Sustained Cerebrovascular and Cognitive Benefits of Resveratrol in Postmenopausal Women
Abstract
1. Background
2. Subjects and Methods
2.1. Study Design
2.2. Study Population
2.3. Screening Visit and Follow-Up Assessments
2.4. Investigational Product and Allocation
2.5. Outcome Assessments
2.5.1. Clinic Blood Pressure and Arterial Compliance
2.5.2. Cerebrovascular Function Assessments with Transcranial Doppler Ultrasound
Basal Cerebral Haemodynamics
Cerebrovascular Responsiveness (CVR)
2.5.3. Cognitive Performance
2.5.4. Blood Biomarker Assessment
2.6. Intervention
2.7. Statistical Analysis
3. Results
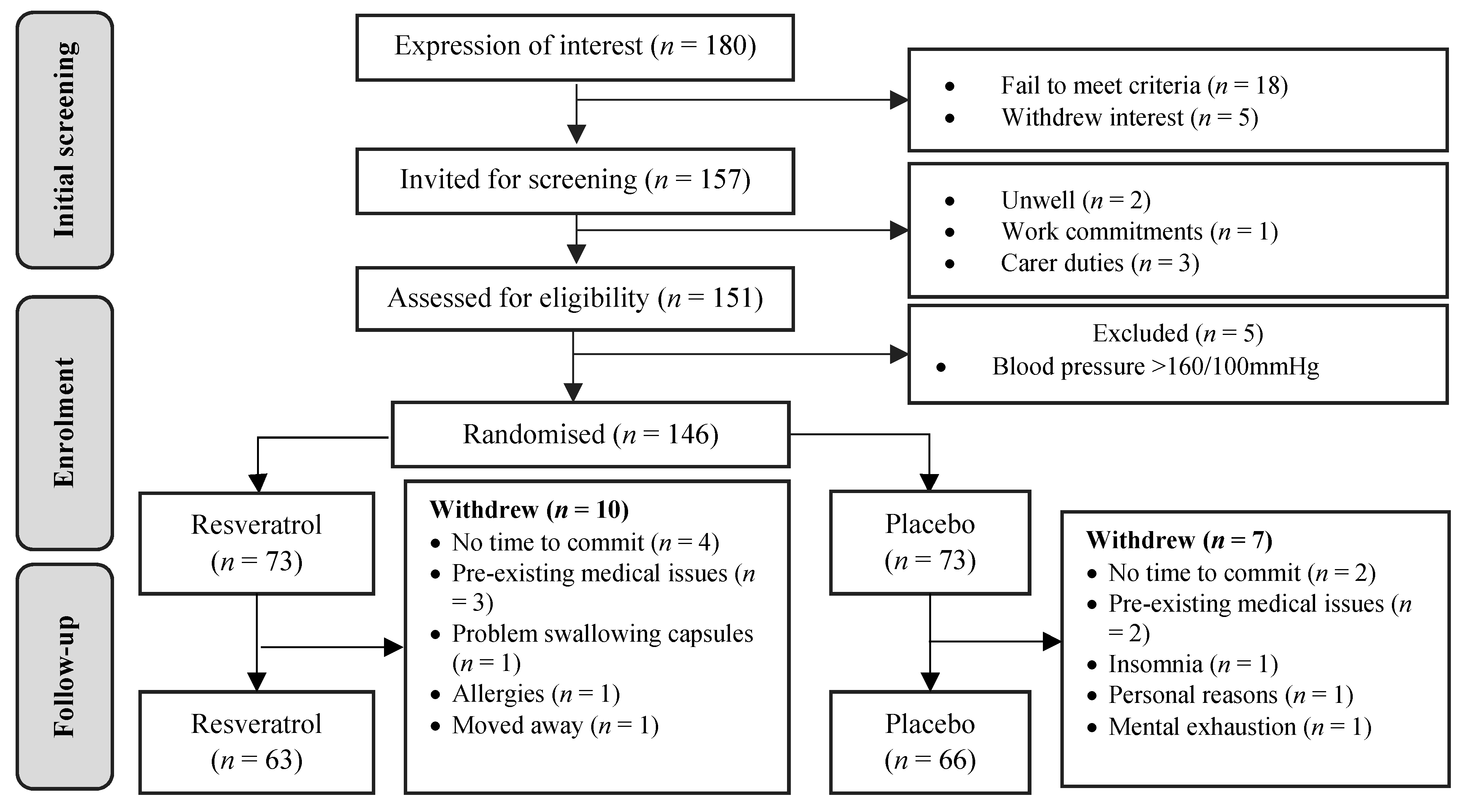
3.1. Participant Disposition
3.2. Baseline Characteristics
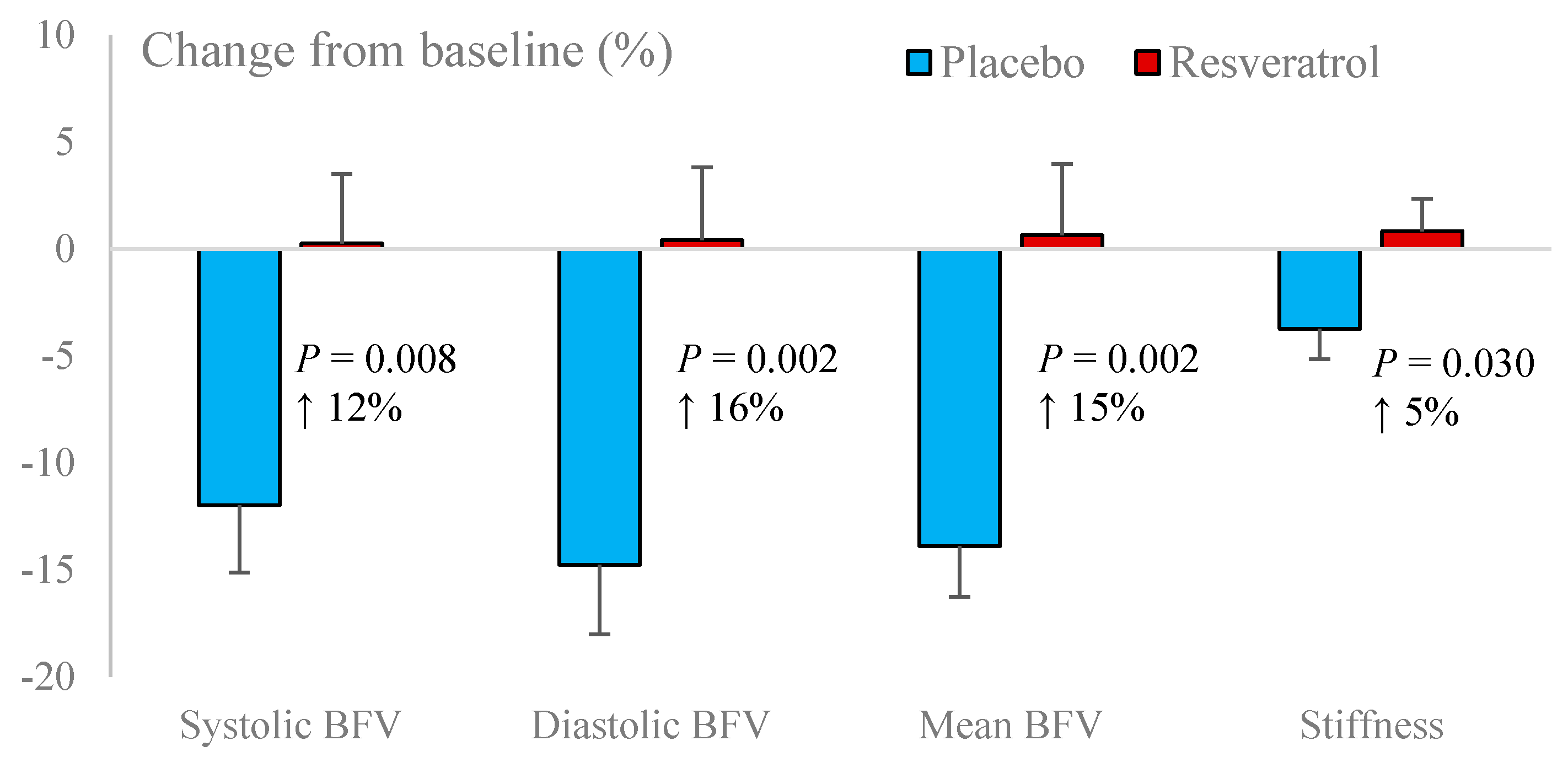
3.3. Systemic Vascular Function
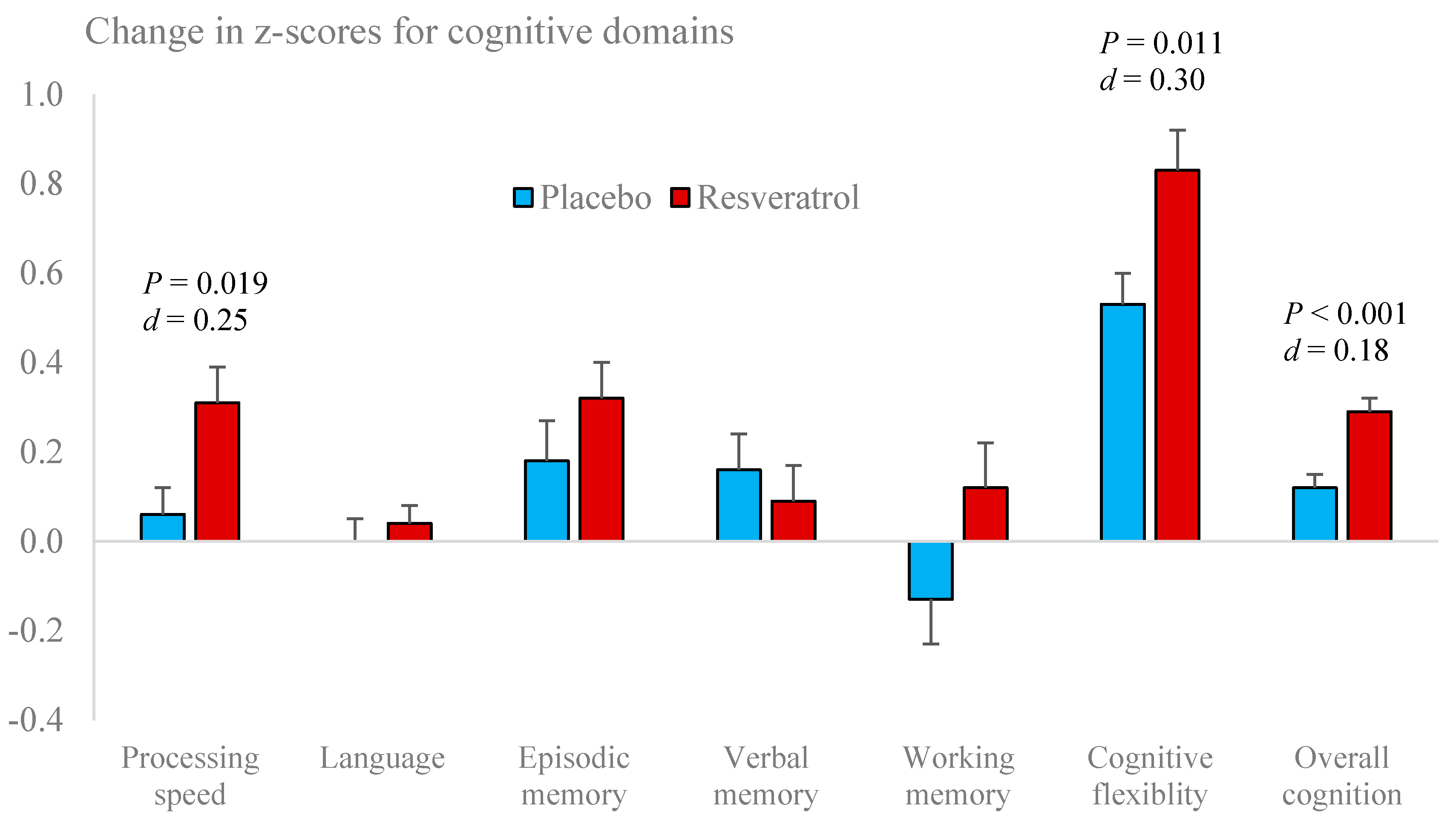
3.4. Cognitive Performance
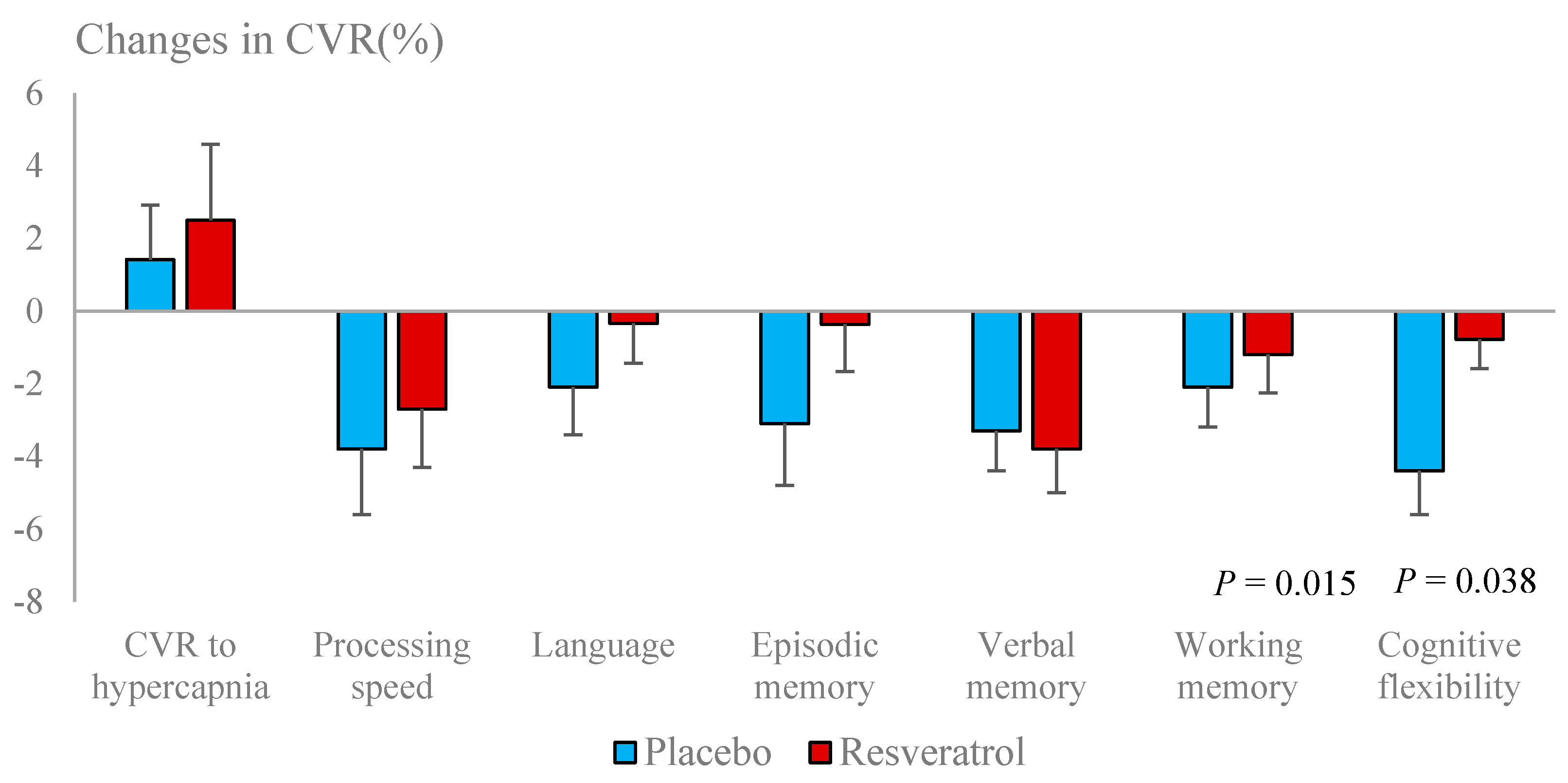
3.5. Cerebrovascular Function
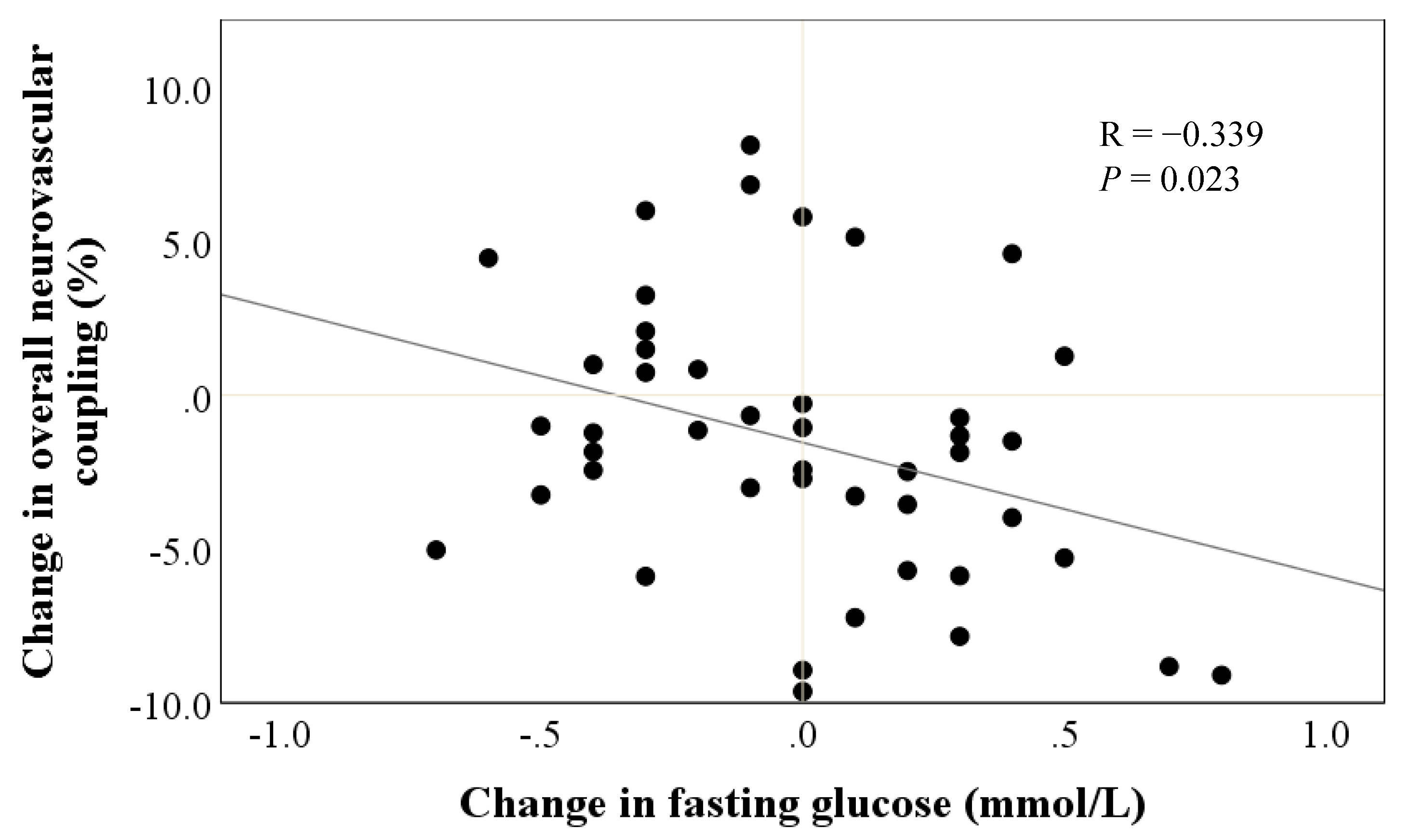
3.6. Cardiometabolic Markers
3.7. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- Colditz, G.A.; Willett, W.C.; Stampfer, M.J.; Rosner, B.; Speizer, F.E.; Hennekens, C.H. Menopause and the risk of coronary heart disease in women. N. Engl. J. Med. 1987, 316, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Singh, M. Sex differences in cognitive impairment and Alzheimer’s disease. Front. Neuroendocrinol. 2014, 35, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Mattei, P.; Sudano, I.; Bernini, G.; Pinto, S.; Salvetti, A. Menopause is associated with endothelial dysfunction in women. Hypertension 1996, 28, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Matteis, M.; Troisi, E.; Monaldo, B.C.; Caltagirone, C.; Silvestrini, M. Age and sex differences in cerebral hemodynamics: A transcranial Doppler study. Stroke 1998, 29, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Veves, A.; Caballaro, A.E.; Smakowski, P.; LoGerfo, F.W. Estrogen improves endothelial function. J. Vasc. Surg. 1998, 27, 1141–1147. [Google Scholar] [CrossRef]
- Silvestrini, M.; Paolino, I.; Vernieri, F.; Pedone, C.; Baruffaldi, R.; Gobbi, B.; Cagnetti, C.; Provinciali, L.; Bartolini, M. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology 2009, 72, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Evans, H.M.; Howe, P.R. Poor cerebrovascular function is an early marker of cognitive decline in healthy postmenopausal women. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2016, 2, 162–168. [Google Scholar] [CrossRef]
- Weber, M.T.; Maki, P.M.; McDermott, M.P. Cognition and mood in perimenopause: A systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol. 2014, 142, 90–98. [Google Scholar] [CrossRef]
- Wong, R.; Howe, P.; Buckley, J.; Coates, A.; Kunz, I.; Berry, N. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 851–856. [Google Scholar] [CrossRef]
- Xia, N.; Förstermann, U.; Li, H. Resveratrol and endothelial nitric oxide. Molecules 2014, 19, 16102–16121. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Flöel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Wong, R.; Nealon, R.; Scholey, A.; Howe, P. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 393–399. [Google Scholar] [CrossRef]
- Wong, R.; Raederstorff, D.; Howe, P. Acute resveratrol consumption improves neurovascular coupling capacity in adults with type 2 diabetes mellitus. Nutrients 2016, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.; Howe, P.; Wong, R. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef]
- Hsieh, S.; Schubert, S.; Hoon, C.; Mioshi, E.; Hodges, J.R. Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2013, 36, 242–250. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Treatment allocation by minimisation. BMJ 2005, 330, 843. [Google Scholar] [CrossRef]
- Wagshul, M.E.; Eide, P.K.; Madsen, J.R. The pulsating brain: A review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS 2011, 8, 5. [Google Scholar] [CrossRef]
- Gershon, R.C.; Wagster, M.V.; Hendrie, H.C.; Fox, N.A.; Cook, K.F.; Nowinski, C.J. NIH toolbox for assessment of neurological and behavioral function. Neurology 2013, 80, S2–S6. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gotzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ (Clin. Res. Ed) 2010, 340, c332. [Google Scholar] [CrossRef]
- Harada, C.N.; Love, M.C.N.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T. Consequences of age-related cognitive declines. Annu. Rev. Psychol. 2012, 63, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Reay, J.L.; Williamson, G.; Dew, T.; Zhang, W.; Kennedy, D.O. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 2015, 114, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Ruitenberg, A.; den Heijer, T.; Bakker, S.L.; van Swieten, J.C.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Cerebral hypoperfusion and clinical onset of dementia: The Rotterdam Study. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2005, 57, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Poels, M.M.; Ikram, M.A.; Vernooij, M.W.; Krestin, G.P.; Hofman, A.; Messen, W.J.; Van der Lugt, A.; Breteler, M.M. Total cerebral blood flow in relation to cognitive function: The Rotterdam Scan Study. J. Cereb. Blood Flow Metab. 2008, 28, 1652–1655. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.; Reyhan, T.; Ramli, Y.; Prihartono, J.; Kurniawan, M. Middle cerebral artery pulsatility index as predictor of cognitive impairment in hypertensive patients. Front. Neurol. 2018, 9, 538. [Google Scholar] [CrossRef]
- Chung, C.-P.; Lee, H.-Y.; Lin, P.-C.; Wang, P.-N. Cerebral artery pulsatility is associated with cognitive impairment and predicts dementia in individuals with subjective memory decline or mild cognitive impairment. J. Alzheimer’s Dis. 2017, 60, 625–632. [Google Scholar] [CrossRef]
- Trudeau, F.; Gagnon, S.; Massicotte, G. Hippocampal synaptic plasticity and glutamate receptor regulation: Influences of diabetes mellitus. Eur. J. Pharm. 2004, 490, 177–186. [Google Scholar] [CrossRef]
- Gröschel, K.; Terborg, C.; Schnaudigel, S.; Ringer, T.; Riecker, A.; Witte, O.W.; Kastrup, A. Effects of physiological aging and cerebrovascular risk factors on the hemodynamic response to brain activation: A functional transcranial Doppler study. Eur. J. Neurol. 2007, 14, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Richiardi, J.; Monsch, A.U.; Haas, T.; Barkhof, F.; Van de Ville, D.; Radü, E.W.; Kressig, R.W.; Haller, S. Altered cerebrovascular reactivity velocity in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2015, 36, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.R.; Muldoon, M.F.; Ryan, C.; Price, J.C.; Greer, P.; Sutton-Tyrrell, K.; Van Der Veen, F.M.; Meltzer, C.C. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology 2005, 64, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.; Stefano, G. Evidence that Alzheimer’s disease is a microvascular disorder: The role of constitutive nitric oxide. Brain Res. Rev. 2000, 34, 119–136. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Berry, N.M.; Coates, A.M.; Buckley, J.D.; Bryan, J.; Kunz, I.; Howe, P.R. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J. Hypertens. 2013, 31, 1819–1827. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Wong, R.H.; Howe, P.R. Resveratrol Counteracts Insulin Resistance—Potential Role of the Circulation. Nutrients 2018, 10, 1160. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. 2017, 14, 60. [Google Scholar] [CrossRef]
- Deng, J.-Y.; Hsieh, P.-S.; Huang, J.-P.; Lu, L.-S.; Hung, L.-M. Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and-independent pathways. Diabetes 2008, 57, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
| Cognitive Domains | NIH-ToolBox Assessment | Other Assessment |
|---|---|---|
| Processing speed | Pattern Comparison Speed Test | Trail Making Task A |
| Language | Picture Vocabulary Test Oral Reading Recognition Test | |
| Working memory | List Sorting Working Memory Test | Forward Spatial Span test |
| Episodic memory | Picture Sequence Memory Test | |
| Verbal memory | Rey’s Auditory Verbal Learning Test (immediate recall and 30-minute delayed recall) | |
| Cognitive flexibility | Dimensional Change Card Sort Test Flanker Inhibitory Control and Attention Test | Trail Making Task B |
| Participant’s Characteristics | Total (n = 146) | Placebo (n = 73) | Resveratrol (n = 73) |
|---|---|---|---|
| Age (years) | 64 ± 1 | 64 ± 1 | 64 ± 1 |
| Years since cessation of menses | 15 ± 1 | 15 ± 1 | 15 ± 1 |
| Years of education | 17 ± 0.3 | 17 ± 0.5 | 17 ± 0.5 |
| ACE-III score (%) | 93 ± 0.5 | 93 ± 0.7 | 93 ± 0.5 |
| BMI (kg/m2) | 25.6 ± 0.3 | 25.8 ± 0.5 | 25.4 ± 0.5 |
| Systolic blood pressure (mmHg) | 124 ± 1 | 125 ± 2 | 123 ± 2 |
| Diastolic blood pressure (mmHg) | 68 ± 1 | 69 ± 1 | 67 ± 1 |
| Large artery compliance (mL/mmHg×10) | 12.6 ± 0.36 | 12.1 ± 0.38 | 13.1 ± 0.67 |
| Small artery compliance (mL/mmHg×100) | 3.7 ± 0.17 | 3.7 ± 0.29 | 3.5 ± 0.18 |
| Month 0 | Month 12 | |||
|---|---|---|---|---|
| Cognitive Domains | Placebo (n = 73) | Resveratrol (n = 73) | Placebo (n = 66) | Resveratrol (n = 63) |
| ● Component Tasks | ||||
| Processing Speed | 0.05 ± 0.09 | −0.08 ± 0.10 | 0.23 ± 0.10 | 0.24 ± 0.09 * |
| ● PCT | 0.08 ± 0.12 | −0.13 ± 0.13 | 0.15 ± 0.13 | 0.32 ± 0.11 * |
| ● TMT A | 0.02 ± 0.13 | −0.02 ± 0.11 | 0.31 ± 0.11 | 0.16 ± 0.11 |
| Language | −0.03 ± 0.10 | 0.03 ± 0.11 | 0.04 ± 0.11 | 0.09 ± 0.11 |
| ● PVT | −0.09 ± 0.12 | 0.09 ± 0.12 | 0.05 ± 0.13 | 0.20 ± 0.13 |
| ● ORR | 0.02 ± 0.12 | −0.02 ± 0.12 | 0.04 ± 0.10 | −0.02 ± 0.11 |
| Episodic Memory | −0.49 ± 0.09 | −0.47 ± 0.10 | −0.27 ± 0.10 | −0.19 ± 0.11 |
| ● PSM | −0.49 ± 0.09 | −0.47 ± 0.10 | −0.27 ± 0.10 | −0.19 ± 0.11 |
| Verbal Memory | 0.001 ± 0.1 | −0.001 ± 0.1 | 0.17 ± 0.12 | 0.12 ± 0.10 |
| ● RAVLT immediate | 0.02 ± 0.07 | −0.02 ± 0.07 | 0.07 ± 0.06 | 0.07 ± 0.06 |
| ● RAVLT delayed | −0.02 ± 0.15 | 0.02 ± 0.15 | 0.28 ± 0.19 | 0.17 ± 0.16 |
| Working Memory | 0.04 ± 0.10 | −0.04 ± 0.10 | 0.06 ± 0.09 | 0.05 ± 0.09 |
| ● LSWM | 0.11 ± 0.11 | −0.11 ± 0.12 | 0.08 ± 0.14 | −0.03 ± 0.13 |
| ● FSS | −0.04 ± 0.12 | 0.04 ± 0.11 | 0.06 ± 0.12 | 0.12 ± 0.10 |
| Cognitive Flexibility | −0.03 ± 0.12 | 0.03 ± 0.08 | 0.78 ± 0.08 | 0.82 ± 0.07 * |
| ● DCCS | −0.05 ± 0.14 | 0.05 ± 0.09 | 0.10 ± 0.13 | 0.23 ± 0.10 |
| ● FICA | −0.08 ± 0.12 | 0.08 ± 0.11 | 0.22 ± 0.11 | 0.26 ± 0.12 |
| ● TMT performance | 0.02 ± 0.21 | −0.02 ± 0.19 | 2.01 ± 0.10 | 1.97 ± 0.11 |
| Overall Cognitive Performance | −0.08 ± 0.07 | −0.09 ± 0.05 | 0.17 ± 0.06 | 0.18 ± 0.05 * |
| Cerebral Hemodynamics | Month 0 | Month 12 | ||
|---|---|---|---|---|
| Resting conditions | Placebo (n = 51) | Resveratrol (n = 47) | Placebo (n = 51) | Resveratrol (n = 47) |
| Systolic blood flow velocity (cm/s) | 81.3 ± 2.7 | 76.0 ± 2.9 | 71.8 ± 1.9 | 76.2 ± 3.3 |
| Diastolic blood flow velocity (cm/s) | 36.0 ± 1.4 | 32.7 ± 1.3 | 30.9 ± 0.95 | 32.8 ± 1.4 |
| Mean blood flow velocity (cm/s) | 53.8 ± 1.9 | 49.6 ± 1.9 | 46.6 ± 1.3 | 49.9 ± 2.1 |
| Pulsatility index | 0.84 ± 0.02 | 0.87 ± 0.03 | 0.88 ± 0.02 | 0.86 ± 0.02 |
| Cerebrovascular responses to hypercapnia (%) | Placebo (n = 44) | Resveratrol (n = 41) | Placebo (n = 44) | Resveratrol (n = 41) |
| 45.8 ± 1.7 | 46.4 ± 2.3 | 47.2 ± 2.03 | 48.9 ± 2.05 | |
| Neurovascular coupling capacity (%) | Placebo (n = 45) | Resveratrol (n = 46) | Placebo (n = 45) | Resveratrol (n = 46) |
| Processing speed | 19.2 ± 1.4 | 19.6 ± 1.4 | 15.4 ± 1.3 | 16.9 ± 1.2 |
| Language | 14.0 ± 1.1 | 12.0 ± 0.97 | 11.9 ± 0.95 | 11.6 ± 0.63 |
| Episodic memory | 16.6 ± 1.3 | 14.5 ± 1.2 | 13.5 ± 1.07 | 14.2 ± 1.2 |
| Verbal memory | 15.9 ± 1.2 | 16.2 ± 1.02 | 12.6 ± 0.89 | 12.4 ± 0.85 |
| Working memory | 15.5 ± 1.02 | 14.2 ± 0.94 | 13.4 ± 0.83 | 13.0 ± 0.64 |
| Cognitive flexibility | 16.1 ± 0.94 | 14.3 ± 0.92 | 11.7 ± 0.77 | 13.5 ± 0.74 |
| Overall cognition | 16.4 ± 0.73 | 15.0 ± 0.63 | 12.8 ± 0.69 | 13.5 ± 0.59 |
| Month 0 | Month 12 | Δ Month 12–Month 0 | |||||
|---|---|---|---|---|---|---|---|
| Fasting Serum Biomarkers | Placebo (n = 65) | Resveratrol (n = 59) | Placebo (n = 65) | Resveratrol (n= 59) | Placebo (n = 65) | Resveratrol (n = 59) | P-Value |
| Glucose (mmol/L) | 5.0 ± 0.07 | 5.0 ± 0.07 | 5.0 ± 0.06 | 5.0 ± 0.06 | 0.05 ± 0.06 | −0.00 ± 0.04 | 0.499 |
| Insulin (mIU/L) | 7.2 ± 0.46 | 7.9 ± 0.61 | 8.0 ± 0.42 | 8.1 ± 0.59 | 0.82 ± 0.42 | 0.16 ± 0.50 | 0.308 |
| HOMA-IR | 1.6 ± 0.12 | 1.8 ± 0.16 | 1.8 ± 0.11 | 1.8 ± 0.14 | 0.20 ± 010 | 0.01 ± 0.12 | 0.236 |
| Triglycerides (mmol/L) | 1.1 ± 0.06 | 1.2 ± 0.07 | 1.2 ± 0.06 | 1.3 ± 0.07 | 0.08 ± 0.04 | 0.10 ± 0.05 | 0.800 |
| Total cholesterol (mmol/L) | 5.7 ± 0.11 | 5.6 ± 0.18 | 5.7 ± 0.12 | 5.6 ± 0.18 | −0.03 ± 0.07 | −0.01 ± 0.08 | 0.901 |
| LDL-cholesterol (mmol/L) | 3.7 ± 0.11 | 3.5 ± 0.17 | 3.6 ± 0.11 | 3.5 ± 0.17 | −0.08 ± 0.07 | −0.03 ± 0.06 | 0.561 |
| HDL-cholesterol (mmol/L) | 1.6 ± 0.04 | 1.6 ± 0.05 | 1.6 ± 0.05 | 1.6 ± 0.05 | 0.02 ± 0.02 | −0.03 ± 0.02 | 0.168 |
| Hs-CRP (mg/L) | 2.0 ± 0.24 | 2.4 ± 0.67 | 2.2 ± 0.43 | 2.5 ± 0.34 | 0.22 ± 0.43 | 0.10 ± 0.54 | 0.862 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thaung Zaw, J.J.; Howe, P.R.C.; Wong, R.H.X. Sustained Cerebrovascular and Cognitive Benefits of Resveratrol in Postmenopausal Women. Nutrients 2020, 12, 828. https://doi.org/10.3390/nu12030828
Thaung Zaw JJ, Howe PRC, Wong RHX. Sustained Cerebrovascular and Cognitive Benefits of Resveratrol in Postmenopausal Women. Nutrients. 2020; 12(3):828. https://doi.org/10.3390/nu12030828
Chicago/Turabian StyleThaung Zaw, Jay Jay, Peter R. C. Howe, and Rachel H. X. Wong. 2020. "Sustained Cerebrovascular and Cognitive Benefits of Resveratrol in Postmenopausal Women" Nutrients 12, no. 3: 828. https://doi.org/10.3390/nu12030828
APA StyleThaung Zaw, J. J., Howe, P. R. C., & Wong, R. H. X. (2020). Sustained Cerebrovascular and Cognitive Benefits of Resveratrol in Postmenopausal Women. Nutrients, 12(3), 828. https://doi.org/10.3390/nu12030828






