Feeding the Outer Bran Fraction of Rice Alters Hepatic Carbohydrate Metabolism in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet Preparation
2.2. Animals and Treatments
2.3. Dietary Digestibility
2.4. Determination of Organic acid Content in Cecal Content
2.5. Sample Preparation for Gas Chromatography/Mass Spectrometry Analysis
2.6. GC/MS Analysis
2.7. Data Processing
2.8. RNA Extraction and Quantitative Real-Time PCR
2.9. Statistical Analysis
3. Results
3.1. The Effects of Feeding OBFR on Growth Performance and Feed Digestibility
3.2. The Effects of Feeding OBFR on pH and Organic Acid Concentration of Cecal Contents
3.3. Untargeted GC/MS-Based Metabolomics Analysis in Cecal Contents, Plasma, and Liver
3.4. The Effects of Feeding OBFR on Gene Expressions Encoding Metabolic Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spiller, R.C. Pharmacology of dietary fibre. Pharmacol. Ther. 1994, 62, 407–427. [Google Scholar] [CrossRef]
- Southgate, D.A.T.; Branch, W.J.; Hill, M.J.; Drasar, B.S.; Walters, R.L.; Davies, P.S.; Baird, I.M. Metabolic responses to dietary supplements of bran. Metabolism 1976, 25, 1129–1135. [Google Scholar] [CrossRef]
- Baer, D.J.; Rumpler, W.V.; Miles, C.W.; Fahey, G.C., Jr. Dietary fiber decreases the metabolizable energy content and nutrient digestibility of mixed diets fed to humans. J. Nutr. 1997, 127, 579–586. [Google Scholar] [CrossRef]
- Southgate, D.A.T.; Durnin, J.V.G.A. Calorie conversion factors. An experimental reassessment of the factors used in the calculation of the energy value of human diets. Br. J. Nutr. 1970, 2, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Tenorio, M.D.; Espinosa-Martos, I.; Rupérez, P. Health-promoting effects of a dietary fiber concentrate from the soybean byproduct okara in rats. J. Agric. Food Chem. 2008, 56, 7495–7501. [Google Scholar] [CrossRef] [PubMed]
- Ijiri, D.; Nojima, T.; Kawaguchi, M.; Yamauchi, Y.; Fujita, Y.; Ijiri, S.; Ohtsuka, A. Effects of feeding outer bran fraction of rice on lipid accumulation and fecal excretion in rats. Biosci. Biotechnol. Biochem. 2015, 79, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Mazza, G.; Maguer, M.L. Functional Foods: Biochemical and Processing Aspects; CRC Press: Boca Raton, FL, USA, 1998; pp. 71–72. [Google Scholar]
- Satchithanandam, S.; Klurfeld, D.M.; Calvert, R.J.; Cassidy, M.M. Effects of dietary fibers on gastrointestinal mucin in rats. Nutr. Res. 1996, 16, 1163–1177. [Google Scholar] [CrossRef]
- Topping, D.L.; Illman, R.J.; Roach, P.D.; Trimble, R.P.; Kambouris, A.; Nestel, P.J. Modulation of the hypolipidemic effect of fish oils by dietary fiber in fats: Studies with rice and wheat bran. J. Nutr. 1990, 120, 325–330. [Google Scholar] [CrossRef]
- Chen, C.W.; Cheng, H.H.J. A rice bran oil diet increases LDL-receptor and HMG-CoA reductase mRNA expressions and insulin sensitivity in rats with streptozotocin/nicotinamide-induced type 2 diabetes. J. Nutr. 2006, 136, 1472–1476. [Google Scholar] [CrossRef]
- Tomlin, J.; Read, N.W. Comparison of the effects of colonic function caused by feeding rice bran and wheat bran. Eur. J. Clin. Nutr. 1998, 42, 857–861. [Google Scholar]
- Kestin, M.; Moss, R.; Clifton, P.M.; Nestel, P.J. Comparative effects of three cereal brans on plasma lipids, blood pressure, and glucose metabolism in mildly hypercholesterolemic men. Am. J. Clin. Nutr. 1990, 52, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Rong, N.; Ausman, L.M.; Nicolosi, R.J. Oryzanol decreases cholesterol absorption and aortic fatty streaks in hamsters. Lipids 1997, 32, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Knuckles, B.E. Effect of phytate and other myo-inositol phosphate esters on lipase activity. J. Food Sci. 1998, 53, 250–252. [Google Scholar] [CrossRef]
- Mukherjee, M. Human digestive and metabolic lipases—A brief review. J. Mol. Catal. B Enzym. 2003, 5, 369–376. [Google Scholar] [CrossRef]
- Shibuya, N.; Nakane, R.; Yasui, A.; Tanaka, K.; Iwasaki, T. Comparative Studies on Cell Wall Preparations from Rice Bran, Germ, and Endosperm. Cereal. Chem. 1985, 62, 252–258. [Google Scholar]
- Anderson, J.W.; Jones, A.E.; Riddell-Mason, S. Ten different dietary fibers have significantly different effects on serum and liver lipids of cholesterol-fed rats. J. Nutr. 1994, 124, 78–83. [Google Scholar] [CrossRef]
- Miwa, M.; Hiyama, C.; Yamamoto, M. High-performance liquid chromatography of short-and long-chain fatty acids as 2-nitrophenylhydrazides. J. Chromatogr. A 1985, 321, 165–174. [Google Scholar] [CrossRef]
- Shigematsu, M.; Nakagawa, R.; Tomonaga, S.; Funaba, M.; Matsui, T. Fluctuations in metabolite content in the liver of magnesium-deficient rats. Br. J. Nutr. 2016, 116, 1694–1699. [Google Scholar] [CrossRef]
- Imura, M.; Iwakiri, R.; Bamba, T.; Fukusaki, E. Metabolomics approach to reduce the Crabtree effect in continuous culture of Saccharomyces cerevisiae. J. Biosci. Bioeng. 2018, 126, 183–188. [Google Scholar] [CrossRef]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwar, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, S.; Ijiri, D.; Nakashima, K.; Kawaguchi, M.; Ishimaru, Y.; Furukawa, A.; Ohtsuka, A. Clenbuterol changes phosphorylated FOXO1 localization and decreases protein degradation in the skeletal muscle of neonatal chicks. Biosci. Biotechnol. Biochem. 2016, 80, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 5 September 2019).
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Hoover, W.H.; Heitmann, R.N. Effects of dietary fiber levels on weight gain, cecal volume and volatile fatty acid production in rabbits. J. Nutr. 1972, 102, 375–379. [Google Scholar] [CrossRef]
- Nagasawa, T.; Yoshizawa, F.; Nishizawa, N. Plasma Nτ-methylhistidine concentration is a sensitive index of myofibrillar protein degradation during starvation in rats. Biosci. Biotechnol. Biochem. 1996, 60, 501–502. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Fukushima, M.; Fujii, S.; Yoshimura, Y.; Endo, T.; Nakano, M. Effect of rice bran on intraintestinal fermentation and cholesterol metabolism in cecectomized rats. Nutr. Res. 1999, 19, 235–245. [Google Scholar] [CrossRef]
- Han, F.; Wang, Y.; Han, Y.; Zhao, J.; Han, F.; Song, G.; Jiang, P.; Miao, H. Effects of Whole-Grain Rice and Wheat on Composition of Gut Microbiota and Short-Chain Fatty Acids in Rats. J. Agric. Food Chem. 2018, 66, 6326–6335. [Google Scholar] [CrossRef]
- Dawson, A.M.; Holdsworth, C.D.; Webb, J. Absorption of Short Chain Fatty Acids in Man. Proc. Soc. Exp. Biol. Med. 1964, 117, 97–100. [Google Scholar] [CrossRef]
- Ruppin, H.; Bar-Meir, S.; Soergel, K.H.; Wood, C.M.; Schmitt, M.G., Jr. Absorption of short-chain fatty acids by the colon. Gastroenterology 1980, 78, 1500–1507. [Google Scholar] [CrossRef]
- Rechkemmer, G.; Rönnau, K.; Engelhardt, W.V. Fermentation of polysaccharides and absorption of short chain fatty acids in the mammalian hindgut. Comp. Biochem. Physiol. A 1988, 90, 563–568. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Murase, M.; Kimura, Y.; Nagata, Y. Determination of portal short-chain fatty acids in rats fed various dietary fibers by capillary gas chromatography. J. Chromatogr. B Biomed. Appl. 1995, 664, 415–420. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Masutomi, N.; Tsutsui, N.; Sakairi, T.; Mitchell, M.; Milburn, M.V.; Ryals, J.A.; Beebe, K.D.; Guo, L. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol. Pathol. 2009, 37, 521–535. [Google Scholar] [CrossRef]
- Abbiss, H.; Maker, G.L.; Gummer, J.P.A.; Rawlinson, C.; Musk, G.C.; Fleming, P.A.; Phillips, J.K.; Boyce, M.C.; Trengove, R.D. Untargeted gas chromatography-mass spectrometry-based metabolomics analysis of kidney and liver tissue from the Lewis Polycystic Kidney rat. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1118, 25–32. [Google Scholar] [CrossRef]
- Ghaisas, S.; Langley, M.R.; Palanisamy, B.N.; Dutta, S.; Narayanaswamy, K.; Plummer, P.J.; Sarkar, S.; Ay, M.; Jin, H.; Anantharam, V.; et al. MitoPark transgenic mouse model recapitulates the gastrointestinal dysfunction and gut-microbiome changes of Parkinson’s disease. Neuro Toxicol. 2019, 75, 186–199. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Intararuchikul, T.; Teerapattarakan, N.; Rodsiri, R.; Tantisira, M.; Wohlgemuth, G.; Fiehn, O.; Tansawat, R. Effects of Centella asiatica extract on antioxidant status and liver metabolome of rotenone-treated rats using GC-MS. Biomed. Chromatogr. 2019, 33, e4395. [Google Scholar] [CrossRef]
- Yoon, S.J.; Long, N.P.; Jung, K.H.; Kim, H.M.; Hong, Y.J.; Fang, Z.; Kim, S.J.; Kim, T.J.; Anh, N.H.; Hong, S.S.; et al. Systemic and Local Metabolic Alterations in Sleep-Deprivation-Induced Stress: A Multiplatform Mass-Spectrometry-Based Lipidomics and Metabolomics Approach. J. Proteome Res. 2019, 18, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Shimano, H. SREBPs: Physiology and pathophysiology of the SREBP family. FEBS J. 2009, 276, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.C.; Kien, C.L.; Bouthillier, L.; Levy, E. Short-chain fatty acids: Ready for prime time? Nutr. Clin. Pract. 2006, 21, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef]
- Suryawan, A.; Hawes, J.W.; Harris, R.A.; Shimomura, Y.; Jenkins, A.E.; Hutson, S.M. A molecular model of human branched-chain amino acid metabolism. Am. J. Clin. Nutr. 1998, 68, 72–81. [Google Scholar] [CrossRef]



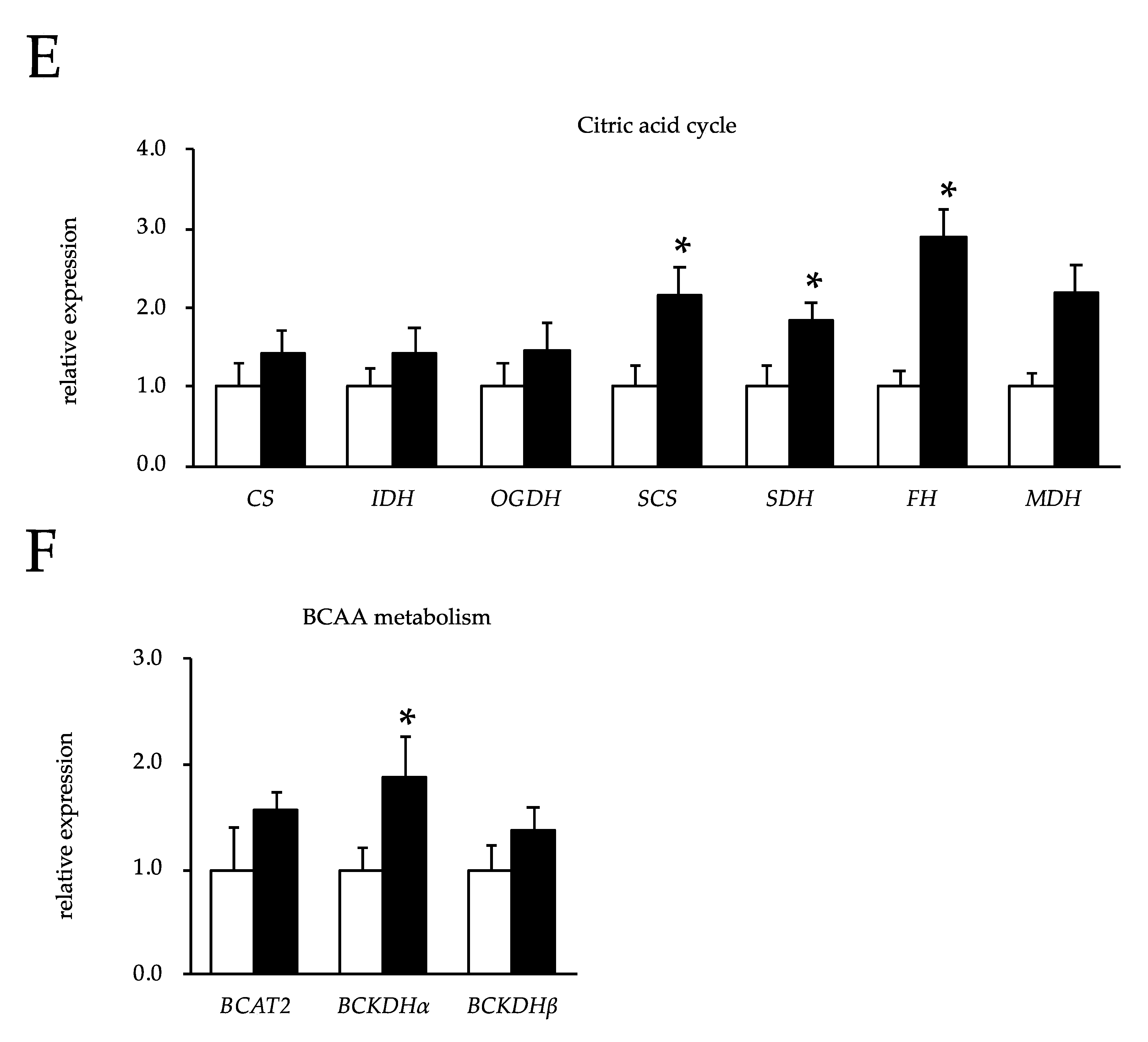
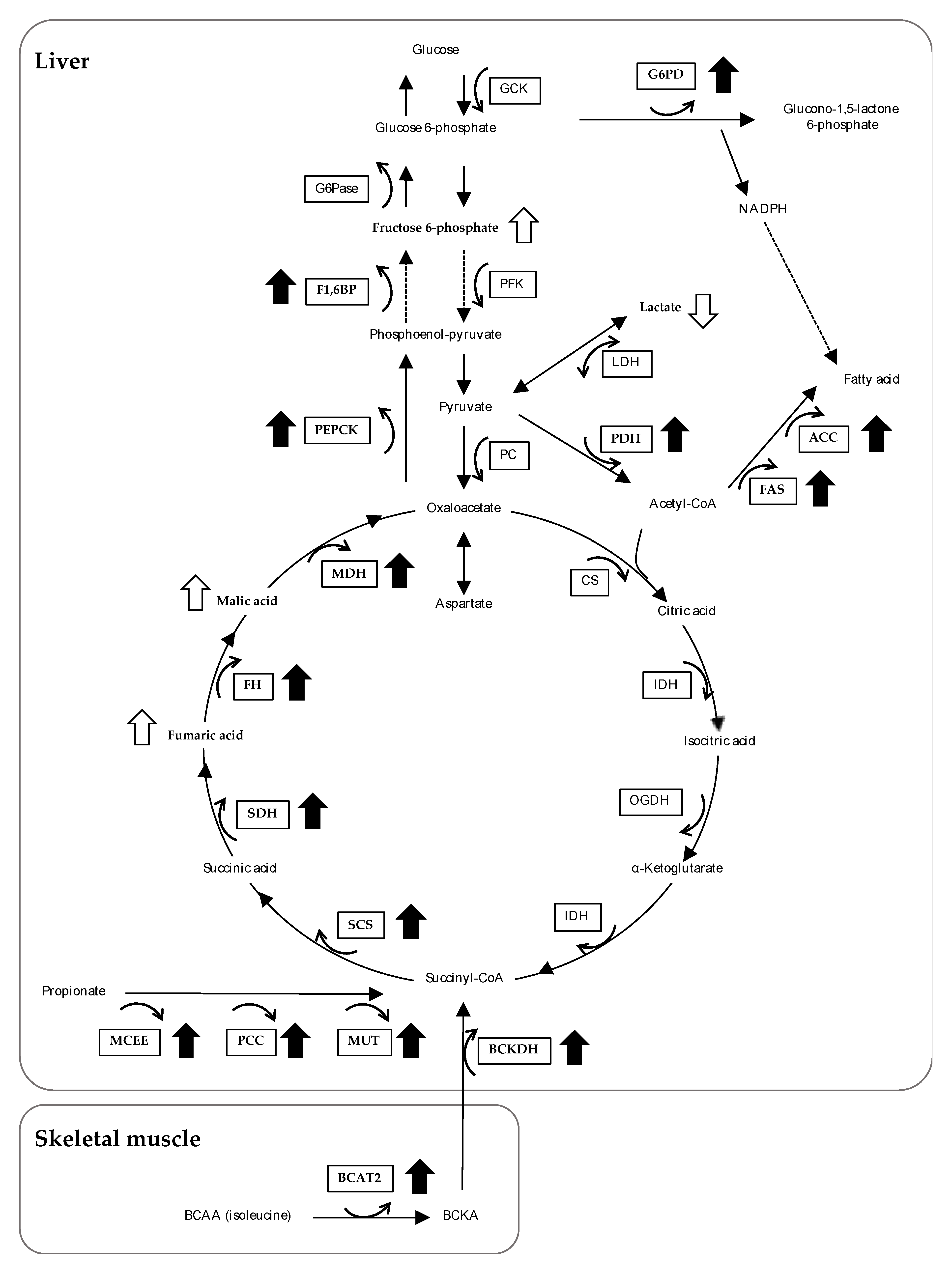
| Control Diet | OBFR 1 Diet | |
|---|---|---|
| Ingredients (g/100 g) | ||
| α-Cornstarch | 52.95 | 50.97 |
| Casein | 20.00 | 19.27 |
| Sucrose | 10.00 | 10.00 |
| Corn oil | 7.00 | 6.09 |
| Cellulose | 5.00 | 3.62 |
| Mineral mix (AIN-93G)2 | 3.50 | 3.50 |
| Vitamin mix (AIN-93G)3 | 1.00 | 1.00 |
| L-Cystine | 0.30 | 0.30 |
| Choline chloride | 0.25 | 0.25 |
| OBFR | 5.00 | |
| Analyzed contents 4 | ||
| Moisture (g/100 g) | 8.44 | 8.89 |
| Crude protein (g/100 g) | 17.38 | 17.20 |
| Ether extract (g/100 g%) | 6.09 | 7.06 |
| Crude ash (g/100 g) | 2.92 | 3.57 |
| Nitrogen free extract (g/100 g) | 63.22 | 60.77 |
| Crude fiber (g/100 g) | 1.95 | 2.52 |
| Nutral detergent fiber (g/100 g) | 6.67 | 6.90 |
| Gross energy (Mcal/kg) | 4.33 | 4.29 |
| Control | OBFR | |||||
|---|---|---|---|---|---|---|
| (n = 7) | (n = 7) | |||||
| Growth performance | ||||||
| Final body weight (g) | 387.30 | ± | 8.61 | 383.32 | ± | 7.51 |
| Body weight gain (g/21 days) | 115.10 | ± | 5.08 | 111.05 | ± | 5.30 |
| Food intake (g/21 days) | 454.78 | ± | 13.75 | 460.28 | ± | 14.48 |
| Food efficiency | 0.25 | ± | 0.01 | 0.24 | ± | 0.01 * |
| Tissue weight | ||||||
| Heart (g/100 g body weight) | 0.28 | ± | 0.01 | 0.28 | ± | 0.01 |
| Liver (g/100 g body weight) | 3.38 | ± | 0.14 | 3.53 | ± | 0.15 |
| Kidney (g/100 g body weight) | 0.62 | ± | 0.02 | 0.63 | ± | 0.02 |
| Abdominal fat (g/100 g body weight) | 5.69 | ± | 0.47 | 5.99 | ± | 0.46 |
| Soleus muscle (g/100 g body weight) | 0.03 | ± | 0.00 | 0.03 | ± | 0.00 |
| Plasma | ||||||
| Glucose (mg/dL) | 99.67 | ± | 1.02 | 87.67 | ± | 7.87 |
| Triacylglycerol (mg /dL) | 185.08 | ± | 22.81 | 198.20 | ± | 22.80 |
| Total cholesterol (mg/dL) | 103.17 | ± | 8.20 | 98.33 | ± | 11.66 |
| 3-Methylhisitidine (nmol/μL) | 5.38 | ± | 0.40 | 4.95 | ± | 0.21 |
| Control | OBFR | |||||
|---|---|---|---|---|---|---|
| (n = 7) | (n = 7) | |||||
| Lactic acid | 0.38 | ± | 0.09 | 1.14 | ± | 0.17 * |
| Acetic acid | 39.75 | ± | 2.01 | 147.67 | ± | 6.97 * |
| Propionic acid | 35.05 | ± | 2.72 | 90.26 | ± | 6.15 * |
| Isobutyric acid | 7.20 | ± | 0.28 | 7.12 | ± | 0.52 |
| Butyric acid | 18.49 | ± | 2.20 | 84.13 | ± | 6.84 * |
| Isovaleric acid | 5.67 | ± | 0.31 | 8.65 | ± | 0.43 |
| Valeric acid | 4.65 | ± | 0.22 | 6.12 | ± | 0.91 |
| Total organic acid | 105.61 | ± | 4.23 | 345.09 | ± | 14.55 * |
| Control | OBFR | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Increased metabolites | |||||||
| Sucrose | 100 | ± | 22 | 166 | ± | 17 | 0.049 |
| 3-Hydroxybenzoic acid | 100 | ± | 20 | 167 | ± | 16 | 0.024 |
| Glyceric acid | 100 | ± | 18 | 169 | ± | 13 | 0.011 |
| Glutaric acid | 100 | ± | 17 | 238 | ± | 36 | 0.006 |
| Mannitol | 100 | ± | 19 | 258 | ± | 50 | 0.015 |
| Sarcosine | 100 | ± | 11 | 267 | ± | 64 | 0.033 |
| Adenine | 100 | ± | 29 | 274 | ± | 59 | 0.024 |
| Hypoxanthine | 100 | ± | 34 | 511 | ± | 173 | 0.048 |
| Isoleucine | 100 | ± | 48 | 549 | ± | 173 | 0.017 |
| 3-Hydroxyphenylacetic acid | 100 | ± | 33 | 737 | ± | 124 | 0.001 |
| Decreased metabolites | |||||||
| Adenosine | 100 | ± | 19 | 10 | ± | 0 | 0.002 |
| Glycolic acid | 100 | ± | 32 | 17 | ± | 3 | 0.042 |
| Inosine | 100 | ± | 23 | 27 | ± | 7 | 0.014 |
| 2-Aminoisobutyric acid | 100 | ± | 18 | 38 | ± | 13 | 0.021 |
| Proline | 100 | ± | 12 | 53 | ± | 11 | 0.020 |
| Valine | 100 | ± | 13 | 55 | ± | 9 | 0.035 |
| 5-Aminovaleric acid | 100 | ± | 10 | 58 | ± | 5 | 0.004 |
| Thymine | 100 | ± | 7 | 59 | ± | 6 | 0.001 |
| Ornithine | 100 | ± | 12 | 64 | ± | 10 | 0.048 |
| Serine | 100 | ± | 9 | 65 | ± | 6 | 0.011 |
| Glycine | 100 | ± | 11 | 65 | ± | 7 | 0.023 |
| Pyroglutamic acid | 100 | ± | 8 | 75 | ± | 6 | 0.030 |
| Control | OBFR | p Value | |||||
|---|---|---|---|---|---|---|---|
| Increased metabolites | |||||||
| Mannose | 100 | ± | 17 | 465 | ± | 59 | 0.006 |
| Arabitol | 100 | ± | 4 | 179 | ± | 22 | 0.009 |
| Psicose | 100 | ± | 7 | 175 | ± | 24 | 0.015 |
| Sorbose | 100 | ± | 38 | 256 | ± | 40 | 0.032 |
| Sorbitol | 100 | ± | 11 | 254 | ± | 65 | 0.047 |
| Inositol | 100 | ± | 16 | 239 | ± | 50 | 0.029 |
| Nonanoic acid | 100 | ± | 22 | 234 | ± | 50 | 0.039 |
| Myristic acid | 100 | ± | 17 | 229 | ± | 49 | 0.039 |
| Palmitic acid | 100 | ± | 13 | 215 | ± | 35 | 0.016 |
| Eicosanoic acid | 100 | ± | 24 | 241 | ± | 55 | 0.047 |
| Decreased metabolites | |||||||
| Succinic acid | 100 | ± | 13 | 25 | ± | 16 | 0.011 |
| β-Alanine | 100 | ± | 13 | 44 | ± | 7 | 0.013 |
| Isoleucine | 100 | ± | 31 | 22 | ± | 7 | 0.040 |
| Control | OBFR | p Value | |||||
|---|---|---|---|---|---|---|---|
| Increased metabolites | |||||||
| Malic acid | 100 | ± | 7 | 134 | ± | 8 | 0.009 |
| Arabinose-5-phosphate | 100 | ± | 15 | 146 | ± | 10 | 0.022 |
| Ascorbic acid | 100 | ± | 41 | 229 | ± | 30 | 0.027 |
| N-Acetyl glucosamine | 100 | ± | 13 | 271 | ± | 55 | 0.027 |
| Uracil | 100 | ± | 31 | 183 | ± | 15 | 0.030 |
| Uridine | 100 | ± | 46 | 201 | ± | 13 | 0.030 |
| Glucono-1,5-lactone | 100 | ± | 35 | 316 | ± | 67 | 0.030 |
| Fumaric acid | 100 | ± | 10 | 135 | ± | 11 | 0.036 |
| Fructose 6-phosphate | 100 | ± | 25 | 216 | ± | 44 | 0.040 |
| Nonanoric acid | 100 | ± | 41 | 245 | ± | 52 | 0.045 |
| Decreased metabolites | |||||||
| Gluconic acid | 100 | ± | 21 | 35 | ± | 4 | 0.010 |
| Lactic acid | 100 | ± | 35 | 15 | ± | 13 | 0.020 |
| Tryptophan | 100 | ± | 8 | 63 | ± | 16 | 0.040 |
| Isoleucine | 100 | ± | 9 | 77 | ± | 9 | 0.040 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawaguchi, M.; Nishikoba, N.; Shimamoto, S.; Tomonaga, S.; Kohrogi, R.; Yamauchi, Y.; Fujita, Y.; Ohtsuka, A.; Ijiri, D. Feeding the Outer Bran Fraction of Rice Alters Hepatic Carbohydrate Metabolism in Rats. Nutrients 2020, 12, 430. https://doi.org/10.3390/nu12020430
Kawaguchi M, Nishikoba N, Shimamoto S, Tomonaga S, Kohrogi R, Yamauchi Y, Fujita Y, Ohtsuka A, Ijiri D. Feeding the Outer Bran Fraction of Rice Alters Hepatic Carbohydrate Metabolism in Rats. Nutrients. 2020; 12(2):430. https://doi.org/10.3390/nu12020430
Chicago/Turabian StyleKawaguchi, Mana, Nao Nishikoba, Saki Shimamoto, Shozo Tomonaga, Rukana Kohrogi, Yoko Yamauchi, Yoshikazu Fujita, Akira Ohtsuka, and Daichi Ijiri. 2020. "Feeding the Outer Bran Fraction of Rice Alters Hepatic Carbohydrate Metabolism in Rats" Nutrients 12, no. 2: 430. https://doi.org/10.3390/nu12020430
APA StyleKawaguchi, M., Nishikoba, N., Shimamoto, S., Tomonaga, S., Kohrogi, R., Yamauchi, Y., Fujita, Y., Ohtsuka, A., & Ijiri, D. (2020). Feeding the Outer Bran Fraction of Rice Alters Hepatic Carbohydrate Metabolism in Rats. Nutrients, 12(2), 430. https://doi.org/10.3390/nu12020430






