Fluoxetine Mimics the Anorectic Action of Estrogen and Its Regulation of Circadian Feeding in Ovariectomized Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Surgical Procedure
2.2. Experimental Design and Protocol
2.3. Immunohistochemistry
2.4. Statistics
3. Results
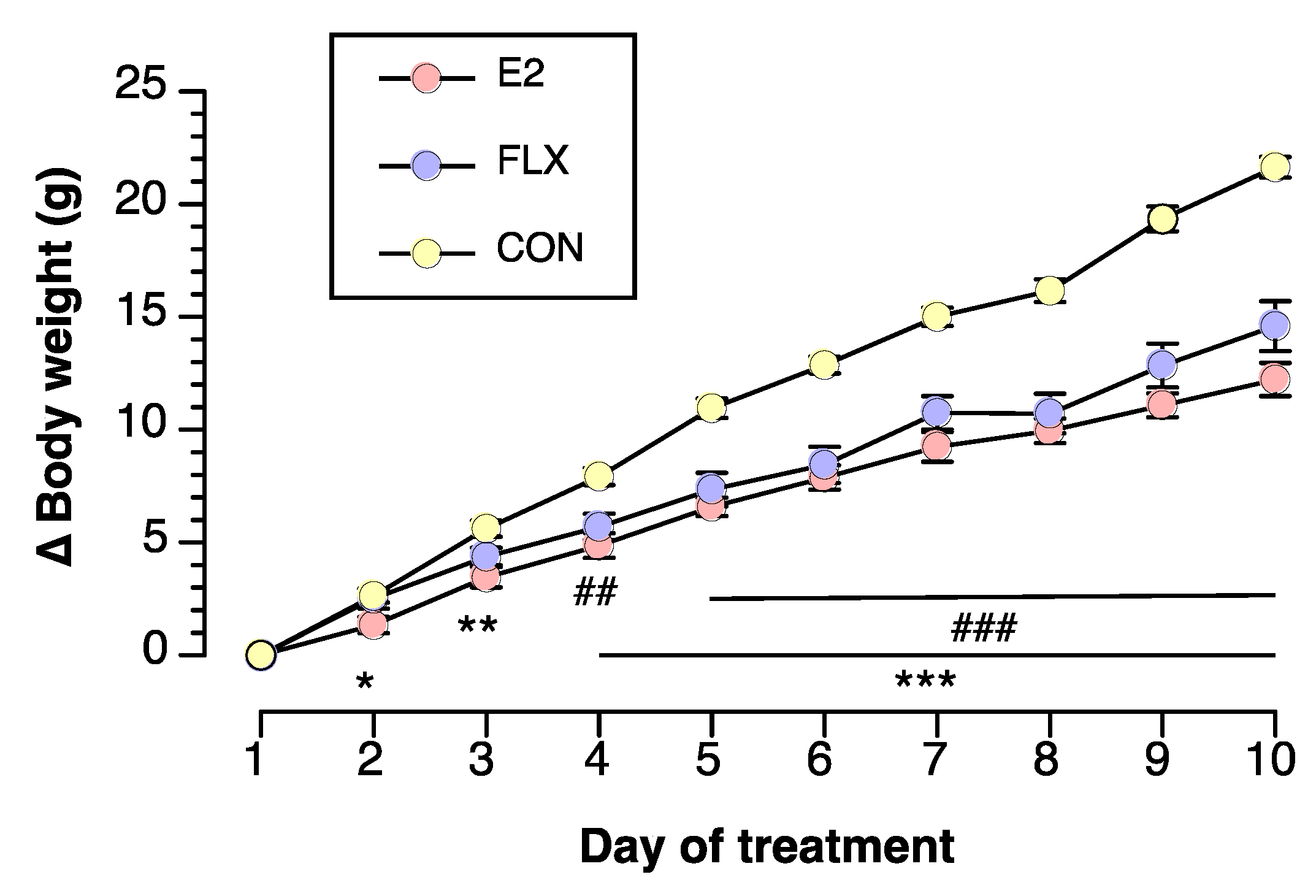
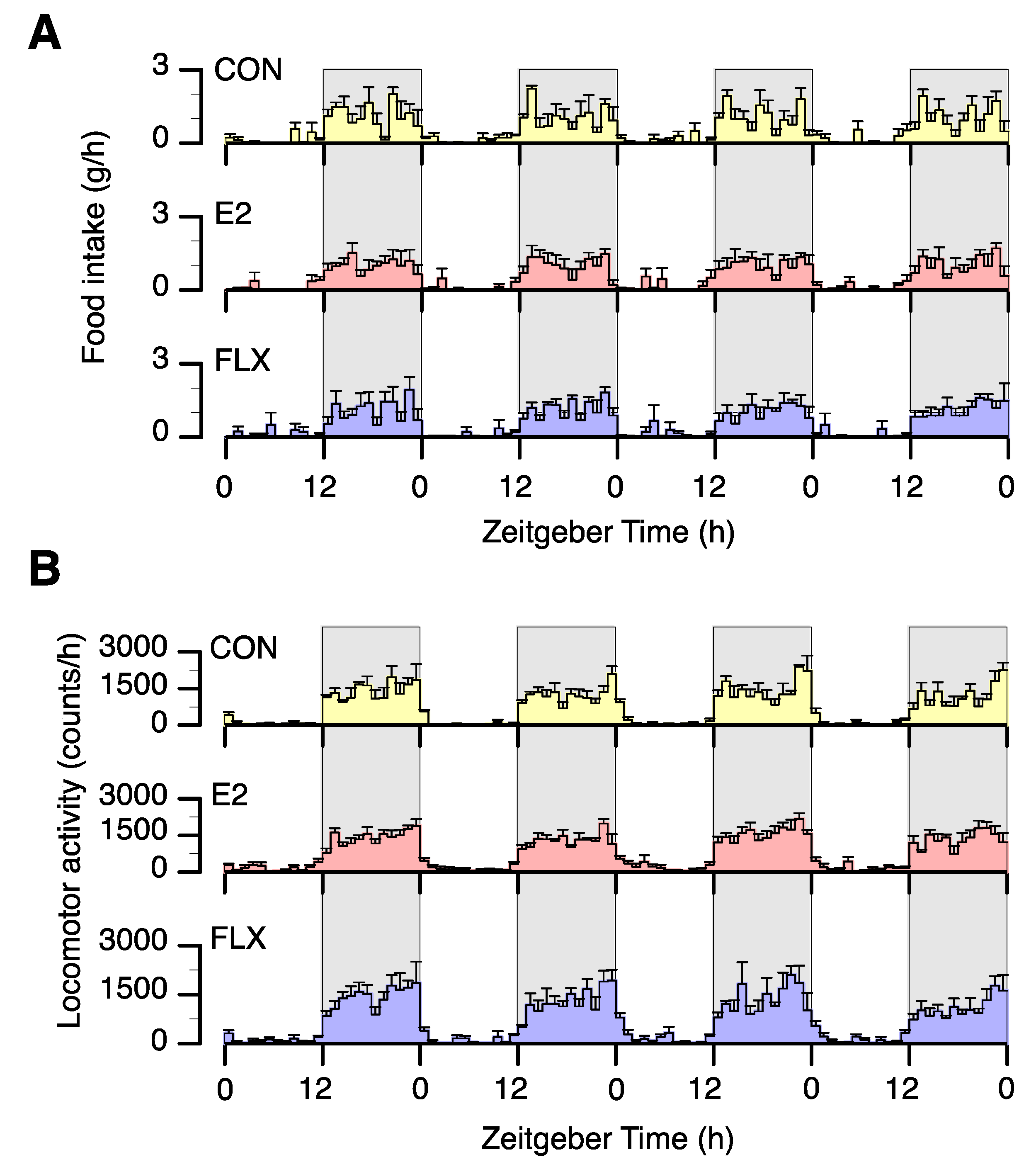
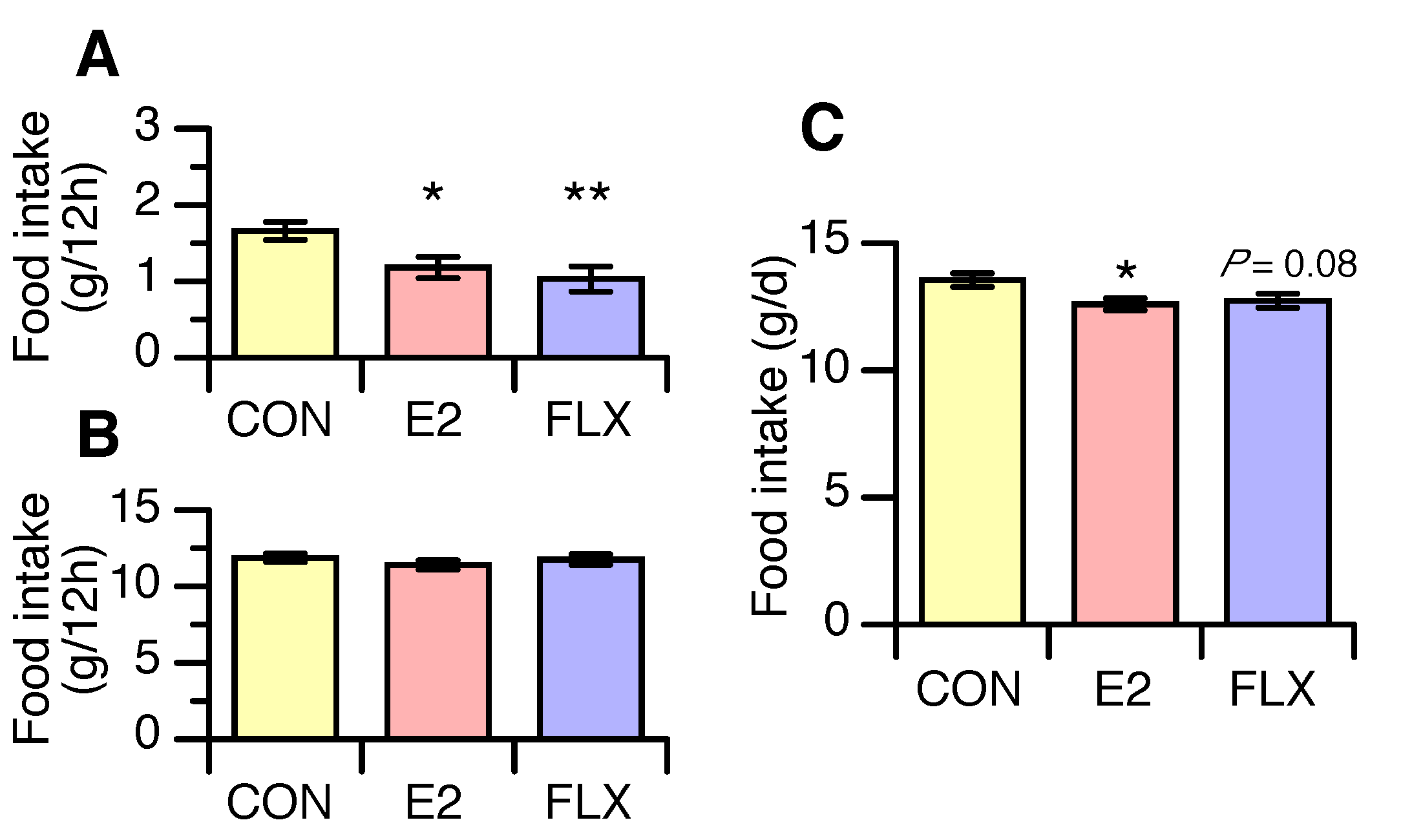
3.1. Effects of E2 and FLX on Body Weight and Feeding Behavior
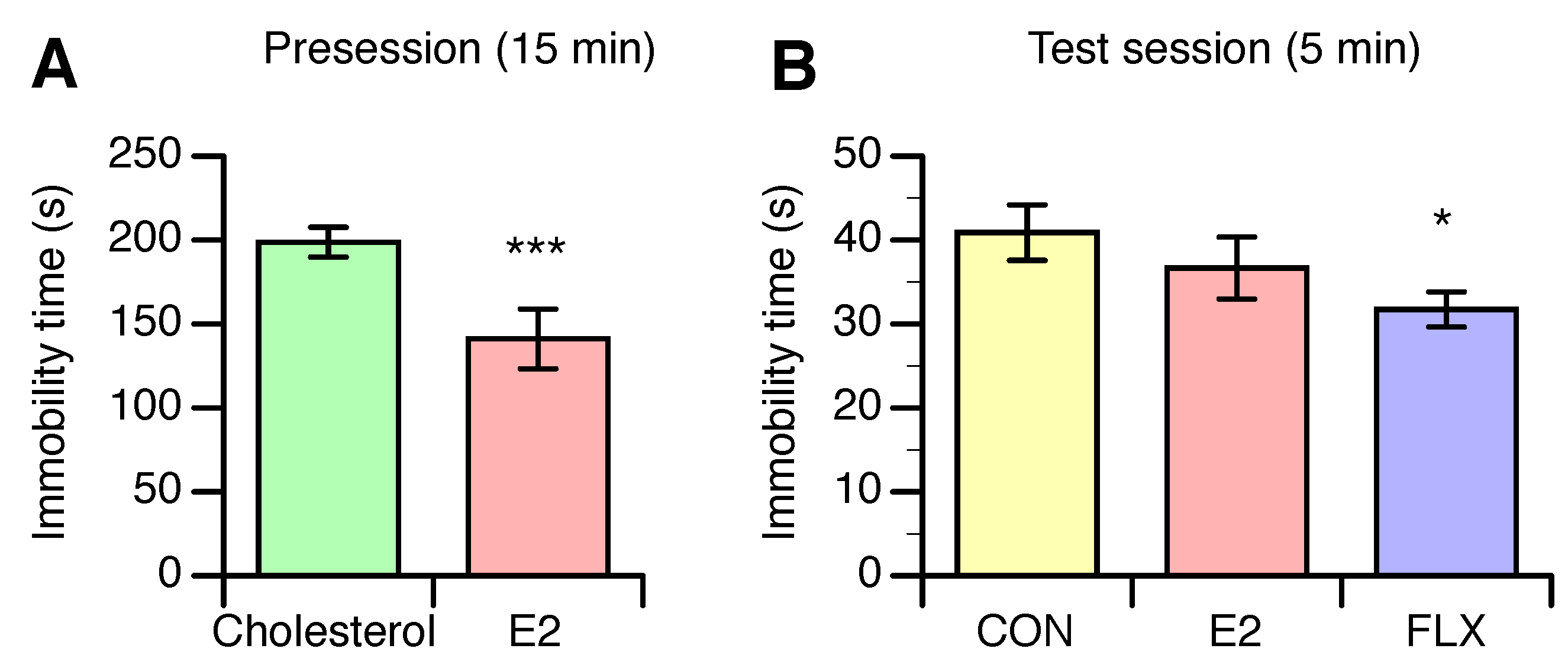
3.2. Effects of E2 and FLX on Depression-Like Behavior
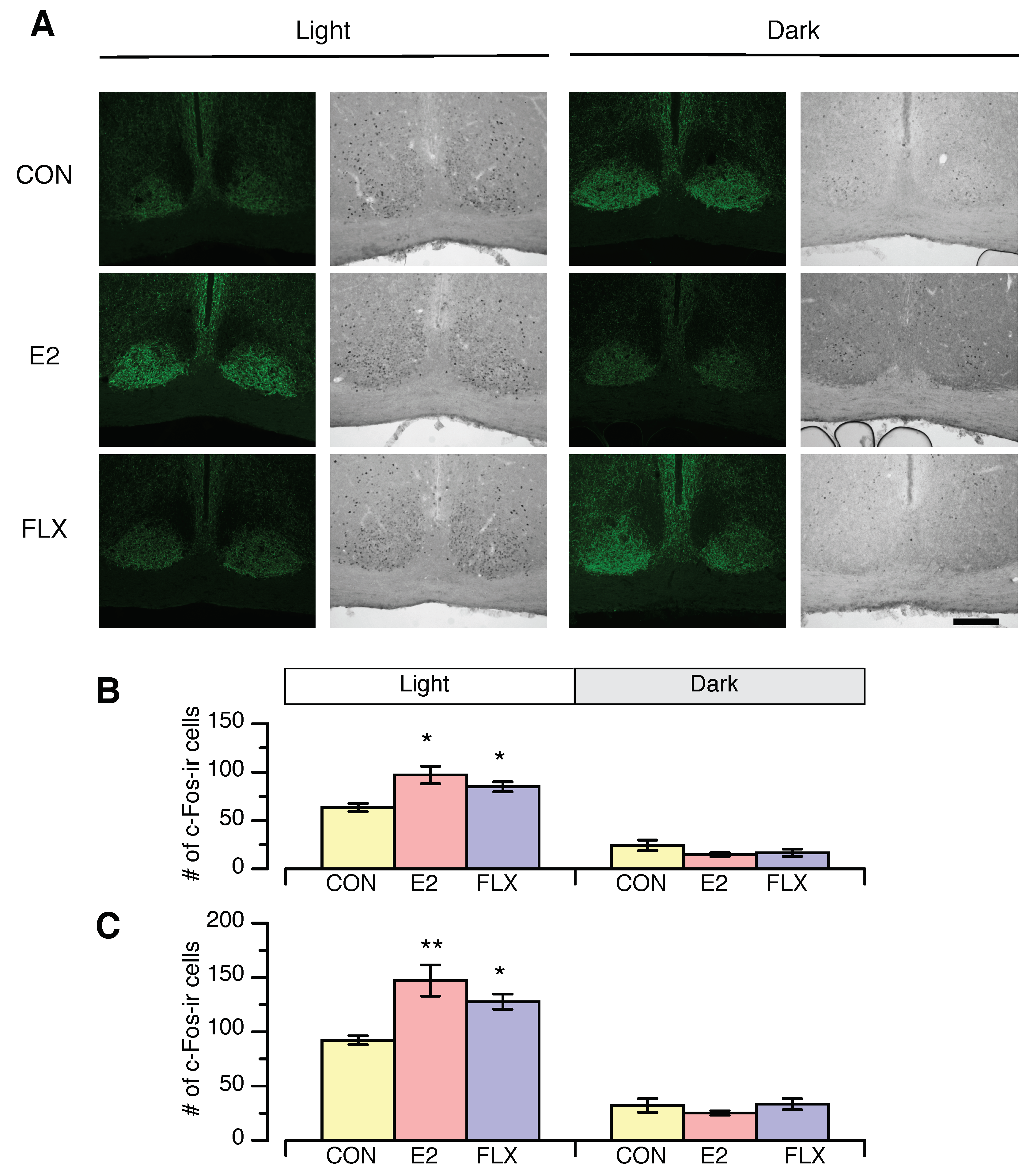
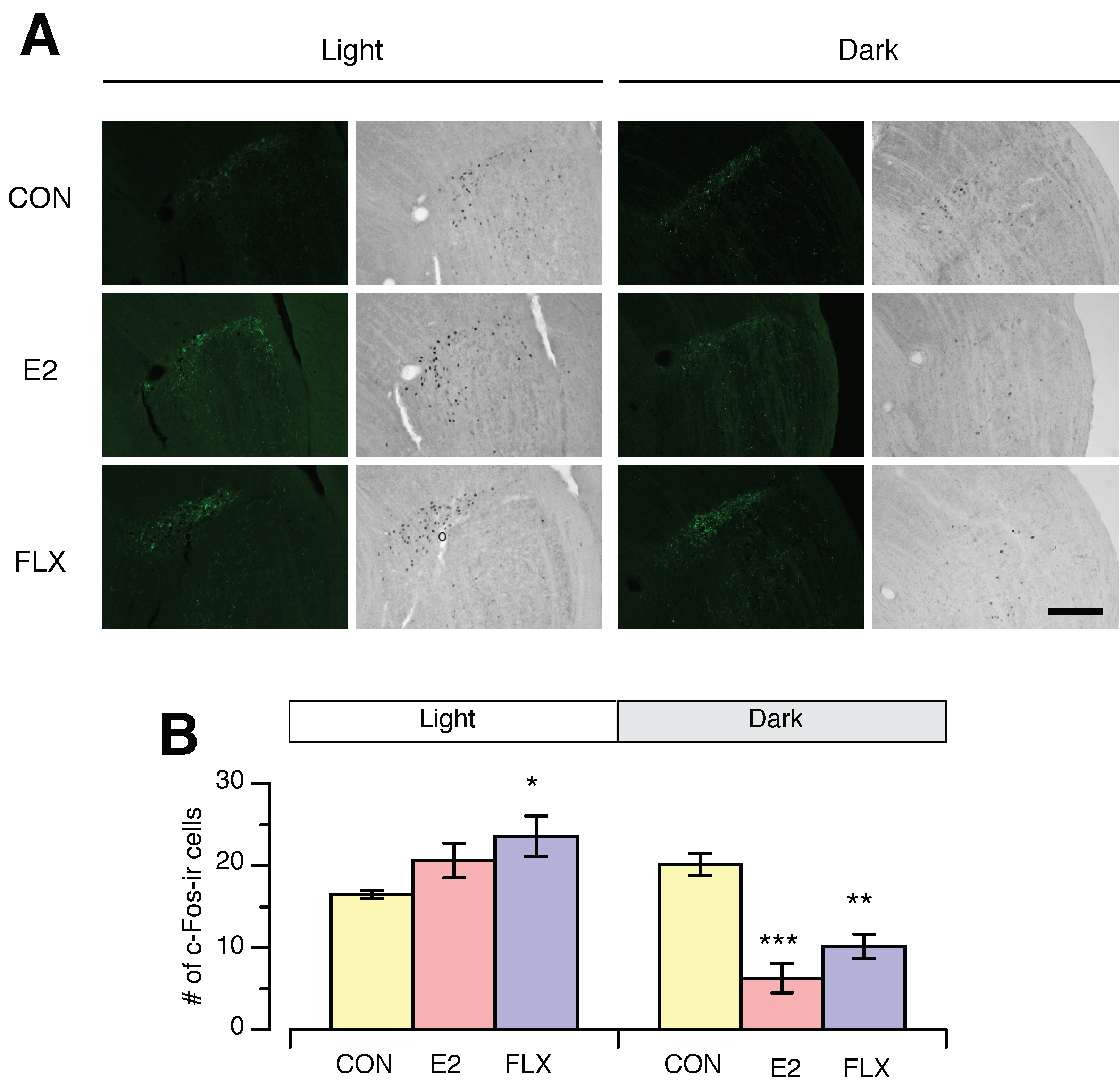
3.3. Effects of E2 and FLX on c-Fos Expression in the SCN and IGL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brown, L.; Clegg, D.J. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid Biochem. Mol. Boil. 2009, 122, 65–73. [Google Scholar] [CrossRef]
- Lobo, R.A. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008, 60, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Rosner, B.A.; Monson, R.R.; Speizer, F.E.; Hennekens, C.H. A Prospective Study of Obesity and Risk of Coronary Heart Disease in Women. N. Engl. J. Med. 1990, 322, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Poehlman, E.T. Menopause, energy expenditure, and body composition. Acta Obstet. Gynecol. Scand. 2002, 81, 603–611. [Google Scholar] [CrossRef]
- Silvestri, R.; Aricò, I.; Bonanni, E.; Bonsignore, M.; Caretto, M.; Caruso, D.; Di Perri, M.; Galletta, S.; Lecca, R.; Lombardi, C.; et al. Italian Association of Sleep Medicine (AIMS) position statement and guideline on the treatment of menopausal sleep disorders. Maturitas 2019, 129, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Tena-Sempere, M. Estrogens and the control of energy homeostasis: A brain perspective. Trends Endocrinol. Metab. 2015, 26, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nedungadi, T.P.; Zhu, L.; Sobhani, N.; Irani, B.G.; Davis, K.E.; Zhang, X.; Zou, F.; Gent, L.M.; Hahner, L.D.; et al. Distinct Hypothalamic Neurons Mediate Estrogenic Effects on Energy Homeostasis and Reproduction. Cell Metab. 2011, 14, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.; Garfield, A.S.; Marston, O.J.; Shaw, J.; Heisler, L. Brain serotonin system in the coordination of food intake and body weight. Pharmacol. Biochem. Behav. 2010, 97, 84–91. [Google Scholar] [CrossRef]
- Carter, W.P.; Hudson, J.I.; Lalonde, J.K.; Pindyck, L.; McElroy, S.L.; Pope, H.G., Jr. Pharmacologic treatment of binge eating disorder. Int. J. Eat. Disord. 2003, 34, S74–S88. [Google Scholar] [CrossRef]
- Clifton, P.; Barnfield, A.M.C.; Philcox, L. A behavioural profile of fluoxetine-induced anorexia. Psychopharmacology 1989, 97, 89–95. [Google Scholar] [CrossRef]
- Garfield, A.S.; Heisler, L. Pharmacological targeting of the serotonergic system for the treatment of obesity. J. Physiol. 2008, 587, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, I.; Schulz, C.; Jarry, H.; Lehnert, H. The effects of the selective serotonin reuptake-inhibitor fluvoxamine on body weight in Zucker rats are mediated by corticotropin-releasing hormone. Int. J. Obes. 2001, 25, 1566–1569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Estradacamarena, E.; López-Rubalcava, C.; Fernández-Guasti, A. Facilitating antidepressant-like actions of estrogens are mediated by 5-HT1A and estrogen receptors in the rat forced swimming test. Psychoneuroendocrinology 2006, 31, 905–914. [Google Scholar] [CrossRef]
- Sheng, Z.; Kawano, J.; Yanai, A.; Fujinaga, R.; Tanaka, M.; Watanabe, Y.; Shinoda, K. Expression of estrogen receptors (α, β) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci. Res. 2004, 49, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Donner, N.C.; Handa, R.J. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience 2009, 163, 705–718. [Google Scholar] [CrossRef]
- Bertrand, P.; Paranavitane, U.T.; Chavez, C.; Gogos, A.; Jones, M.; Buuse, M.V.D. The effect of low estrogen state on serotonin transporter function in mouse hippocampus: A behavioral and electrochemical study. Brain Res. 2005, 1064, 10–20. [Google Scholar] [CrossRef]
- Biegon, A.; McEwen, B. Modulation by estradiol of serotonin receptors in brain. J. Neurosci. 1982, 2, 199–205. [Google Scholar] [CrossRef]
- Cyr, M.; Landry, M.; Di Paolo, T. Modulation by Estrogen-Receptor Directed Drugs of 5-Hydroxytryptamine-2A Receptors in Rat Brain. Neuropsychopharmacology 2000, 23, 69–78. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Johnston, J.D.; Ordovás, J.M.; Scheer, F.A.J.L.; Turek, F.W. Circadian Rhythms, Metabolism, and Chrononutrition in Rodents and Humans123. Adv. Nutr. 2016, 7, 399–406. [Google Scholar] [CrossRef]
- Sasaki, T. Neural and Molecular Mechanisms Involved in Controlling the Quality of Feeding Behavior: Diet Selection and Feeding Patterns. Nutrients 2017, 9, 1151. [Google Scholar] [CrossRef] [PubMed]
- Coomans, C.; Berg, S.V.D.; Lucassen, E.A.; Houben, T.; Pronk, A.C.; Van Der Spek, R.; Kalsbeek, A.; Biermasz, N.R.; Van Dijk, K.W.; Romijn, J.A.; et al. The Suprachiasmatic Nucleus Controls Circadian Energy Metabolism and Hepatic Insulin Sensitivity. Diabetes 2013, 62, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Mabuchi, K.; Takano, A.; Hara, Y.; Negishi, H.; Morimoto, K.; Ueno, T.; Uchiyama, S.; Takamata, A. S-equol Exerts Estradiol-Like Anorectic Action with Minimal Stimulation of Estrogen Receptor-α in Ovariectomized Rats. Front. Endocrinol. 2017, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Takamata, A.; Torii, K.; Miyake, K.; Morimoto, K. Chronic oestrogen replacement in ovariectomised rats attenuates food intake and augments c-Fos expression in the suprachiasmatic nucleus specifically during the light phase. Br. J. Nutr. 2011, 106, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, R.; Serlie, M.; Kalsbeek, A.; La Fleur, S. Serotonin, a possible intermediate between disturbed circadian rhythms and metabolic disease. Neuroscience 2015, 301, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Abizaid, A.; Mezei, G.; Thanarajasingam, G.; Horvath, T.L. Estrogen enhances light-induced activation of dorsal raphe serotonergic neurons. Eur. J. Neurosci. 2005, 21, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Golombek, D.A.; Rosenstein, R.E. Physiology of Circadian Entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Nishimura, Y.; Mabuchi, K.; Taguchi, S.; Ikeda, S.; Aida, E.; Negishi, H.; Takamata, A. Involvement of orexin-A neurons but not melanin-concentrating hormone neurons in the short-term regulation of food intake in rats. J. Physiol. Sci. 2014, 64, 203–211. [Google Scholar] [CrossRef]
- Abizaid, A.; Mezei, G.; Horvath, T.L. Estradiol enhances light-induced expression of transcription factors in the SCN. Brain Res. 2004, 1010, 35–44. [Google Scholar] [CrossRef]
- Cao, X.; Xu, P.; Oyola, M.G.; Xia, Y.; Yan, X.; Saito, K.; Zou, F.; Wang, C.; Yang, Y.; Hinton, A.; et al. Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice. J. Clin. Investig. 2014, 124, 4351–4362. [Google Scholar] [CrossRef] [PubMed]
- Eckel, L.A.; Rivera, H.M.; Atchley, D.P.D. The anorectic effect of fenfluramine is influenced by sex and stage of the estrous cycle in rats. Am. J. Physiol. Integr. Comp. Physiol. 2005, 288, R1486–R1491. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, M.; Debonnel, G. Oestrogen and Testosterone Modulate the Firing Activity of Dorsal Raphe Nucleus Serotonergic Neurones in Both Male and Female Rats. J. Neuroendocr. 2005, 17, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cui, G.; Jin, B.; Wang, K.; Chen, X.; Sun, Y.; Qin, L.; Bai, W. Estradiol Valerate and Remifemin ameliorate ovariectomy-induced decrease in a serotonin dorsal raphe–preoptic hypothalamus pathway in rats. Ann. Anat. Anat. Anz. 2016, 208, 31–39. [Google Scholar] [CrossRef]
- Pop, A.; Lupu, D.I.; Cherfan, J.; Kiss, B.; Loghin, F. Estrogenic/antiestrogenic activity of selected selective serotonin reuptake inhibitors. Clujul Med 2015, 88, 381–385. [Google Scholar] [CrossRef]
- Cuesta, M.; Clesse, D.; Pévet, P.; Challet, E. New light on the serotonergic paradox in the rat circadian system. J. Neurochem. 2009, 110, 231–243. [Google Scholar] [CrossRef]
- Meyer-Bernstein, E.; Morin, L. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J. Neurosci. 1996, 16, 2097–2111. [Google Scholar] [CrossRef]
- Tabuchi, S.; Tsunematsu, T.; Kilduff, T.S.; Sugio, S.; Xu, M.; Tanaka, K.F.; Takahashi, S.; Tominaga, M.; Yamanaka, A. Influence of Inhibitory Serotonergic Inputs to Orexin/Hypocretin Neurons on the Diurnal Rhythm of Sleep and Wakefulness. Sleep 2013, 36, 1391–1404. [Google Scholar] [CrossRef]
- Igarashi, A.; Omura, N.; Miura, M.; Mima, N.; Nishimura, Y.; Mabuchi, K.; Takamata, A. Effect of Estradiol Replacement on Diurnal Sleep/Wake Pattern in Ovariectomized Rats Measured with a Subcutaneously Implanted Acceleration Sensor. FASEB J. 2015, 29, 676. [Google Scholar]
- Glass, J.D.; Guinn, J.; Kaur, G.; Francl, J.M. On the intrinsic regulation of neuropeptide Y release in the mammalian suprachiasmatic nucleus circadian clock. Eur. J. Neurosci. 2010, 31, 1117–1126. [Google Scholar] [CrossRef]
- Smith, V.M.; Jeffers, R.T.; Antle, M.C. Serotonergic enhancement of circadian responses to light: Role of the raphe and intergeniculate leaflet. Eur. J. Neurosci. 2015, 42, 2805–2817. [Google Scholar] [CrossRef] [PubMed]
- Horvath, T.L.; Diano, S.; Sakamoto, H.; Shughrue, P.J.; Merchenthaler, I. Estrogen receptor beta and progesterone receptor mRNA in the intergeniculate leaflet of the female rat. Brain Res. 1999, 844, 196–200. [Google Scholar] [CrossRef]
- Cai, W.; Rambaud, J.; Teboul, M.; Masse, I.; Benoit, G.; Gustafsson, J.-Å.; Delaunay, F.; Laudet, V.; Pongratz, I. Expression Levels of Estrogen Receptor β Are Modulated by Components of the Molecular Clock. Mol. Cell. Boil. 2007, 28, 784–793. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, Y.; Mabuchi, K.; Omura, N.; Igarashi, A.; Miura, M.; Mima, N.; Negishi, H.; Morimoto, K.; Takamata, A. Fluoxetine Mimics the Anorectic Action of Estrogen and Its Regulation of Circadian Feeding in Ovariectomized Female Rats. Nutrients 2020, 12, 849. https://doi.org/10.3390/nu12030849
Nishimura Y, Mabuchi K, Omura N, Igarashi A, Miura M, Mima N, Negishi H, Morimoto K, Takamata A. Fluoxetine Mimics the Anorectic Action of Estrogen and Its Regulation of Circadian Feeding in Ovariectomized Female Rats. Nutrients. 2020; 12(3):849. https://doi.org/10.3390/nu12030849
Chicago/Turabian StyleNishimura, Yuri, Kaori Mabuchi, Natsumi Omura, Ayako Igarashi, Megumi Miura, Nanako Mima, Hiroko Negishi, Keiko Morimoto, and Akira Takamata. 2020. "Fluoxetine Mimics the Anorectic Action of Estrogen and Its Regulation of Circadian Feeding in Ovariectomized Female Rats" Nutrients 12, no. 3: 849. https://doi.org/10.3390/nu12030849
APA StyleNishimura, Y., Mabuchi, K., Omura, N., Igarashi, A., Miura, M., Mima, N., Negishi, H., Morimoto, K., & Takamata, A. (2020). Fluoxetine Mimics the Anorectic Action of Estrogen and Its Regulation of Circadian Feeding in Ovariectomized Female Rats. Nutrients, 12(3), 849. https://doi.org/10.3390/nu12030849




