Safety and Effectiveness of the Prolonged Treatment of Children with a Ketogenic Diet
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
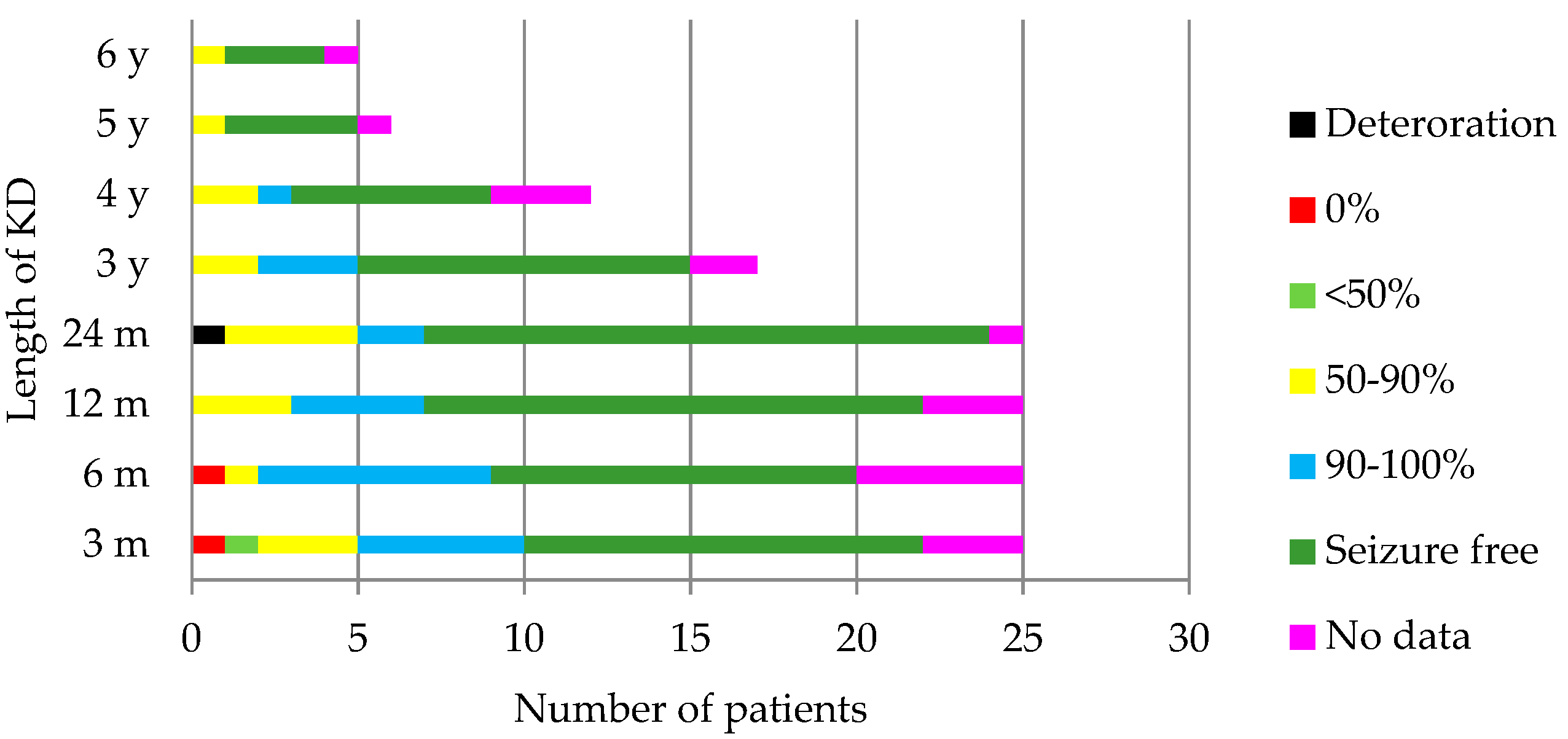
4.1. Outcome of the KD
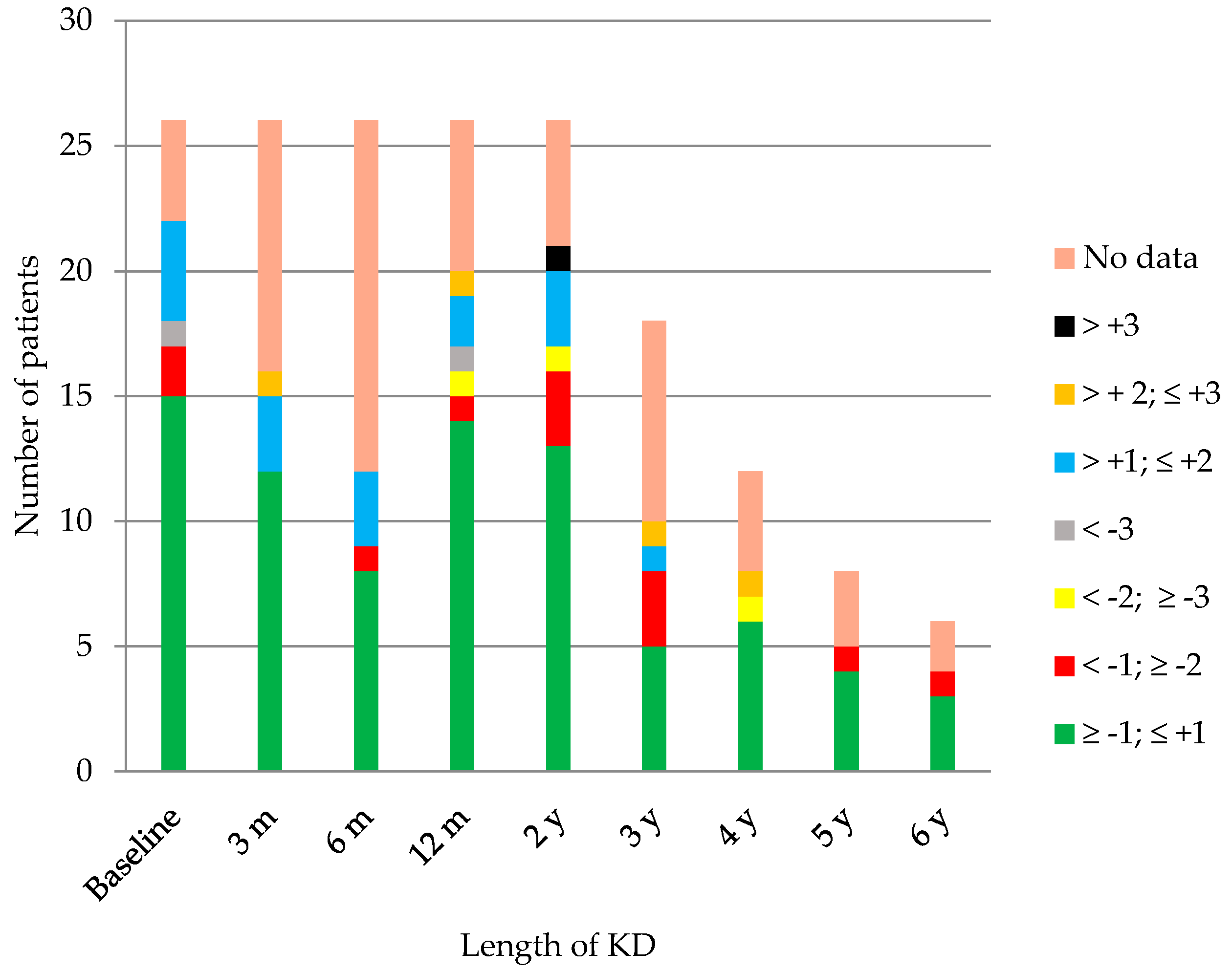
4.2. Side Effects
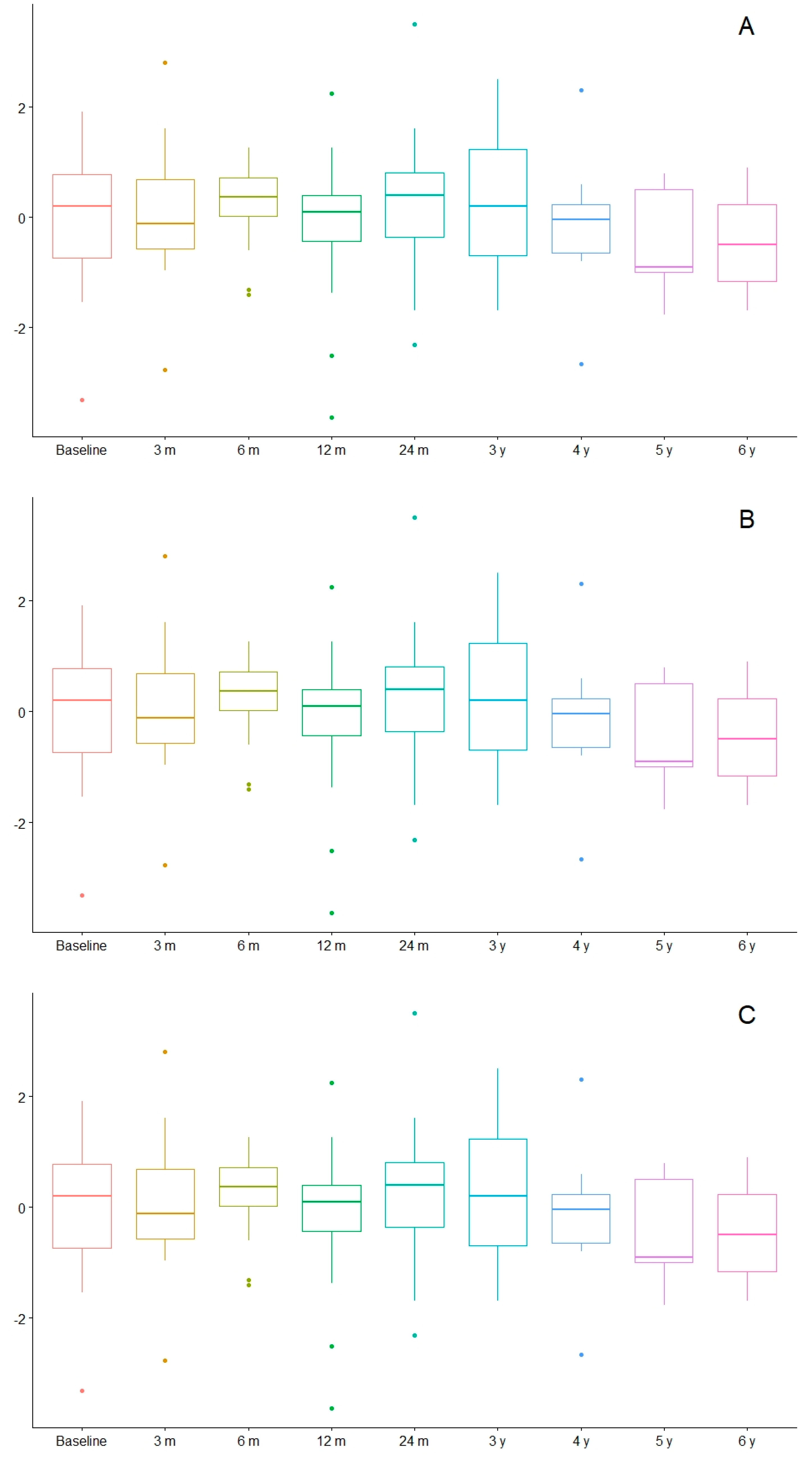
4.3. Nutritional Evolution
4.3.1. Nutritional Status before the KD
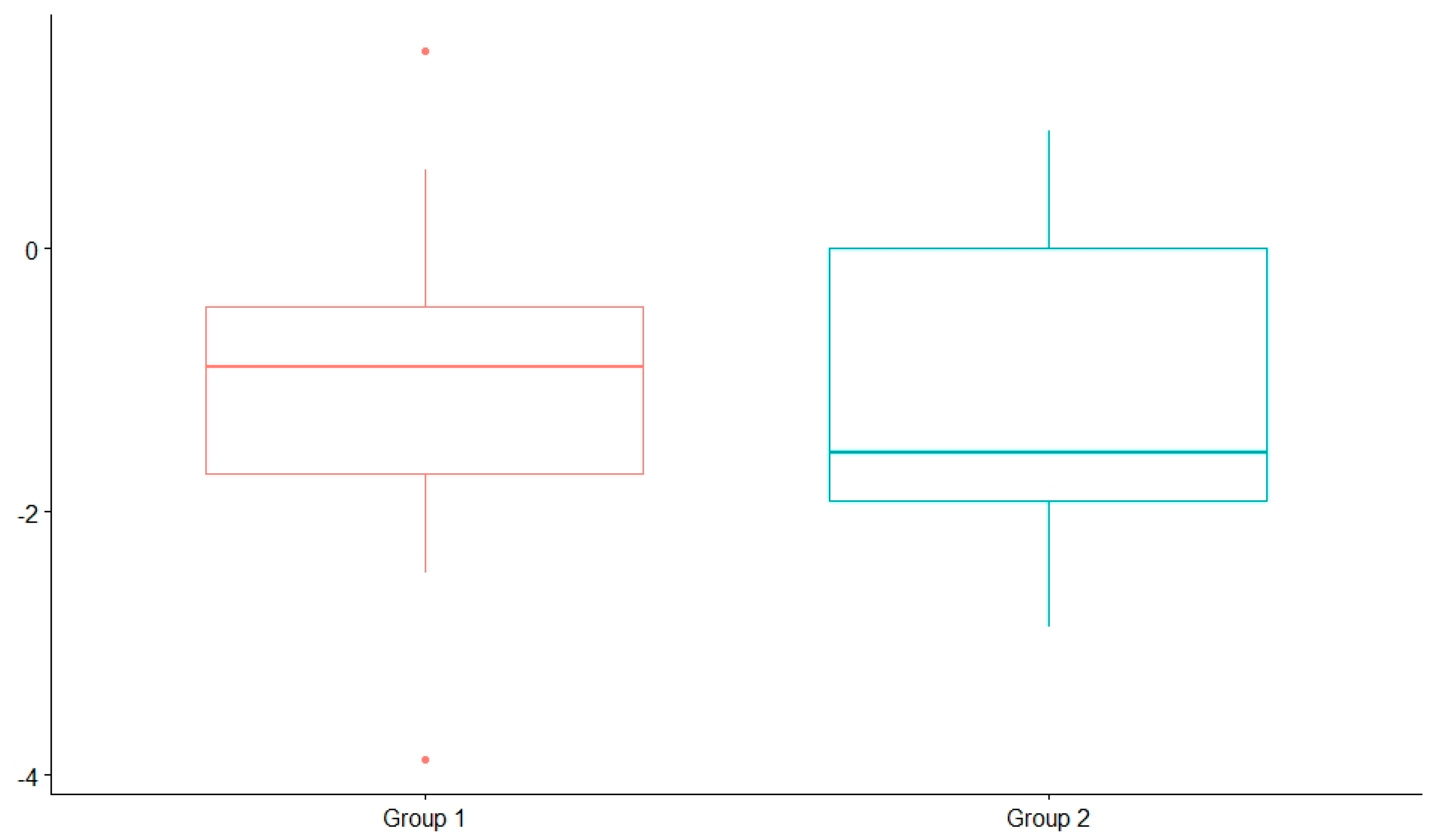
4.3.2. Nutritional Status after the Introduction of the KD
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kossoff, E.H.; McGrogan, J.R. Worldwide use of the ketogenic diet. Epilepsia 2005, 46, 280–289. [Google Scholar] [CrossRef]
- Kossoff, E.H. International consensus statement on clinical implementation of the ketogenic diet: agreement, flexibility, and controversy. Epilepsia 2008, 49 (Suppl. 8), 11–13. [Google Scholar] [CrossRef]
- Huttenlocher, P.R.; Wilbourn, A.J.; Signore, J.M. Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 1971, 21, 1097–1103. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Krauss, G.L.; McGrogan, J.R.; Freeman, J.M. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology 2003, 61, 1789–1791. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; Dorward, J.L. The modified Atkins diet. Epilepsia 2008, 49 (Suppl. 8), 37–41. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, H.H.; Thiele, E.A. Low-glycemic index treatment: A liberalized ketogenic diet for treatment of intractable epilepsy. Neurology 2005, 65, 1810–1812. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Yoon, J.R.; Lee, E.J.; Lee, J.S.; Kim, J.T.; Kim, H.D.; Kang, H.C. Efficacy of the classic ketogenic and the modified Atkins diets in refractory childhood epilepsy. Epilepsia 2016, 57, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Masino, S.A.; Rho, J.M. Mechanisms of Ketogenic Diet Action. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012; Available online: http://www.ncbi.nlm.nih.gov/books/NBK98219/PubMed (accessed on 2 November 2018).
- Nangia, S.; Caraballo, R.H.; Kang, H.C.; Nordli, D.R.; Scheffer, I.E. Is the ketogenic diet effective in specific epilepsy syndromes? Epilepsy Res. 2012, 100, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Thammongkol, S.; Vears, D.F.; Bicknell-Royle, J.; Nation, J.; Draffin, K.; Stewart, K.G.; Scheffer, I.E.; Mackay, M.T. Efficacy of the ketogenic diet: Which epilepsies respond? Epilepsia 2012, 53, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Dressler, A.; Trimmel-Schwahofer, P.; Reithofer, E.; Mühlebner, A.; Gröppel, G.; Reiter-Fink, E.; Benninger, F.; Grassl, R.; Feucht, M. Efficacy and tolerability of the ketogenic diet in Dravet syndrome—Comparison with various standard antiepileptic drug regimen. Epilepsy Res. 2015, 109, 81–89. [Google Scholar] [CrossRef]
- Caraballo, R.H.; Cersosimo, R.O.; Sakr, D.; Cresta, A.; Escobal, N.; Fejerman, N. Ketogenic diet in patients with Dravet syndrome. Epilepsia 2005, 46, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Eun, S.H.; Kang, H.C.; Kim, D.W.; Kim, H.D. Ketogenic diet for treatment of infantile spasms. Brain Dev. 2006, 28, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.M.; Turner, Z.; Hamdy, R.F.; Kossoff, E.H. Infantile spasms treated with the ketogenic diet: Prospective single-center experience in 104 consecutive infants. Epilepsia 2010, 51, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, R.H.; Cersosimo, R.O.; Sakr, D.; Cresta, A.; Escobal, N.; Fejerman, N. Ketogenic diet in patients with myoclonic-astatic epilepsy. Epileptic Disord. 2006, 8, 151–155. [Google Scholar] [PubMed]
- Kilaru, S.; Bergqvist, A.G. Current treatment of myoclonic astatic epilepsy: Clinical experience at the children’s hospital of Philadelphia. Epilepsia 2007, 48, 1703–1707. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Thiele, E.A.; Pfeifer, H.H.; McGrogan, J.R.; Freeman, J.M. Tuberous sclerosis complex and the ketogenic diet. Epilepsia 2005, 46, 1684–1686. [Google Scholar] [CrossRef]
- Millichap, J.J.; Millichap, J.G. Ketogenic diet as preferred treatment of FIRES. Pediatr. Neurol. Briefs 2015, 29, 3. [Google Scholar] [CrossRef][Green Version]
- Nabbout, R.; Mazzuca, M.; Hubert, P.; Peudennier, S.; Allaire, C.; Flurin, V.; Aberastury, M.; Silva, W.; Dulac, O. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES). Epilepsia 2010, 51, 2033–2037. [Google Scholar] [CrossRef]
- Klepper, J.; Leiendecker, B. Glut1 deficiency syndrome and novel ketogenic diets. J. Child Neurol. 2013, 28, 1045–1048. [Google Scholar] [CrossRef]
- Sofou, K.; Dahlin, M.; Hallböök, T.; Lindefeldt, M.; Viggedal, G.; Darin, N. Ketogenic diet in pyruvate dehydrogenase complex deficiency: Short- and long-term outcomes. J. Inherit. Metab. Dis. 2017, 40, 237–245. [Google Scholar] [CrossRef]
- Kang, H.C.; Chung, D.E.; Kim, D.W.; Kim, H.D. Early and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia 2004, 45, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity clinic: A practical guide. J. Trasnl. Med. 2019, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Groesbeck, D.K.; Bluml, R.M.; Kossoff, E.H. Long-term use of the ketogenic diet in the treatment of epilepsy. Dev. Med. Child Neurol. 2006, 48, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Epifanio, G.; Auricchio, G.; Federico, R.R.; Resicato, G.; Pascotto, A. Plasma free carnitine in epilepsy children, adolescents and young adults treated with old and new antiepileptic drugs with or without ketogenic diet. Brain Dev. 2006, 28, 358–365. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Booth, G.; Sánchez, A.C.; Woodbury-Kolb, J. Carnitine levels and the ketogenic diet. Epilepsia 2001, 42, 1445–1451. [Google Scholar] [CrossRef]
- Couch, S.C.; Schwarzman, F.; Carroll, J.; Koenigsberger, D.; Nordli, D.R.; Deckelbaum, R.J.; DeFelice, A.R. Growth and nutritional outcomes of children treated with the ketogenic diet. J. Am. Diet Assoc. 1999, 99, 1573–1575. [Google Scholar] [CrossRef]
- Liu, Y.M.; Williams, S.; Basualdo-Hammond, C.; Stephens, D.; Curtis, R. A prospective study: Growth and nutritional status of children treated with the ketogenic diet. J. Am. Diet Assoc. 2003, 103, 707–712. [Google Scholar] [CrossRef]
- Peterson, S.J.; Tangney, C.C.; Pimentel-Zablah, E.M.; Hjelmgren, B.; Booth, G.; Berry-Kravis, E. Changes in growth and seizure reduction in children on the ketogenic diet as a treatment for intractable epilepsy. J. Am. Diet Assoc. 2005, 105, 718–725. [Google Scholar] [CrossRef]
- Spulber, G.; Spulber, S.; Hagenas, L.; Amark, P.; Dahlin, M. Growth dependence on insulin-like growth factor-1 during the ketogenic diet. Epilepsia 2009, 50, 297–303. [Google Scholar] [CrossRef]
- Vining, E.P.; Pyzik, P.; McGrogan, J.; Hladky, H.; Anand, A.; Kriegler, S.; Freeman, J.M. Growth of children on the ketogenic diet. Dev. Med. Child Neurol. 2008, 44, 796–802. [Google Scholar] [CrossRef]
- Ferraris, C.; Guglielmetti, M.; Pasca, L.; De Giorgis, V.; Ferraro, O.E.; Brambilla, I.; Leone, A.; De Amicis, R.; Bertoli, S.; Veggiotti, P.; et al. Impact of the Ketogenic Diet on Linear Growth in Children: A Single-Center Retrospective Analysis of 34 Cases. Nutrients 2019, 11, 1442. [Google Scholar] [CrossRef] [PubMed]
- Svedlund, A.; Hallböök, T.; Magnusson, P.; Dahlgren, J.; Swolin-Eide, D. Prospective study of growth and bone mass in Swedish children treated with the modified Atkins diet. Eur. J. Paediatr. Neurol. 2019, 23, 629–638. [Google Scholar] [CrossRef]
- World Health Organization, Food and Agriculture Organization of the United Nations, United Nations University. Human Energy Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation, Rome, Italy, 17–24 October 2001. 2004. Available online: www.fao.org/ag/agn/nutrition/requirements_pubs_en.stm (accessed on 15 July 2019).
- Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition (2002: Geneva, Switzerland). Protein and Amino Acid Requirements in Human Nutrition: Report of a Join FAO/WHO/UNU Expert Consultation; WHO Technical Report Series; no. 935; WHO Press: Geneva, Switzerland, 2007. [Google Scholar]
- Armeno, M.; Caraballo, R.; Vaccarezza, M.; Alberti, M.J.; Ríos, V.; Galicchio, S.; de Grandis, E.S.; Mestre, G.; Escobal, N.; Matarrese, P.; et al. Consenso nacional sobre dieta cetogénica. Rev. Neurol. 2014, 59, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Holliday, M.A.; Segar, W.E. Maintenance need for water in parenteral fluid therapy. Pediatrics 1957, 19, 823. [Google Scholar] [CrossRef]
- Huttenlocher, P.R. Ketonaemia and seizures: Metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr. Res. 1976, 10, 536–540. [Google Scholar] [CrossRef]
- WHO|The WHO Child Growth Standards. WHO. Available online: http://www.who.int/childgrowth/standards/en/ (accessed on 3 June 2019).
- WHO|Growth Reference Data for 5–19 Years [Internet]. WHO. Available online: https://www.who.int/growthref/en/ (accessed on 3 June 2019).
- World Health Organization. International Classification of Diseases 11th Revision-Beta Browser [Internet]. Available online: http://apps.who.int/classifications/icd11/browse/l-m/en (accessed on 3 June 2019).
- Sivaraju, A.; Nussbaum, I.; Cardoza, C.S.; Mattson, R.H. Substantial and sustained seizure reduction with ketogenic diet in a patient with Ohtahara syndrome. Epilepsy Behav. Case Rep. 2015, 3, 43–45. [Google Scholar] [CrossRef][Green Version]
- Seo, J.H.; Lee, Y.M.; Lee, J.S.; Kim, S.H.; Kim, H.D. A case of Ohtahara syndrome with mitochondrial respiratory chain complex I deficiency. Brain Dev. 2010, 32, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.C.; Lee, Y.M.; Kim, H.D.; Lee, J.S.; Slama, A. Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia 2007, 48, 82–88. [Google Scholar] [CrossRef]
- Caraballo, R.; Vaccarezza, M.; Cersósimo, R.; Rios, V.; Soraru, A.; Arroyo, H.; Agosta, G.; Escobal, N.; Demartini, M.; Maxit, C.; et al. Long-term followup of the ketogenic diet for refractory epilepsy: Multicenter Argentinean experience in 216 pediatric patients. Seizure 2011, 20, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; Pyzik, P.L.; Furth, S.L.; Hladky, H.D.; Freeman, J.M.; Vining, E.P. Kidney stones, carbonic anhydrase inhibitors, and the ketogenic diet. Epilepsia 2002, 43, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Sampath, A.; Kossoff, E.H.; Furth, S.L.; Pyzik, P.L.; Vining, E.P. Kidney stones and the ketogenic diet: Risk factors and prevention. J. Child Neurol. 2007, 22, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Furth, S.L.; Casey, J.C.; Pyzik, P.L.; Neu, A.M.; Docimo, S.G.; Vining, E.P.; Freeman, J.M.; Fivush, B.A. Risk factors for urolithiasis in children on the ketogenic diet. Pediatri. Nephrol. 2000, 15, 125–128. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.A.; Pyzik, P.L.; Rubenstein, J.E.; Hamdy, R.F.; Kossoff, E.H. Empiric use of oral potassium citrate reduces symptomatic kidney stone incidence with the ketogenic diet. Pediatrics 2009, 124, e300–e304. [Google Scholar] [CrossRef] [PubMed]
- Guzel, O.; Yılmaz, U.; Uysal, U.; Arslan, N. The effect of olive oil-based ketogenic diet on serum lipid levels in epileptic children. Neurol. Sci. 2016, 37, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Kwiterovich, P.O.; Vining, E.P.; Pyzik, P.; Skolasky, R.; Freeman, J.M. Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA 2003, 290, 912–920. [Google Scholar] [CrossRef]
- Heussinger, N.; Della Marina, A.; Beyerlin, A.; Leiendecker, B.; Hermann-Alves, S.; Dalla Pozza, R.; Klepper, J. 10 patients, 10 years—Long term follow-up of cardiovascular risk factors in Glut1 deficiency treated with ketogenic diet therapies: A prospective, multicenter case series. Clin. Nutr. 2018, 37, 2246–2251. [Google Scholar] [CrossRef]
- Stevens, C.E.; Turner, Z.; Kossoff, E.H. Hepatic dysfunction as a complication of combined ketogenic diet and valproic acid. Pediatr. Neurol. 2016, 54, 82–84. [Google Scholar] [CrossRef]
- Neal, E.G.; Chaffe, H.M.; Edwards, N.; Lawson, M.S.; Schwartz, R.H.; Cross, J.H. Growth of children on classical and medium chain triglyceride diets. Pediatrics 2008, 122, e334–e340. [Google Scholar] [CrossRef]
- Williams, S.; Basualdo-Hammond, C.; Curtis, R.; Schuller, R. Growth retardation in children with epilepsy on the ketogenic diet: A retrospective chart review. J. Am. Diet Assoc. 2002, 102, 405–407. [Google Scholar] [CrossRef]
- Groleau, V.; Schall, J.I.; Stallings, V.A.; Bergqvist, C.A. Long-term impact of the ketogenic diet on growth and resting energy expenditure in children with intractable epilepsy. Dev. Med. Child Neurol. 2014, 56, 898–904. [Google Scholar] [CrossRef]
- Kim, J.T.; Kang, H.C.; Song, J.E.; Lee, M.J.; Lee, Y.J.; Lee, E.J.; Lee, J.S.; Kim, H.D. Catch-up growth after long-term implementation and weaning from ketogenic diet in pediatric epileptic patients. Clin. Nutr. 2013, 32, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, A.G.; Schall, J.I.; Stallings, V.A. Vitamin D status in children with intractable epilepsy, and impact of the ketogenic diet. Epilepsia 2007, 48, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, S.S.; Neal, E.G.; Fitzsimmons, G.; Chaffe, H.M.; Jeanes, Y.M.; Aitkenhead, H.; Cross, J.H. The effect of the classical and medium chain triglyceride ketogenic diet on vitamin and mineral levels. J. Hum. Nutr. Diet 2012, 25, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Arslan, N.; Kose, E.; Guzel, O. The effect of ketogenic diet on serum selenium levels in patients with intractable epilepsy. Biol. Trace Elem. Res. 2017, 178, 1–6. [Google Scholar] [CrossRef]
- Bergqvist, A.G.; Chee, C.M.; Lutchka, L.; Rychik, J.; Stallings, V.A. Selenium deficiency with cardiomyopathy: A complication of the ketogenic diet. Epilepsia 2003, 44, 618–620. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef]
- Veggiotti, P.; De Giorgis, V. Dietary treatments and new therapeutic perspective in GLUT1 deficiency syndrome. Curr. Treat Options Neurol. 2014, 16, 291. [Google Scholar] [CrossRef]
- Leen, W.G.; Taher, M.; Verbeek, M.M.; Kamsteeg, E.J.; van de Warrenburg, B.P.; Willemsen, M.A. GLUT1 deficiency syndrome into adulthood: A follow-up study. J. Neurol. 2014, 261, 589–599. [Google Scholar] [CrossRef]
- Patel, A.; Pyzik, P.L.; Turner, Z.; Rubenstein, J.E.; Kossoff, E.H. Long-term outcomes of children treated with the ketogenic diet in the past. Epilepsia 2010, 51, 1277–1282. [Google Scholar] [CrossRef]
| Patients with KD (n = 26) | |
|---|---|
| Sex | 17 M (65.38%) |
| 9 F (34.62%) | |
| Type of seizure | GTC, 1 (4%) |
| Myoclonic, 7 (28%) | |
| Tonic, 4 (16%) | |
| Focal onset, 5 (20%) | |
| Atonic, 1 (4%) | |
| Typical absences, 1 (4%) | |
| Clonic, 1 (4%) | |
| Various, 5 (20%) | |
| Median age of epilepsy debut (range) | 0.97 years (2 days to 4 years) |
| Median age at introduction of KD (range) | 4 years (3 months to 12.6 years) |
| Type of KD | CKD 3:1, 11 (42.31%) |
| CKD 4:1, 4 (15.38%) | |
| MAD, 11 (42.31%) | |
| Administration route | Oral, 21 (80.77%) |
| Nasogastric tube, 2 (7.69%) | |
| Gastrostomy (G-tube), 3 (11.54%) | |
| Median length of KD (range) | 3.91 years (2–9.4 years) |
| Present situation | Off of the diet, 10 (38.5%) |
| Active, 16 (61.5%) |
| Etiology | Frequency | Percent | Specific Etiology |
|---|---|---|---|
| Unknown | 4 | 16.00 | |
| Metabolic | 10 | 40.00 | 7 GLUT1DS |
| 3 mitochondrial diseases | |||
| Structural | 5 | 20.00 | 1 cortical dysplasia |
| 4 hypoxic-ischemic structural brain abnormalities (2 perinatal problems, 1 meningitis, 1 sepsis) | |||
| Genetic disorders | 4 | 16.00 | 3 gene disorders: |
| 1 KCNQ2 gene mutation | |||
| 1 CDKL5 gene mutation | |||
| 1 Noonan syndrome (PTPN) | |||
| 1 chromosome disorders: | |||
| 1 Down syndrome | |||
| Infection | 2 | 8.00 | 1 herpes simplex virus |
| 1 Streptococcus pneumoniae |
| 1 Year | 2 Years | 3 Years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 50%–90% | 90%–100% | 100% | 50%–90% | 90%–100% | 100% | 50%–90% | 90%–100% | 100% | |
| Unknown | - | - | 3/4 | - | - | 3/4 | - | 1/2 | 1/2 |
| Metabolic | 1/10 | 1/10 | 7/10 | - | 1/10 | 9/10 | - | 1/7 | 6/7 |
| Structural | 1/5 | 1/5 | 2/5 | 2/5 | - | 2/5 | - | 1/1 | - |
| Genetic disorders | - | 1/4 | 3/4 | - | 1/4 | 3/4 | - | - | 2/2 |
| Infection | 1/2 | 1/2 | - | 2/2 | - | - | 2/2 | - | - |
| Time on the Diet | 3 Months | 6 Months | 12 Months | 2 Years | 3 Years | 4 Years | 5 Years | 6 Years |
|---|---|---|---|---|---|---|---|---|
| Total of patients suffering side effects | 15/26 | 13/26 | 21/26 | 22/26 | 11/18 | 7/12 | 3/8 | 3/6 |
| Constipation | 4 | 3 | 3 | 2 | 1 | - | 2 | 1 |
| Abdominal Pain | - | - | - | 1 | - | - | - | - |
| Nausea/vomiting | 1 | 1 | 1 | 1 | - | 1 | - | - |
| Diarrhea | - | - | - | 1 | - | - | - | - |
| Hypoglycemia | - | - | - | - | - | 1 | - | - |
| Hypercholesterolemia | 6 | 5 | 10 | 8 | 6 | 4 | 1 | 1 |
| Hypertriglyceridemia | 3 | 1 | 2 | 2 | 2 | - | - | 1 |
| Elevated AST, ALT or GGT | 3 | 2 | 1 | 2 | 1 | - | - | - |
| Hypercalciuria | 6 | 6 | 9 | 11 | 4 | 3 | - | 1 |
| Protein in urine | 1 | 4 | 3 | 3 | 1 | 1 | 1 | - |
| Fatigue | 1 | - | - | - | - | - | - | - |
| Anorexia | 1 | - | - | - | - | - | - | - |
| Hyperuricemia | 1 | - | 2 | 4 | 3 | 2 | 1 | 1 |
| Metabolic acidosis (pH) | 2 | - | 4 | 4 | 2 | - | - | - |
| Basal | 2 Years on KD | Paired Differences | ||
|---|---|---|---|---|
| Median ± SD | Median ± SD | p-Value * | ||
| (Minimum–Maximum) | ||||
| Weight | −0.252 ± 1.10 | −0.477 ± 1.33 | −0.257 ± 1.297841 | 0.3869 |
| (−2.65 – 1.95) | (−3.31 – 3.00) | |||
| Height | −0.279 ± 1.21 | −1.03 ± 1.27 | −0.644 ± 1.431242 | 0.04681 |
| (−2.4 – 2.14) | (−3.88 – 1.5) | |||
| BMI | −0.00364 ± 1.21 | 0.197 ± 1.25 | 0.248 ± 1.630604 | 0.5046 |
| (−3.31 – 1.91) | (−2.31 – 3.5) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz Herrero, J.; Cañedo Villarroya, E.; García Peñas, J.J.; García Alcolea, B.; Gómez Fernández, B.; Puerta Macfarland, L.A.; Pedrón Giner, C. Safety and Effectiveness of the Prolonged Treatment of Children with a Ketogenic Diet. Nutrients 2020, 12, 306. https://doi.org/10.3390/nu12020306
Ruiz Herrero J, Cañedo Villarroya E, García Peñas JJ, García Alcolea B, Gómez Fernández B, Puerta Macfarland LA, Pedrón Giner C. Safety and Effectiveness of the Prolonged Treatment of Children with a Ketogenic Diet. Nutrients. 2020; 12(2):306. https://doi.org/10.3390/nu12020306
Chicago/Turabian StyleRuiz Herrero, Jana, Elvira Cañedo Villarroya, Juan José García Peñas, Beatriz García Alcolea, Begoña Gómez Fernández, Laura Andrea Puerta Macfarland, and Consuelo Pedrón Giner. 2020. "Safety and Effectiveness of the Prolonged Treatment of Children with a Ketogenic Diet" Nutrients 12, no. 2: 306. https://doi.org/10.3390/nu12020306
APA StyleRuiz Herrero, J., Cañedo Villarroya, E., García Peñas, J. J., García Alcolea, B., Gómez Fernández, B., Puerta Macfarland, L. A., & Pedrón Giner, C. (2020). Safety and Effectiveness of the Prolonged Treatment of Children with a Ketogenic Diet. Nutrients, 12(2), 306. https://doi.org/10.3390/nu12020306





