its original approach, aiming to highlight differences and similarities of these two dietary patterns as well as to stress both the common and distinct molecular pathways related to cognitive function. Secondly, despite our study consisting of a bibliographic and nonsystematic review, it used broad inclusion criteria aimed at developing a comprehensive and complete idea about the effect of both diets on cognition. Thirdly, unlike several previously published works) [135,136,137], it considered both dietary patterns holistically rather than the association between specific macronutrient or micronutrient intakes and cognitive function (in terms of improvement or deterioration). We believe that such an approach could be more suggestive of a wider and more complete message on the efficacy of the whole dietary approach rather than of single foods or supplements.
This review also has some limitations that might have affected the reporting of our results. Among them, it should be mentioned that dietary assessment methods varied from one study to the other, and this might have an effect on the outcomes and conclusions of the studies. More generically, the substantial problem in using the review approach for analyzing correlations and biological mechanisms is the heterogeneity in methodology, population, and outcome measurements between the retrieved studies.
Several issues must be considered in order to correctly interpret the reported literature in a meaningful way. By revising current scientific literature, in fact, we realized that overall evidence is not strong enough to show that any type of nutritional approach can significantly reduce the incidence of AD or other neurodegenerative diseases [24,138,139]. The reasons why we cannot, at this point, make exhaustive conclusions on this regard are multifaceted.
One issue is that most studies on the effects of diet on dementia are based on dietary questionnaires completed by participants who already may have trouble recalling what they ate or have memory problems [140].
Additionally, it may be challenging to assess the efficacy of specific dietary patterns such as KD for an adequately long study period, considering certain constraints of the application of such diets in older people with concomitant illness.
Finally, we believe that making a choice between different and specific eating patterns could be an incorrect simplification for a complex problem, which instead should be handled with personalized approaches in order to address individual nutritional needs. Many researchers, in fact, speculate that making healthy food choices (and not following specific diets) may improve the modifiable risk factors for cognitive deterioration such as dyslipidemia, diabetes, atherosclerosis, which may in turn reduce the risk of MCI or AD.
6. Conclusions
Current scientific literature does not provide exhaustive conclusions on the impact of any dietary patterns such as MedDi and KD on neurodegenerative disorders like AD.
Nevertheless, several useful clinical messages emerge from the careful review of published papers and are summarized as follows: (1) Even if the vast majority of evidence supports MedDi as the nutritional pattern, which has been demonstrated to be fairly efficacious in the preservation of several cognitive functions, other nutritional patterns such as KD have also been associated with beneficial effects. (2) Although the consumption of KD has been very popular in the last few years, KD should be recommended only in specific cases and for a limited time, considering both the insufficient available data in human models (especially in the elderly) and the possible risks related to its prolonged use. (3) MedDi, independently from the effectiveness in reducing the risk of development and progression of cognitive impairment, has already been demonstrated to reduce risk for cardiovascular disease and mortality and can be recommended to patients—despite their age, clinical conditions, and comorbidities. Furthermore, it can be tailored on the majority of the cultural habits within the different countries and regions, and along with physical activity it represents an overall healthy lifestyle more than a simple dietary pattern.
More research and clinical trials are needed to determine to what degree a certain dietary pattern can be able to prevent AD or to slow its progression.
Author Contributions
Conceptualization, F.V., M.G., L.F., and A.T.; resources, F.V., M.G., M.H., L.F., and A.T. original draft preparation, F.V., M.G., L.F., and A.T.; review and editing, F.V., M.H., L.F., and A.T.; supervision, F.V., L.F., and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
A.T. is a recipient of a post-doctoral fellowship founded by the Department of Clinical and Experimental Medicine, University of Catania, Catania (Italy).
Conflicts of Interest
The authors declare no conflict of interest.
References
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Therneau, T.M.; Weigand, S.D.; Wiste, H.J.; Knopman, D.S.; Vemuri, P.; Lowe, V.J.; Mielke, M.M.; Roberts, R.O.; Machulda, M.M.; et al. Prevalence of Biologically vs Clinically Defined Alzheimer Spectrum Entities Using the National Institute on Aging-Alzheimer’s Association Research Framework. JAMA Neurol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, L.; Rosset, I.; Roriz-Cruz, M. Global epidemiology of dementia: Alzheimer’s and vascular types. BioMed Res. Int. 2014, 2014, 908915. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- World Health Organization endorses global action plan on rising incidence of dementia. Nurs. Older People 2017, 29, 7. [CrossRef]
- Fong, T.G.; Vasunilashorn, S.M.; Libermann, T.; Marcantonio, E.R.; Inouye, S.K. Delirium and Alzheimer disease: A proposed model for shared pathophysiology. Int. J. Geriatr. Psychiatry 2019, 34, 781–789. [Google Scholar] [CrossRef]
- Kern, S.; Zetterberg, H.; Kern, J.; Zettergren, A.; Waern, M.; Hoglund, K.; Andreasson, U.; Wetterberg, H.; Borjesson-Hanson, A.; Blennow, K.; et al. Prevalence of preclinical Alzheimer disease: Comparison of current classification systems. Neurology 2018, 90, e1682–e1691. [Google Scholar] [CrossRef]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Frittitta, L. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Role of Insulin Signalling and Therapeutic Implications. Int. J. Mol. Sci. 2018, 19, 3306. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, S.; Zhu, Z.; Xu, J. Multi-target design strategies for the improved treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 176, 228–247. [Google Scholar] [CrossRef]
- Shukla, M.; Htoo, H.H.; Wintachai, P.; Hernandez, J.F.; Dubois, C.; Postina, R.; Xu, H.; Checler, F.; Smith, D.R.; Govitrapong, P.; et al. Melatonin stimulates the nonamyloidogenic processing of betaAPP through the positive transcriptional regulation of ADAM10 and ADAM17. J. Pineal Res. 2015, 58, 151–165. [Google Scholar] [CrossRef]
- Pimenova, A.A.; Thathiah, A.; De Strooper, B.; Tesseur, I. Regulation of amyloid precursor protein processing by serotonin signaling. PLoS ONE 2014, 9, e87014. [Google Scholar] [CrossRef] [PubMed]
- Godyn, J.; Jonczyk, J.; Panek, D.; Malawska, B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol. Rep. PR 2016, 68, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Soobiah, C.; Berliner, S.; Ho, J.M.; Ng, C.H.; Ashoor, H.M.; Chen, M.H.; Hemmelgarn, B.; Straus, S.E. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: A systematic review and meta-analysis. CMAJ Can. Med Assoc. J. 2013, 185, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.C.; Yu, J.T.; Wang, H.F.; Tan, M.S.; Meng, X.F.; Wang, C.; Jiang, T.; Zhu, X.C.; Tan, L. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. JAD 2014, 41, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.R.; Bangen, K.J.; Weigand, A.J.; Edmonds, E.C.; Sundermann, E.; Wong, C.G.; Eppig, J.; Werhane, M.L.; Delano-Wood, L.; Bondi, M.W.; et al. Type II Diabetes Interacts With Alzheimer Disease Risk Factors to Predict Functional Decline. Alzheimer Dis. Assoc. Disord. 2019. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.K.; Myung, W.; Yoo, J.H.; Shin, S.J.; Na, D.L.; Kim, S.Y.; Lee, J.H.; Kim, S.Y.; Han, S.H.; et al. Risk Factors of Behavioral and Psychological Symptoms in Patients with Alzheimer Disease: The Clinical Research of Dementia of South Korea Study. Korean J. Fam. Med. 2019, 40, 16–21. [Google Scholar] [CrossRef]
- Srisuwan, P. Primary prevention of dementia: Focus on modifiable risk factors. J. Med Assoc. Thail. 2013, 96, 251–258. [Google Scholar]
- Gill, S.S.; Seitz, D.P. Lifestyles and Cognitive Health: What Older Individuals Can Do to Optimize Cognitive Outcomes. JAMA 2015, 314, 774–775. [Google Scholar] [CrossRef]
- Barnard, N.D.; Bush, A.I.; Ceccarelli, A.; Cooper, J.; de Jager, C.A.; Erickson, K.I.; Fraser, G.; Kesler, S.; Levin, S.M.; Lucey, B.; et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol. Aging 2014, 35 (Suppl. S2), S74–S78. [Google Scholar] [CrossRef]
- Roman, G.C.; Jackson, R.E.; Gadhia, R.; Roman, A.N.; Reis, J. Mediterranean diet: The role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease—A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Mediterranean and MIND Diets Containing Olive Biophenols Reduces the Prevalence of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 2797. [Google Scholar] [CrossRef] [PubMed]
- Rusek, M.; Pluta, R.; Ulamek-Koziol, M.; Czuczwar, S.J. Ketogenic Diet in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 3892. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, A.L.; Caffa, I.; Cea, M.; Nencioni, A.; Odetti, P.; Monacelli, F. Nutrients in the Prevention of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2019, 2019, 9874159. [Google Scholar] [CrossRef]
- Wlodarek, D. Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef]
- Madhusudhanan, J.; Suresh, G.; Devanathan, V. Neurodegeneration in type 2 diabetes: Alzheimer’s as a case study. Brain Behav. 2020, e01577. [Google Scholar] [CrossRef]
- Zammit, A.R.; Katz, M.J.; Bitzer, M.; Lipton, R.B. Cognitive Impairment and Dementia in Older Adults with Chronic Kidney Disease: A Review. Alzheimer Dis. Assoc. Disord. 2016, 30, 357–366. [Google Scholar] [CrossRef]
- Yang, M.; Li, C.; Zhang, Y.; Ren, J. Interrelationship between Alzheimer’s disease and cardiac dysfunction: The brain-heart continuum? Acta Biochim. Biophys. Sin. 2020, 52, 1–8. [Google Scholar] [CrossRef]
- Zhang, F.; Niu, L.; Li, S.; Le, W. Pathological Impacts of Chronic Hypoxia on Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.D. Health effects of dietary risks in 195 countries, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Petersson, S.D.; Philippou, E. Mediterranean Diet, Cognitive Function, and Dementia: A Systematic Review of the Evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Radd-Vagenas, S.; Duffy, S.L.; Naismith, S.L.; Brew, B.J.; Flood, V.M.; Fiatarone Singh, M.A. Effect of the Mediterranean diet on cognition and brain morphology and function: A systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2018, 107, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Bryan, J.; Wilson, C.; Hodgson, J.M.; Davis, C.R.; Murphy, K.J. The Mediterranean Diet and Cognitive Function among Healthy Older Adults in a 6-Month Randomised Controlled Trial: The MedLey Study. Nutrients 2016, 8, 579. [Google Scholar] [CrossRef]
- Marseglia, A.; Xu, W.; Fratiglioni, L.; Fabbri, C.; Berendsen, A.A.M.; Bialecka-Debek, A.; Jennings, A.; Gillings, R.; Meunier, N.; Caumon, E.; et al. Effect of the NU-AGE Diet on Cognitive Functioning in Older Adults: A Randomized Controlled Trial. Front. Physiol. 2018, 9, 349. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martinez-Gonzalez, M.A.; Martinez-Lapiscina, E.H.; Fito, M.; Perez-Heras, A.; Salas-Salvado, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef]
- Martinez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvado, J.; San Julian, B.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M.A. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Eichenbaum, H. The episodic memory system: Neurocircuitry and disorders. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 86–104. [Google Scholar] [CrossRef]
- Loughrey, D.G.; Lavecchia, S.; Brennan, S.; Lawlor, B.A.; Kelly, M.E. The Impact of the Mediterranean Diet on the Cognitive Functioning of Healthy Older Adults: A Systematic Review and Meta-Analysis. Adv. Nutr. 2017, 8, 571–586. [Google Scholar] [CrossRef]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. JAD 2014, 39, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch. Neurol. 2006, 63, 1709–1717. [Google Scholar] [CrossRef]
- Gardener, S.; Gu, Y.; Rainey-Smith, S.R.; Keogh, J.B.; Clifton, P.M.; Mathieson, S.L.; Taddei, K.; Mondal, A.; Ward, V.K.; Scarmeas, N.; et al. Adherence to a Mediterranean diet and Alzheimer’s disease risk in an Australian population. Transl. Psychiatry 2012, 2, e164. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef]
- Scarmeas, N.; Luchsinger, J.A.; Schupf, N.; Brickman, A.M.; Cosentino, S.; Tang, M.X.; Stern, Y. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009, 302, 627–637. [Google Scholar] [CrossRef]
- Lourida, I.; Soni, M.; Thompson-Coon, J.; Purandare, N.; Lang, I.A.; Ukoumunne, O.C.; Llewellyn, D.J. Mediterranean diet, cognitive function, and dementia: A systematic review. Epidemiology 2013, 24, 479–489. [Google Scholar] [CrossRef]
- Scarmeas, N.; Luchsinger, J.A.; Mayeux, R.; Stern, Y. Mediterranean diet and Alzheimer disease mortality. Neurology 2007, 69, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Feart, C.; Samieri, C.; Rondeau, V.; Amieva, H.; Portet, F.; Dartigues, J.F.; Scarmeas, N.; Barberger-Gateau, P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009, 302, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Karlstrom, B.; Kilander, L.; Byberg, L.; Cederholm, T.; Sjogren, P. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J. Alzheimer’s Dis. JAD 2015, 43, 109–119. [Google Scholar] [CrossRef] [PubMed]
- deKoning, L.; Anand, S.S. Vascular viewpoint. Vasc. Med. 2004, 9, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D. Adherence to Mediterranean diet and risk of developing cognitive disorders: An updated systematic review and meta-analysis of prospective cohort studies. Sci. Rep. 2017, 7, 41317. [Google Scholar] [CrossRef]
- Cao, L.; Tan, L.; Wang, H.F.; Jiang, T.; Zhu, X.C.; Lu, H.; Tan, M.S.; Yu, J.T. Dietary Patterns and Risk of Dementia: A Systematic Review and Meta-Analysis of Cohort Studies. Mol. Neurobiol. 2016, 53, 6144–6154. [Google Scholar] [CrossRef]
- van de Rest, O.; Berendsen, A.A.; Haveman-Nies, A.; de Groot, L.C. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Guyer, H.; Langa, K.M.; Yaffe, K. Neuroprotective Diets Are Associated with Better Cognitive Function: The Health and Retirement Study. J. Am. Geriatr. Soc. 2017, 65, 1857–1862. [Google Scholar] [CrossRef]
- Katsiardanis, K.; Diamantaras, A.A.; Dessypris, N.; Michelakos, T.; Anastasiou, A.; Katsiardani, K.P.; Kanavidis, P.; Papadopoulos, F.C.; Stefanadis, C.; Panagiotakos, D.B.; et al. Cognitive impairment and dietary habits among elders: The Velestino Study. J. Med. Food 2013, 16, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Okereke, O.I.; E. Devore, E.; Grodstein, F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J. Nutr. 2013, 143, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Wengreen, H.; Munger, R.G.; Cutler, A.; Quach, A.; Bowles, A.; Corcoran, C.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A. Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: The Cache County Study on Memory, Health and Aging. Am. J. Clin. Nutr. 2013, 98, 1263–1271. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Judd, S.; Letter, A.J.; Alexandrov, A.V.; Howard, G.; Nahab, F.; Unverzagt, F.W.; Moy, C.; Howard, V.J.; Kissela, B.; et al. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology 2013, 80, 1684–1692. [Google Scholar] [CrossRef]
- Cherbuin, N.; Anstey, K.J. The Mediterranean diet is not related to cognitive change in a large prospective investigation: The PATH Through Life study. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2012, 20, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Geda, Y.E.; Cerhan, J.R.; Knopman, D.S.; Cha, R.H.; Christianson, T.J.; Pankratz, V.S.; Ivnik, R.J.; Boeve, B.F.; O’Connor, H.M.; et al. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2010, 29, 413–423. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Kyrozis, A.; Stathopoulos, P.; Trichopoulos, D.; Vassilopoulos, D.; Trichopoulou, A. Diet, physical activity and cognitive impairment among elders: The EPIC-Greece cohort (European Prospective Investigation into Cancer and Nutrition). Public Health Nutr. 2008, 11, 1054–1062. [Google Scholar] [CrossRef]
- Sofi, F.; Valecchi, D.; Bacci, D.; Abbate, R.; Gensini, G.F.; Casini, A.; Macchi, C. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J. Intern. Med. 2011, 269, 107–117. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, H.M.; Kim, N.K.; Yang, J.Y.; Noh, J.H.; Ko, K.S.; Rhee, B.D.; Kim, D.J. Factors associated for mild cognitive impairment in older korean adults with type 2 diabetes mellitus. Diabetes Metab. J. 2014, 38, 150–157. [Google Scholar] [CrossRef]
- Li, W.; Huang, E. An Update on Type 2 Diabetes Mellitus as a Risk Factor for Dementia. J. Alzheimer’s Dis. JAD 2016, 53, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Mattei, J.; Bigornia, S.J.; Sotos-Prieto, M.; Scott, T.; Gao, X.; Tucker, K.L. The Mediterranean Diet and 2-Year Change in Cognitive Function by Status of Type 2 Diabetes and Glycemic Control. Diabetes Care 2019, 42, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Vetrani, C.; Costabile, G.; Di Marino, L.; Rivellese, A.A. Nutrition and oxidative stress: A systematic review of human studies. Int. J. Food Sci. Nutr. 2013, 64, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Belalcazar, L.M.; Haffner, S.M.; Lang, W.; Hoogeveen, R.C.; Rushing, J.; Schwenke, D.C.; Tracy, R.P.; Pi-Sunyer, F.X.; Kriska, A.M.; Ballantyne, C.M.; et al. Lifestyle intervention and/or statins for the reduction of C-reactive protein in type 2 diabetes: From the look AHEAD study. Obesity 2013, 21, 944–950. [Google Scholar] [CrossRef]
- Bozzetto, L.; De Natale, C.; Di Capua, L.; Della Corte, G.; Patti, L.; Maione, S.; Riccardi, G.; Rivellese, A.A.; Annuzzi, G. The association of hs-CRP with fasting and postprandial plasma lipids in patients with type 2 diabetes is disrupted by dietary monounsaturated fatty acids. Acta Diabetol. 2013, 50, 273–276. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Gu, Y.; Gardener, S.L.; Doecke, J.D.; Villemagne, V.L.; Brown, B.M.; Taddei, K.; Laws, S.M.; Sohrabi, H.R.; Weinborn, M.; et al. Mediterranean diet adherence and rate of cerebral Abeta-amyloid accumulation: Data from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Transl. Psychiatry 2018, 8, 238. [Google Scholar] [CrossRef]
- Hill, E.; Szoeke, C.; Dennerstein, L.; Campbell, S.; Clifton, P. Adherence to the Mediterranean Diet Is not Related to Beta-Amyloid Deposition: Data from the Women’s Healthy Ageing Project. J. Prev. Alzheimer’s Dis. 2018, 5, 137–141. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Lopez-Legarrea, P.; Fuller, N.R.; Zulet, M.A.; Martinez, J.A.; Caterson, I.D. The influence of Mediterranean, carbohydrate and high protein diets on gut microbiota composition in the treatment of obesity and associated inflammatory state. Asia Pac. J. Clin. Nutr. 2014, 23, 360–368. [Google Scholar] [CrossRef]
- Broom, G.M.; Shaw, I.C.; Rucklidge, J.J. The ketogenic diet as a potential treatment and prevention strategy for Alzheimer’s disease. Nutrition 2019, 60, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.W.; Rothman, T.L.; Behar, K.L.; Stein, D.T.; Hetherington, H.P. Human brain beta-hydroxybutyrate and lactate increase in fasting-induced ketosis. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2000, 20, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Ding, X.Q.; Maudsley, A.A.; Schweiger, U.; Schmitz, B.; Lichtinghagen, R.; Bleich, S.; Lanfermann, H.; Kahl, K.G. Effects of a 72 h fasting on brain metabolism in healthy women studied in vivo with magnetic resonance spectroscopic imaging. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2018, 38, 469–478. [Google Scholar] [CrossRef]
- Gano, L.B.; Patel, M.; Rho, J.M. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 2014, 55, 2211–2228. [Google Scholar] [CrossRef]
- Wheless, J.W. History of the ketogenic diet. Epilepsia 2008, 49 (Suppl. S8), 3–5. [Google Scholar] [CrossRef]
- Hohn, S.; Dozieres-Puyravel, B.; Auvin, S. History of dietary treatment from Wilder’s hypothesis to the first open studies in the 1920s. Epilepsy Behav. 2019, 101, 106588. [Google Scholar] [CrossRef]
- Huttenlocher, P.R.; Wilbourn, A.J.; Signore, J.M. Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 1971, 21, 1097–1103. [Google Scholar] [CrossRef]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 2009, 50, 1109–1117. [Google Scholar] [CrossRef]
- Pinto, A.; Bonucci, A.; Maggi, E.; Corsi, M.; Businaro, R. Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer’s Disease. Antioxidants 2018, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Stafstrom, C.E. Dietary approaches to epilepsy treatment: Old and new options on the menu. Epilepsy Curr. 2004, 4, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; Cervenka, M.C.; Henry, B.J.; Haney, C.A.; Turner, Z. A decade of the modified Atkins diet (2003-2013): Results, insights, and future directions. Epilepsy Behav. 2013, 29, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Milder, J.B.; Liang, L.P.; Patel, M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol. Dis. 2010, 40, 238–244. [Google Scholar] [CrossRef]
- Augustin, K.; Khabbush, A.; Williams, S.; Eaton, S.; Orford, M.; Cross, J.H.; Heales, S.J.R.; Walker, M.C.; Williams, R.S.B. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018, 17, 84–93. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Dangelo, K.; Couch, S.C.; Benoit, S.C.; Clegg, D.J. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol. Aging 2012, 33, 425.e19–425.e27. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Courchesne-Loyer, A.; St-Pierre, V.; Vandenberghe, C.; Pierotti, T.; Fortier, M.; Croteau, E.; Castellano, C.A. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2016, 1367, 12–20. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Courchesne-Loyer, A.; Vandenberghe, C.; St-Pierre, V.; Fortier, M.; Hennebelle, M.; Croteau, E.; Bocti, C.; Fulop, T.; Castellano, C.A. Can Ketones Help Rescue Brain Fuel Supply in Later Life? Implications for Cognitive Health during Aging and the Treatment of Alzheimer’s Disease. Front. Mol. Neurosci. 2016, 9, 53. [Google Scholar] [CrossRef]
- Farasat, S.; Kossoff, E.H.; Pillas, D.J.; Rubenstein, J.E.; Vining, E.P.; Freeman, J.M. The importance of parental expectations of cognitive improvement for their children with epilepsy prior to starting the ketogenic diet. Epilepsy Behav. 2006, 8, 406–410. [Google Scholar] [CrossRef]
- Thompson, L.; Fecske, E.; Salim, M.; Hall, A. Use of the ketogenic diet in the neonatal intensive care unit-Safety and tolerability. Epilepsia 2017, 58, e36–e39. [Google Scholar] [CrossRef]
- Hernandez, A.R.; Hernandez, C.M.; Campos, K.; Truckenbrod, L.; Federico, Q.; Moon, B.; McQuail, J.A.; Maurer, A.P.; Bizon, J.L.; Burke, S.N. A Ketogenic Diet Improves Cognition and Has Biochemical Effects in Prefrontal Cortex That Are Dissociable From Hippocampus. Front. Aging Neurosci. 2018, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017, 26, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, I.; Wera, S.; Van Leuven, F.; Henderson, S.T. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr. Metab. 2005, 2, 28. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Bergman, C.; Lee, J.H.; Wan, R.; King, M.T.; Mughal, M.R.; Okun, E.; Clarke, K.; Mattson, M.P.; Veech, R.L. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.X.; Maalouf, M.; Han, P.; Zhao, M.; Gao, M.; Dharshaun, T.; Ryan, C.; Whitelegge, J.; Wu, J.; Eisenberg, D.; et al. Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol. Aging 2016, 39, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Brownlow, M.L.; Benner, L.; D’Agostino, D.; Gordon, M.N.; Morgan, D. Ketogenic diet improves motor performance but not cognition in two mouse models of Alzheimer’s pathology. PLoS ONE 2013, 8, e75713. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Stafstrom, C.E.; Fu, D.D.; Hu, Y.; Holmes, G.L. Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatric Res. 2004, 55, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Likhodii, S.S. Claims to identify detrimental effects of the ketogenic diet (KD) on cognitive function in rats. Pediatric Res. 2004, 56, 663–664. [Google Scholar] [CrossRef]
- Reger, M.A.; Henderson, S.T.; Hale, C.; Cholerton, B.; Baker, L.D.; Watson, G.S.; Hyde, K.; Chapman, D.; Craft, S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 2004, 25, 311–314. [Google Scholar] [CrossRef]
- Henderson, S.T.; Vogel, J.L.; Barr, L.J.; Garvin, F.; Jones, J.J.; Costantini, L.C. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab. 2009, 6, 31. [Google Scholar] [CrossRef]
- Newport, M.T.; VanItallie, T.B.; Kashiwaya, Y.; King, M.T.; Veech, R.L. A new way to produce hyperketonemia: Use of ketone ester in a case of Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2015, 11, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Morrill, S.J.; Gibas, K.J. Ketogenic diet rescues cognition in ApoE4+ patient with mild Alzheimer’s disease: A case study. Diabetes Metab. Syndr. 2019, 13, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Matsuo, J.; Ishida, I.; Takano, H.; Yokoi, Y.; Hori, H.; Yoshida, S.; Ashida, K.; Nakamura, K.; Takahashi, T.; et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci. Lett. 2019, 690, 232–236. [Google Scholar] [CrossRef]
- Dahlgren, K.; Gibas, K.J. Ketogenic diet, high intensity interval training (HIIT) and memory training in the treatment of mild cognitive impairment: A case study. Diabetes Metab. Syndr. 2018, 12, 819–822. [Google Scholar] [CrossRef]
- Taylor, M.K.; Sullivan, D.K.; Mahnken, J.D.; Burns, J.M.; Swerdlow, R.H. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimer’s Dement. 2018, 4, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Neth, B.J.; Mintz, A.; Whitlow, C.; Jung, Y.; Solingapuram Sai, K.; Register, T.C.; Kellar, D.; Lockhart, S.N.; Hoscheidt, S.; Maldjian, J.; et al. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer’s disease: A pilot study. Neurobiol. Aging 2020, 86, 54–63. [Google Scholar] [CrossRef]
- Iacovides, S.; Goble, D.; Paterson, B.; Meiring, R.M. Three consecutive weeks of nutritional ketosis has no effect on cognitive function, sleep, and mood compared with a high-carbohydrate, low-fat diet in healthy individuals: A randomized, crossover, controlled trial. Am. J. Clin. Nutr. 2019, 110, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.L.; Chance, B.; Kashiwaya, Y.; Lardy, H.A.; Cahill, G.F., Jr. Ketone bodies, potential therapeutic uses. IUBMB Life 2001, 51, 241–247. [Google Scholar] [CrossRef]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Verdile, G.; Keane, K.N.; Cruzat, V.F.; Medic, S.; Sabale, M.; Rowles, J.; Wijesekara, N.; Martins, R.N.; Fraser, P.E.; Newsholme, P. Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer’s Disease. Mediat. Inflamm. 2015, 2015, 105828. [Google Scholar] [CrossRef]
- Achanta, L.B.; Rae, C.D. beta-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem. Res. 2017, 42, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.G.; Rippy, N.A.; Dorenbos, K.; Concepcion, R.C.; Agarwal, A.K.; Rho, J.M. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann. Neurol. 2004, 55, 576–580. [Google Scholar] [CrossRef]
- Jeong, E.A.; Jeon, B.T.; Shin, H.J.; Kim, N.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Ketogenic diet-induced peroxisome proliferator-activated receptor-gamma activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp. Neurol. 2011, 232, 195–202. [Google Scholar] [CrossRef]
- Ziegler, D.R.; Ribeiro, L.C.; Hagenn, M.; Siqueira, I.R.; Araujo, E.; Torres, I.L.; Gottfried, C.; Netto, C.A.; Goncalves, C.A. Ketogenic diet increases glutathione peroxidase activity in rat hippocampus. Neurochem. Res. 2003, 28, 1793–1797. [Google Scholar] [CrossRef]
- Bough, K.J.; Wetherington, J.; Hassel, B.; Pare, J.F.; Gawryluk, J.W.; Greene, J.G.; Shaw, R.; Smith, Y.; Geiger, J.D.; Dingledine, R.J. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006, 60, 223–235. [Google Scholar] [CrossRef]
- Versele, R.; Corsi, M.; Fuso, A.; Sevin, E.; Businaro, R.; Gosselet, F.; Fenart, L.; Candela, P. Ketone Bodies Promote Amyloid-beta1-40 Clearance in a Human in Vitro Blood-Brain Barrier Model. Int. J. Mol. Sci. 2020, 21, 934. [Google Scholar] [CrossRef]
- Van Gaal, L.; Vansant, G.; Van Acker, K.; De Leeuw, I. Effect of a long term very low calorie diet on glucose/insulin metabolism in obesity. Influence of fat distribution on hepatic insulin extraction. Int. J. Obes. 1989, 13 (Suppl. S2), 47–49. [Google Scholar]
- Talbot, K.; Wang, H.Y.; Kazi, H.; Han, L.Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef]
- Chiu, S.L.; Chen, C.M.; Cline, H.T. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 2008, 58, 708–719. [Google Scholar] [CrossRef]
- Piroli, G.G.; Grillo, C.A.; Reznikov, L.R.; Adams, S.; McEwen, B.S.; Charron, M.J.; Reagan, L.P. Corticosterone impairs insulin-stimulated translocation of GLUT4 in the rat hippocampus. Neuroendocrinology 2007, 85, 71–80. [Google Scholar] [CrossRef]
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, ketogenic diet and food intake control: A complex relationship. Front. Psychol. 2015, 6, 27. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Fourrier, C.; Kropp, C.; Aubert, A.; Sauvant, J.; Vaysse, C.; Chardigny, J.M.; Laye, S.; Joffre, C.; Castanon, N. Rapeseed oil fortified with micronutrients improves cognitive alterations associated with metabolic syndrome. Brain Behav. Immun. 2020, 84, 23–35. [Google Scholar] [CrossRef]
- Huskisson, E.; Maggini, S.; Ruf, M. The influence of micronutrients on cognitive function and performance. J. Int. Med Res. 2007, 35, 1–19. [Google Scholar] [CrossRef]
- Scott, T.M.; Peter, I.; Tucker, K.L.; Arsenault, L.; Bergethon, P.; Bhadelia, R.; Buell, J.; Collins, L.; Dashe, J.F.; Griffith, J.; et al. The Nutrition, Aging, and Memory in Elders (NAME) study: Design and methods for a study of micronutrients and cognitive function in a homebound elderly population. Int. J. Geriatr. Psychiatry 2006, 21, 519–528. [Google Scholar] [CrossRef]
- Otsuka, M. Prevention of Alzheimer’s Disease and Nutrients. Brain Nerve 2016, 68, 809–817. [Google Scholar] [CrossRef]
- George, D.R.; Dangour, A.D.; Smith, L.; Ruddick, J.; Vellas, B.; Whitehouse, P.J. The role of nutrients in the prevention and treatment of Alzheimer’s disease: Methodology for a systematic review. Eur. J. Neurol. 2009, 16 (Suppl. S1), 8–11. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Barbagallo, M. Nutritional prevention of cognitive decline and dementia. Acta Biomed. Atenei Parm. 2018, 89, 276–290. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).