The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment
Abstract
1. Introduction
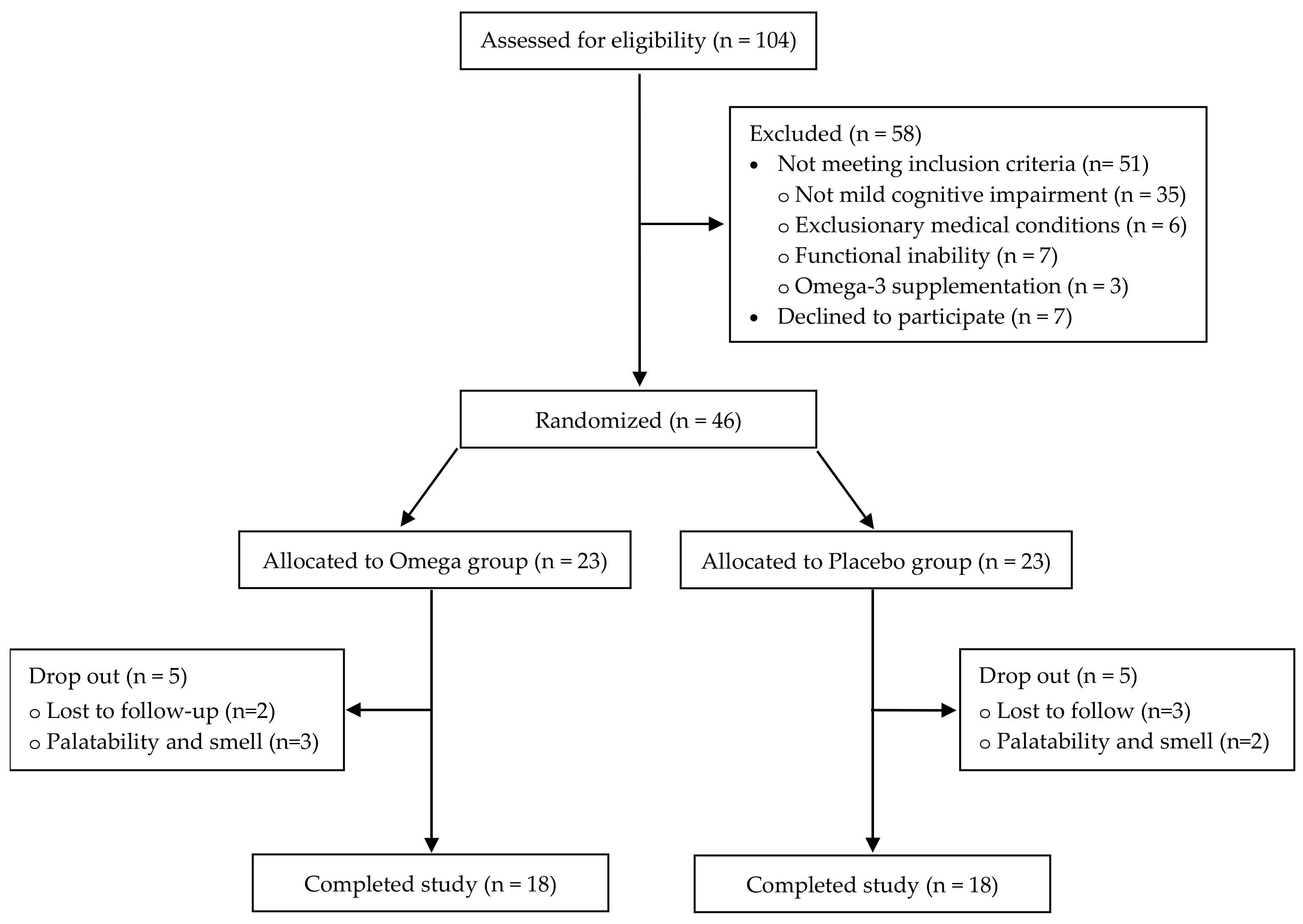
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Outcome Measures
2.3.1. Cognitive Function
2.3.2. Functional Capacity
2.3.3. Body Composition
2.3.4. Quality of Life, Sleep Quality, Daily Sleepiness Status and Fatigue
2.4. Sample Size & Statistical Analysis
3. Results
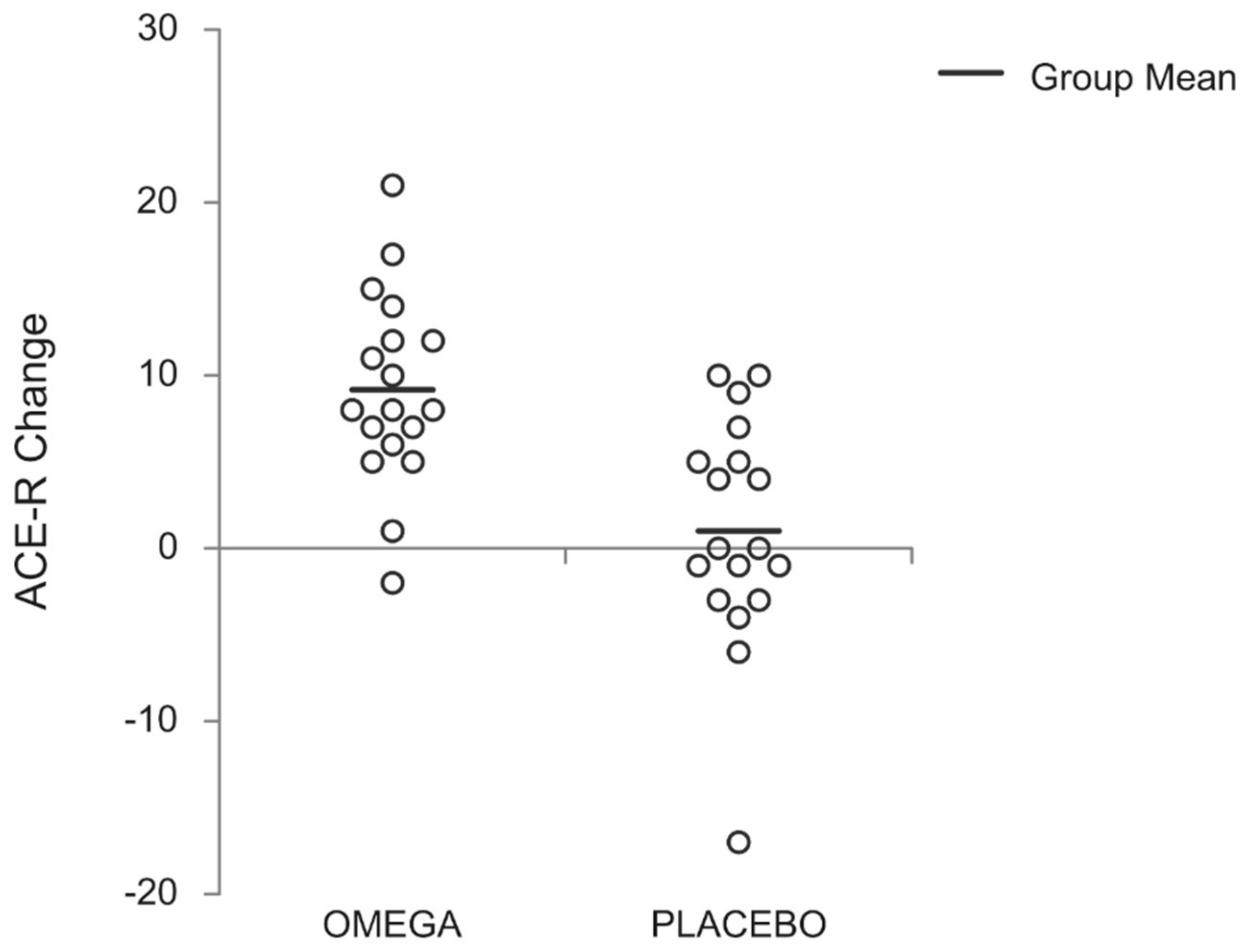
3.1. Cognitive Function
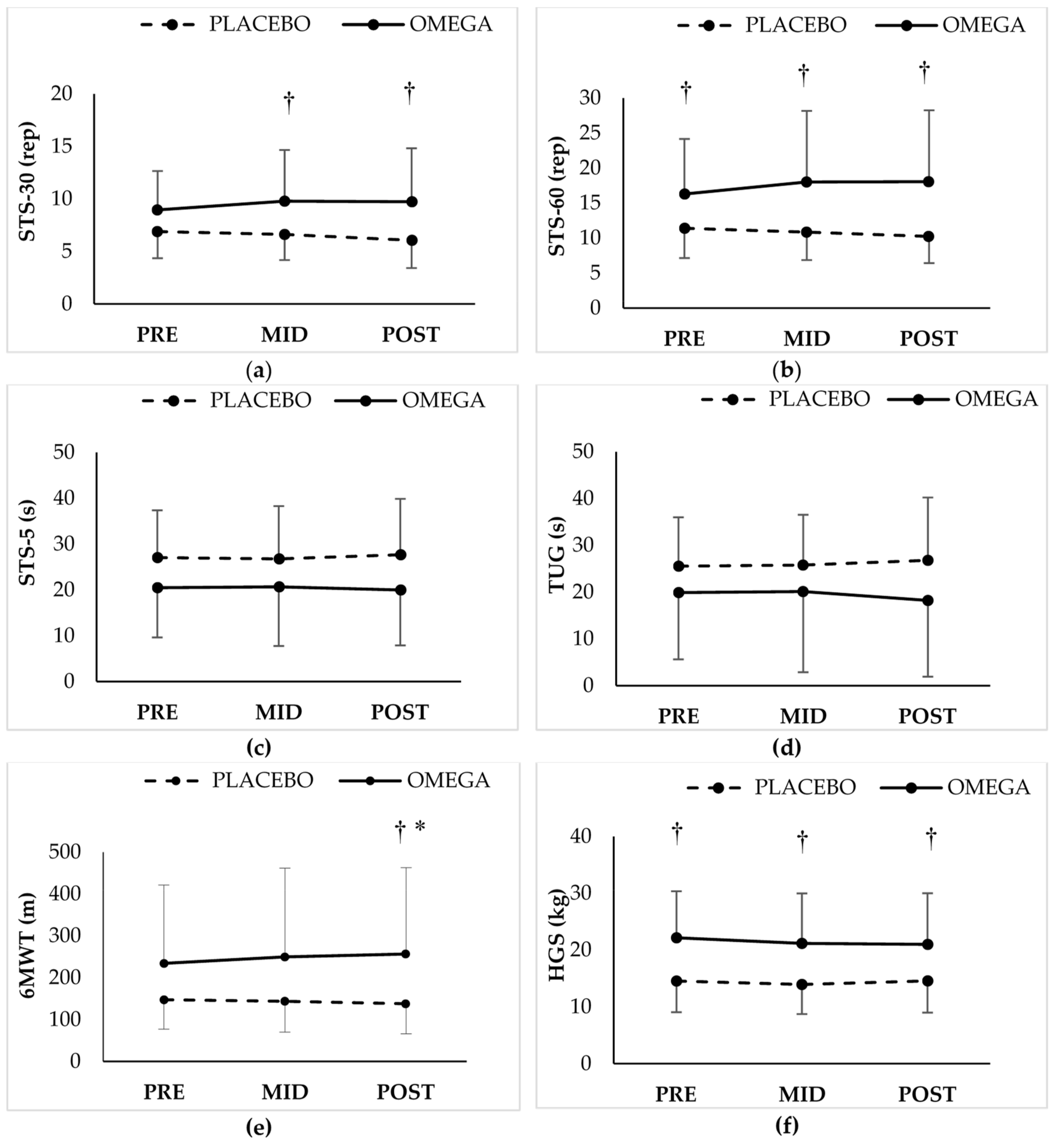
3.2. Functional Capacity
3.3. Body Composition
3.4. Questionnaires
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kontis, V.; Bennett, J.E.; Mathers, C.D.; Li, G.; Foreman, K.; Ezzati, M. Future life expectancy in 35 industrialised countries: Projections with a Bayesian model ensemble. Lancet 2017, 389, 1323–1335. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/ageing/events/world-report-2015-launch/en/ (accessed on 10 November 2019).
- Crimmins, E.M. Lifespan and healthspan: Past, present, and promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chan, P. Understanding the Physiological Links Between Physical Frailty and Cognitive Decline. Aging Dis. 2020, 11, 1–14. [Google Scholar]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders—A review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Fotuhi, M.; Mohassel, P.; Yaffe, K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: A complex association. Nat. Clin. Pract. Neurol. 2009, 5, 140–152. [Google Scholar] [CrossRef]
- Youdim, K.A.; Martin, A.; Joseph, J.A. Essential fatty acids and the brain: Possible health implications. Int. J. Dev. Neurosci. 2000, 18, 383–399. [Google Scholar] [CrossRef]
- Calder, P.C. n−3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Calder, P.C. Dietary modification of inflammation with lipids. Proc. Nutr. Soc. 2002, 61, 345–358. [Google Scholar] [CrossRef]
- Vatassery, G.T. Vitamin E and other endogenous antioxidants in the central nervous system. Geriatrics 1998, 53 (Suppl. 1), S25–S27. [Google Scholar]
- Fukuzawa, K.; Gebicki, J.M. Oxidation of α-tocopherol in micelles and liposomes by the hydroxyl, perhydroxyl, and superoxide free radicals. Arch. Biochem. Biophys. 1983, 226, 242–251. [Google Scholar] [CrossRef]
- Christen, S.; Woodall, A.A.; Shigenaga, M.K.; Southwell-Keely, P.T.; Duncan, M.W.; Ames, B.N. γ-Tocopherol traps mutagenic electrophiles such as NOx and complements α-tocopherol: Physiological implications. Proc. Natl. Acad. Sci. USA 1997, 94, 3217–3222. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Kelly, F.J.; Salonen, J.T.; Neuzil, J.; Zingg, J.M.; Azzi, A. The European perspective on vitamin E: Current knowledge and future research. Am. J. Clin. Nutr. 2002, 76, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Q.; Chu, J.; Zeng, W.; Yang, M.; Zhu, S. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1422–1436. [Google Scholar] [CrossRef]
- Burckhardt, M.; Herke, M.; Wustmann, T.; Watzke, S.; Langer, G.; Fink, A. Omega-3 fatty acids for the treatment of dementia. Cochrane Database Syst. Rev. 2016, 2015. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Gaugler, J.E.; Duval, S.; Anderson, K.A.; Kane, R.L. Predicting nursing home admission in the U.S: A meta-analysis. BMC Geriatr. 2007, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Rolland, Y.; Cruz-Jentoft, A.J.; Bauer, J.M.; Sieber, C.; Cooper, C.; Al-Daghri, N.; Araujo de Carvalho, I.; Bautmans, I.; Bernabei, R.; et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice: A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif. Tissue Int. 2019, 105. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Shin, D.W.; Jeong, S.-M.; Son, K.Y.; Cho, B.; Yoon, J.L.; Park, B.J.; Kwon, I.S.; Lee, J.; Kim, S. Association Between Timed Up and Go Test and Future Dementia Onset. J. Gerontol. Ser. A 2018, 73, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Lalia, A.Z.; Dasari, S.; Robinson, M.M.; Abid, H.; Morse, D.M.; Klaus, K.A.; Lanza, I.R. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging 2017, 9, 1096–1129. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil–derived n−3 PUFA therapy increases muscle mass and function in healthy older adults1. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef]
- Krzymińska-Siemaszko, R.; Czepulis, N.; Lewandowicz, M.; Zasadzka, E.; Suwalska, A.; Witowski, J.; Wieczorowska-Tobis, K. The Effect of a 12-week omega-3 supplementation on body composition, muscle strength and physical performance in elderly individuals with decreased muscle mass. Int. J. Environ. Res. Public Health 2015, 12, 10558–10574. [Google Scholar] [CrossRef]
- Rolland, Y.; Barreto, P.d.S.; Maltais, M.; Guyonnet, S.; Cantet, C.; Andrieu, S.; Vellas, B. Effect of Long-Term Omega 3 Polyunsaturated Fatty Acid Supplementation with or without Multidomain Lifestyle Intervention on Muscle Strength in Older Adults: Secondary Analysis of the Multidomain Alzheimer Preventive Trial (MAPT). Nutrients 2019, 11, 1931. [Google Scholar]
- Logan, S.L.; Spriet, L.L. Omega-3 fatty acid supplementation for 12 weeks increases resting and exercise metabolic rate in healthy community- dwelling older females. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef]
- Hutchins-Wiese, H.L.; Kleppinger, A.; Annis, K.; Liva, E.; Lammi-Keefe, C.J.; Durham, H.A.; Kenny, A.M. The impact of supplemental N-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J. Nutr. Health Aging 2013, 17, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild Cognitive Impairment as a diagnostic utility. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Terada, S.; Honda, H.; Kishimoto, Y.; Takeda, N.; Oshima, E.; Hirayama, K.; Yokota, O.; Uchitomi, Y. Validation of the revised Addenbrooke’s Cognitive Examination (ACE-R) for detecting mild cognitive impairment and dementia in a Japanese population. Int. Psychogeriatrics 2012, 24, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Dawson, K.; Mitchell, J.; Arnold, R.; Hodges, J.R. The Addenbrooke’s Cognitive Examination Revised (ACE-R): A brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry 2006, 21, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Pantzaris, M.C.; Loukaides, G.N.; Ntzani, E.E.; Patrikios, I.S. A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: A randomised, double-blind, placebo-controlled proof-of-concept clinical trial. BMJ Open 2013, 3. [Google Scholar] [CrossRef]
- Konstantinopoulou, E.; Kosmidis, M.H.; Ioannidis, P.; Kiosseoglou, G.; Karacostas, D.; Taskos, N. Adaptation of Addenbrooke’s Cognitive Examination-Revised for the Greek population. Eur. J. Neurol. 2011, 18, 442–447. [Google Scholar] [CrossRef]
- Ahmed, S.; de Jager, C.; Wilcock, G. A comparison of screening tools for the assessment of Mild Cognitive Impairment: Preliminary findings. Neurocase 2012, 18, 336–351. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Mariz, J.; Bull, L.; Mehta, Z.; Rothwell, P.M. MoCA, ACE-R, and MMSE versus the national institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards neuropsychological battery after TIA and stroke. Stroke 2012, 43, 464–469. [Google Scholar] [CrossRef]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Bowie, C.R.; Harvey, P.D. Administration and interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef]
- Bixby, W.R.; Spalding, T.W.; Haufler, A.J.; Deeny, S.P.; Mahlow, P.T.; Zimmerman, J.B.; Hatfield, B.D. The unique relation of physical activity to executive function in older men and women. Med. Sci. Sports Exerc. 2007, 39, 1408–1416. [Google Scholar] [CrossRef]
- Lowery, N.; Ragland, J.D.; Gur, R.C.; Gur, R.E.; Moberg, P.J. Normative data for the symbol cancellation test in young healthy adults. Appl. Neuropsychol. 2004, 11, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Rikli, R.E.; Jones, C.J. Development and Validation of a Functional Fitness Test for Community-Residing Older Adults. J. Aging Phys. Act. 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed ”up & go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Enright, P.L. The six-minute walk test. Respir. Care 2003, 48, 783–785. [Google Scholar]
- McGrath, R.P.; Kraemer, W.J.; Snih, S.A.; Peterson, M.D. Handgrip Strength and Health in Aging Adults. Sports Med. 2018, 48, 1993–2000. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Bayliss, M.S.; McHorney, C.A.; Rogers, W.H.; Raczek, A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the Medical Outcomes Study. Med. Care 1995, 33, AS264–AS279. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, R. A new method of classifying prognostic in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild Cognitive Impairment. N. Engl. J. Med. 2011, 364, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Busse, A.; Hensel, A.; Gühne, U.; Angermeyer, M.C.; Riedel-Heller, S.G. Mild cognitive impairment: Long-term course of four clinical subtypes. Neurology 2006, 67, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Su, K.P.; Cheng, T.C.; Liu, H.C.; Chang, C.J.; Dewey, M.E.; Stewart, R.; Huang, S.Y. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Zhang, X.; Wang, Y.; You, J.; Cui, H.; Zhu, Y.; Pang, W.; Liu, W.; Jiang, Y.; Lu, Q. The n-3 polyunsaturated fatty acids supplementation improved the cognitive function in the Chinese elderly with mild cognitive impairment: A double-blind randomized controlled trial. Nutrients 2017, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Sinn, N.; Milte, C.M.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Petkov, J.; Howe, P.R.C. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Nutr. 2012, 107, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Traykov, L.; Raoux, N.; Latour, F.; Gallo, L.; Hanon, O.; Baudic, S.; Bayle, C.; Wenisch, E.; Remy, P.; Rigaud, A.-S. Executive Functions Deficit in Mild Cognitive Impairment. Cogn. Behav. Neurol. 2007, 20, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Milte, C.M.; Meyer, B.J. Nutritional modulation of cognitive function and mental health. J. Nutr. Biochem. 2013, 24, 725–743. [Google Scholar] [CrossRef]
- Dolopikou, C.F.; Kourtzidis, I.A.; Margaritelis, N.V.; Vrabas, I.S.; Koidou, I.; Kyparos, A.; Theodorou, A.A.; Paschalis, V.; Nikolaidis, M.G. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: A double-blind cross-over study. Eur. J. Nutr. 2019. Available online: https://link.springer.com/article/10.1007/s00394-019-01919-4 (accessed on 10 November 2019). [CrossRef]
- Robertson, D.A.; Savva, G.M.; Kenny, R.A. Frailty and cognitive impairment-A review of the evidence and causal mechanisms. Ageing Res. Rev. 2013, 12, 840–851. [Google Scholar] [CrossRef]
- Kiiti Borges, M.; Oiring de Castro Cezar, N.; Silva Santos Siqueira, A.; Yassuda, M.; Cesari, M.; Aprahamian, I. The Relationship between Physical Frailty and Mild Cognitive Impairment in the Elderly: A Systematic Review. J. Frailty Aging 2019, 8, 192–197. [Google Scholar]
- Rikli, R.E.; Jones, C.J. The Reliability and Validity of a 6-Minute Walk Test as a Measure of Physical Endurance in Older Adults. J. Aging Phys. Act. 1998, 6, 363–375. [Google Scholar] [CrossRef]
- Yazdanyar, A.; Aziz, M.M.; Enright, P.L.; Edmundowicz, D.; Boudreau, R.; Sutton-Tyrell, K.; Kuller, L.; Newman, A.B. Association between 6-minute walk test and all-cause mortality, coronary heart disease-specific mortality, and incident coronary heart disease. J. Aging Health 2014, 26, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Rodacki, C.L.N.; Rodacki, A.L.F.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef] [PubMed]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Turnover in Health, Disuse, and Disease. Front. Nutr. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Philpott, J.D.; Bootsma, N.J.; Rodriguez-Sanchez, N.; Hamilton, D.L.; MacKinlay, E.; Dick, J.; Mettler, S.; Galloway, S.D.R.; Tipton, K.D.; Witard, O.C. Influence of Fish Oil-Derived n-3 Fatty Acid Supplementation on Changes in Body Composition and Muscle Strength During Short-Term Weight Loss in Resistance-Trained Men. Front. Nutr. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Damiano, S.; Muscariello, E.; La Rosa, G.; Di Maro, M.; Mondola, P.; Santillo, M. Dual role of reactive oxygen species in muscle function: Can antioxidant dietary supplements counteract age-related sarcopenia? Int. J. Mol. Sci. 2019, 20, 3815. [Google Scholar] [CrossRef]
- Patten, G.S.; Abeywardena, M.Y.; McMurchie, E.J.; Jahangiri, A. Dietary Fish Oil Increases Acetylcholine- and Eicosanoid-Induced Contractility of Isolated Rat Ileum. J. Nutr. 2002, 132, 2506–2513. [Google Scholar] [CrossRef]
- Yoshino, J.; Smith, G.I.; Kelly, S.C.; Julliand, S.; Reeds, D.N.; Mittendorfer, B. Effect of dietary n-3 PUFA supplementation on the muscle transcriptome in older adults. Physiol. Rep. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Żebrowska, A.; Mizia-Stec, K.; Mizia, M.; Gąsior, Z.; Poprzęcki, S. Omega-3 fatty acids supplementation improves endothelial function and maximal oxygen uptake in endurance-trained athletes. Eur. J. Sport Sci. 2015, 15, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, A.A.; Nikolaidis, M.G.; Paschalis, V.; Koutsias, S.; Panayiotou, G.; Fatouros, I.G.; Koutedakis, Y.; Jamurtas, A.Z. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am. J. Clin. Nutr. 2011, 93, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Shin, Y.O.; Yoon, J.H.; Kim, S.H.; Shin, H.C.; Hwang, H.J. Effect of supplementation with Ecklonia cava polyphenol on endurance performance of college students. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Antioxidants in personalized nutrition and exercise. Adv. Nutr. 2018, 9, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Teng, E.; Tassniyom, K.; Lu, P.H. Reduced quality-of-life ratings in mild cognitive impairment: Analyses of subject and informant responses. Am. J. Geriatr. Psychiatry 2012, 20, 1016–1025. [Google Scholar] [CrossRef]
- Avlund, K. Fatigue in older adults: An early indicator of the aging process? Aging Clin. Exp. Res. 2010, 22, 100–115. [Google Scholar] [CrossRef]
- Ju, Y.-E.S.; Lucey, B.P.; Holtzman, D.M. Sleep and Alzheimer disease pathology—A bidirectional relationship. Nat. Rev. Neurol. 2014, 10, 115–119. [Google Scholar] [CrossRef]
- Martin, J.L.; Fiorentino, L.; Jouldjian, S.; Josephson, K.R.; Alessi, C.A. Sleep quality in residents of assisted living facilities: Effect on quality of life, functional status, and depression. J. Am. Geriatr. Soc. 2010, 58, 829–836. [Google Scholar] [CrossRef]
- Danthiir, V.; Hosking, D.E.; Nettelbeck, T.; Vincent, A.D.; Wilson, C.; O’Callaghan, N.; Calvaresi, E.; Clifton, P.; Wittert, G.A. An 18-mo randomized, double-blind, placebo-controlled trial of DHA-rich fish oil to prevent age-related cognitive decline in cognitively normal older adults. Am. J. Clin. Nutr. 2018, 107, 754–762. [Google Scholar] [CrossRef]
| PLACEBO | OMEGA | |
|---|---|---|
| N | 18 | 18 |
| Sex (F/M) | 12F/6M | 10F/8M |
| Age (years) | 81.2 ± 5.3 | 77.4 ± 9.2 |
| Long-term care facility residents (n) | 13 | 12 |
| ACE-R | 63.9 ± 10.2 | 69.4 ± 11.2 |
| Education (years) | 9.3 ± 4.1 | 8.4 ± 4.1 |
| Charlson comorbidity index | 2.4 ± 1.2 | 1.8 ± 1.7 |
| PLACEBO | OMEGA | |||||
|---|---|---|---|---|---|---|
| PRE | MID | POST | PRE | MID | POST | |
| ACE-R (total score) | 63.9 ± 10.2 | 63.8 ± 9.9 | 64.9 ± 13.3 | 69.4 ± 11.2 | 73.7 ± 15.7 †* | 78.6 ± 14.0 †*# |
| Attention/orientation | 14.5 ± 2.8 | 14.4 ± 3 | 14.6 ± 3.7 | 15.1 ± 2.7 | 15.4 ± 2.8 | 16.4 ± 2.4 |
| Memory | 14.1 ± 4.9 | 13.4 ± 4.6 | 15.0 ± 5.6 | 15.8 ± 3.7 | 17.3 ± 5.9 | 19.1 ± 5.1 * |
| Fluency | 3.6 ± 2.5 | 4.2 ± 2.4 | 3.7 ± 2.2 | 5.2 ± 2.3 | 6.0 ± 3.0 | 6.6 ± 3.0 †* |
| Language | 19.2 ± 3.5 | 19.8 ± 3.6 | 19.7 ± 3.1 | 20.3 ± 4.0 | 21.7 ± 4.5 * | 22.5 ± 3.7 †* |
| Visuospatial | 12.6 ± 2.6 | 12.1 ± 2.5 | 11.9 ± 2.7 | 13.1 ± 2.5 | 13.2 ± 2.3 | 14.1 ± 2.1 †*# |
| MMSE | 23.6 ± 3.2 | 23.3 ± 3.5 | 22.9 ± 4.6 | 24.3 ± 3.5 | 25.2 ± 3.9 | 26.1 ± 3 †* |
| PLACEBO | OMEGA | |||||
|---|---|---|---|---|---|---|
| n | PRE | POST | n | PRE | POST | |
| TMT A (s) | 14 | 251.5 ± 148.7 | 236.4 ± 146.7 | 17 | 168.5 ± 142.6 | 155.7 ± 157 |
| TMT B (s) | 8 | 420.5 ± 203.3 | 415 ± 162.8 | 10 | 263.3 ± 167.4 | 201.6 ± 142.2 |
| STROOP word (score) | 12 | 48.6 ± 15.8 | 51 ± 15.2 | 14 | 55.2 ± 23.3 | 61.3 ± 24.7 * |
| STROOP color (score) | 12 | 33.3 ± 12 | 33.1 ± 13.8 | 14 | 31.1 ± 16.9 | 36.9 ± 17.8 * |
| STROOP color-word (score) | 12 | 14.3 ± 6.9 | 12.5 ± 9.1 | 14 | 14.4 ± 9.8 | 19.1 ± 13.3 |
| STROOP interference (score) | 12 | −4.9 ± 5.9 | −6.9 ± 8.4 | 14 | −5.2 ± 8.6 | −3.6 ± 11.1 |
| Symbol cancellation (errors) | 13 | 53.4 ± 6.7 | 50.0 ± 7.6 | 14 | 37.6 ± 15.8† | 31.6 ± 14.5 *† |
| PLACEBO | OMEGA | |||||
|---|---|---|---|---|---|---|
| PRE | MID | POST | PRE | MID | POST | |
| Body mass (kg) | 70.8 ± 19.2 | 70.2 ± 19.4 | 70.0 ± 19.3 | 71.5 ± 13.0 | 72.1 ± 14 | 72.1 ± 13.7 |
| Body mass index (kg/m2) | 33.9 ± 7.7 | 33.6 ± 7.8 | 33.5 ± 7.8 | 33.5 ± 6.3 | 33.8 ± 6.6 | 33.8 ± 6.6 |
| Trunk fat (%) | 38.5 ± 10.3 | 37.8 ± 9.8 | 38.0 ± 10.3 | 35.4 ± 9.7 | 34.5 ± 9.7 | 35.4 ± 9.5 |
| Total body fat (%) | 41.1 ± 8.3 | 41.3 ± 7.8 | 40.5 ± 7.5 | 37.1 ± 6.2 | 36.0 ± 6.8 † | 35.9 ± 7.3 † |
| Total body water (%) | 50.8 ± 5.8 | 50.9 ± 5.8 | 51.5 ± 5.2 | 53.1 ± 6.0 | 53.8 ± 6.9 | 54.1 ± 7.0 |
| Lean body mass (kg) | 42.8 ± 12.3 | 42.2 ± 12.3 | 42.6 ± 12.1 | 44.7 ± 10.0 | 45.9 ± 11.5 | 45.9 ± 11.2 |
| Fat mass index (kg/m2) | 11.9 ± 4.2 | 11.8 ± 4.2 | 11.6 ± 4.1 | 10.2 ± 2.6 | 9.9 ±2.7 | 9.9 ± 2.8 |
| Lean mass index (kg/m2) | 16.7 ±3.7 | 16.5 ± 3.7 | 16.7 ± 3.7 | 17.1 ± 3.1 | 17.5 ± 3.6 | 17.5 ± 3.4 |
| Waist circumference (cm) | 107.9 ± 12.8 | 107.2 ± 12.6 | 107.0 ± 13.2 | 101.2 ± 10.5 | 103.0 ± 10.6 | 100.1 ± 11.7 |
| Hip circumference (cm) | 108.4 ± 13.3 | 107.6 ± 13.5 | 107.6 ± 13.8 | 105.1 ± 9.9 | 103.7 ± 10.0 | 103.6 ± 10.4 |
| PLACEBO | OMEGA | |||||
|---|---|---|---|---|---|---|
| PRE | MID | POST | PRE | MID | POST | |
| Fatigue | 4.2 ± 1.5 | 4.4 ± 1.5 | 4.6 ± 1.7 | 4.5 ± 1.8 | 3.8 ± 1.7 * | 3.7 ± 1.9 * |
| Sleep quality | 8.5 ± 3.5 | 8.0 ± 3.2 | 8.5 ± 3.4 | 8.7 ± 5.1 | 7.1 ± 4.9 * | 7.4 ± 4.7 |
| Sleepiness | 6.9 ± 4.2 | 7.3 ± 3.5 | 8.5 ± 4.0 | 7.6 ± 5.1 | 6.6 ± 3.5 | 6.1 ± 3.0 |
| Quality of life | ||||||
| Physical health component | 47.5 ± 19.9 | 47.2 ± 17.7 | 45.7 ± 16.8 | 50.8 ± 22.0 | 60.8 ± 21.3 * | 59.2 ± 23.2 * |
| Mental health component | 58.7 ± 18.6 | 60.9 ± 21.2 | 61.2 ± 21.2 | 57.4 ± 20.3 | 60.7 ± 21.1 | 61.9 ± 20.5 |
| SF-36 total score | 51.5 ± 19.4 | 52.6 ± 19.8 | 52.3 ± 18.9 | 53.1 ± 20.3 | 60.4 ± 21 | 60.3 ± 22.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
S. Stavrinou, P.; Andreou, E.; Aphamis, G.; Pantzaris, M.; Ioannou, M.; S. Patrikios, I.; D. Giannaki, C. The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment. Nutrients 2020, 12, 325. https://doi.org/10.3390/nu12020325
S. Stavrinou P, Andreou E, Aphamis G, Pantzaris M, Ioannou M, S. Patrikios I, D. Giannaki C. The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment. Nutrients. 2020; 12(2):325. https://doi.org/10.3390/nu12020325
Chicago/Turabian StyleS. Stavrinou, Pinelopi, Eleni Andreou, George Aphamis, Marios Pantzaris, Melina Ioannou, Ioannis S. Patrikios, and Christoforos D. Giannaki. 2020. "The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment" Nutrients 12, no. 2: 325. https://doi.org/10.3390/nu12020325
APA StyleS. Stavrinou, P., Andreou, E., Aphamis, G., Pantzaris, M., Ioannou, M., S. Patrikios, I., & D. Giannaki, C. (2020). The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment. Nutrients, 12(2), 325. https://doi.org/10.3390/nu12020325








