Diet-Induced Obesity Disrupts Trace Element Homeostasis and Gene Expression in the Olfactory Bulb
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Tissue Collection
2.3. Trace Element Analysis
2.4. RNA Isolation and cDNA Synthesis
2.5. Real-Time Polymerase Chain Reaction (RT-PCR)
2.6. Statistical Analysis
3. Results
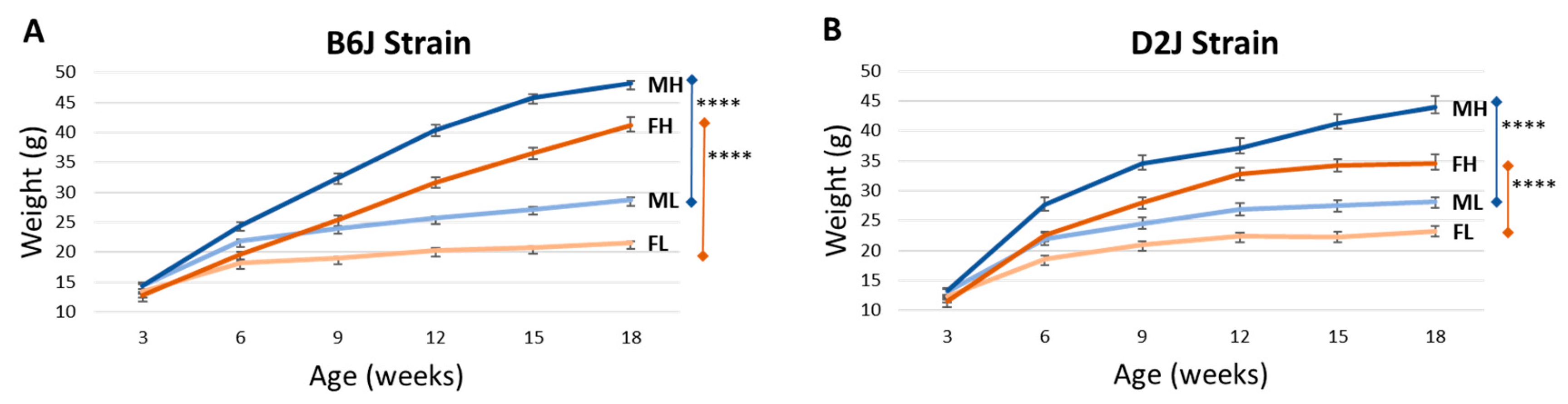
3.1. Weight Gain
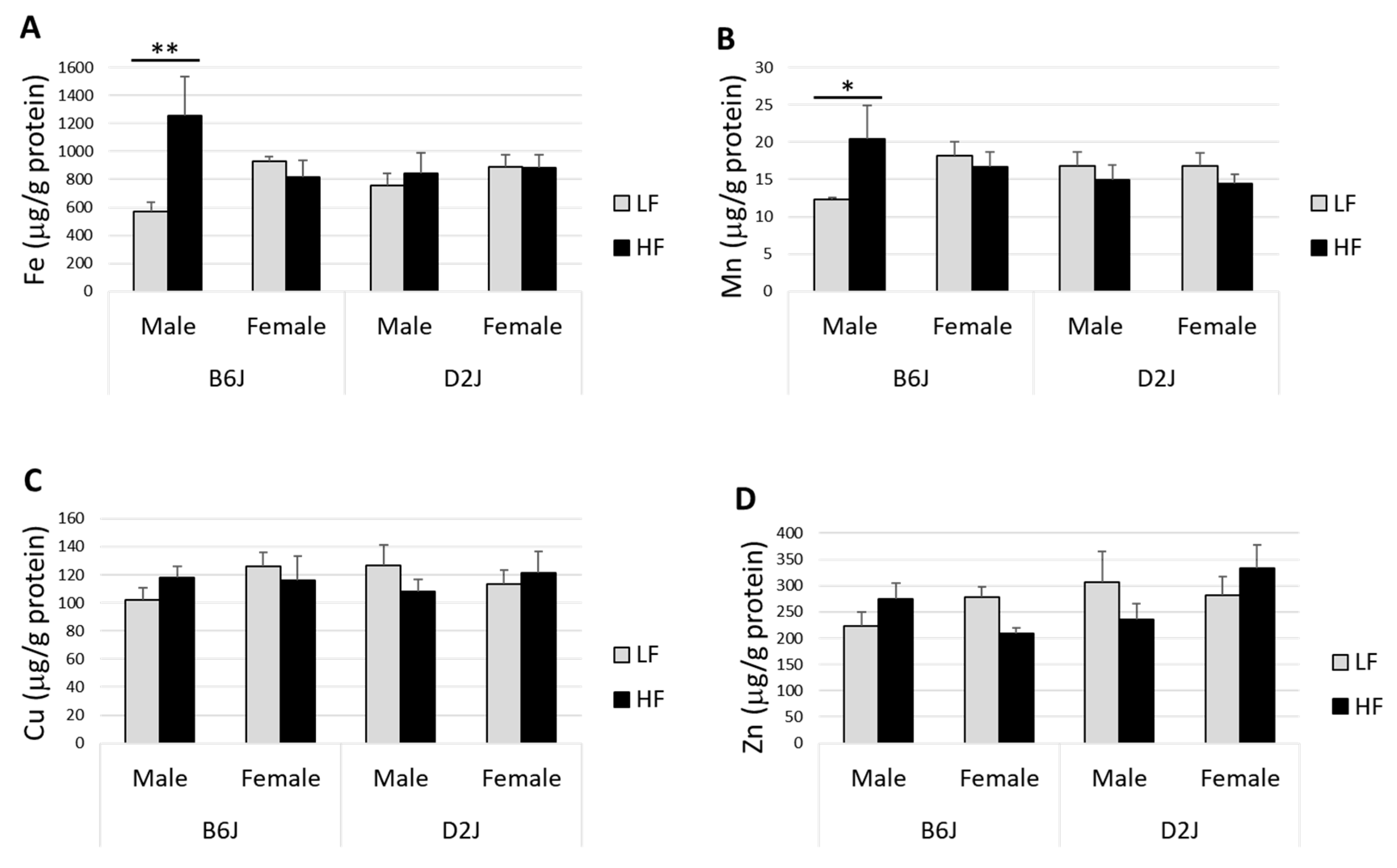
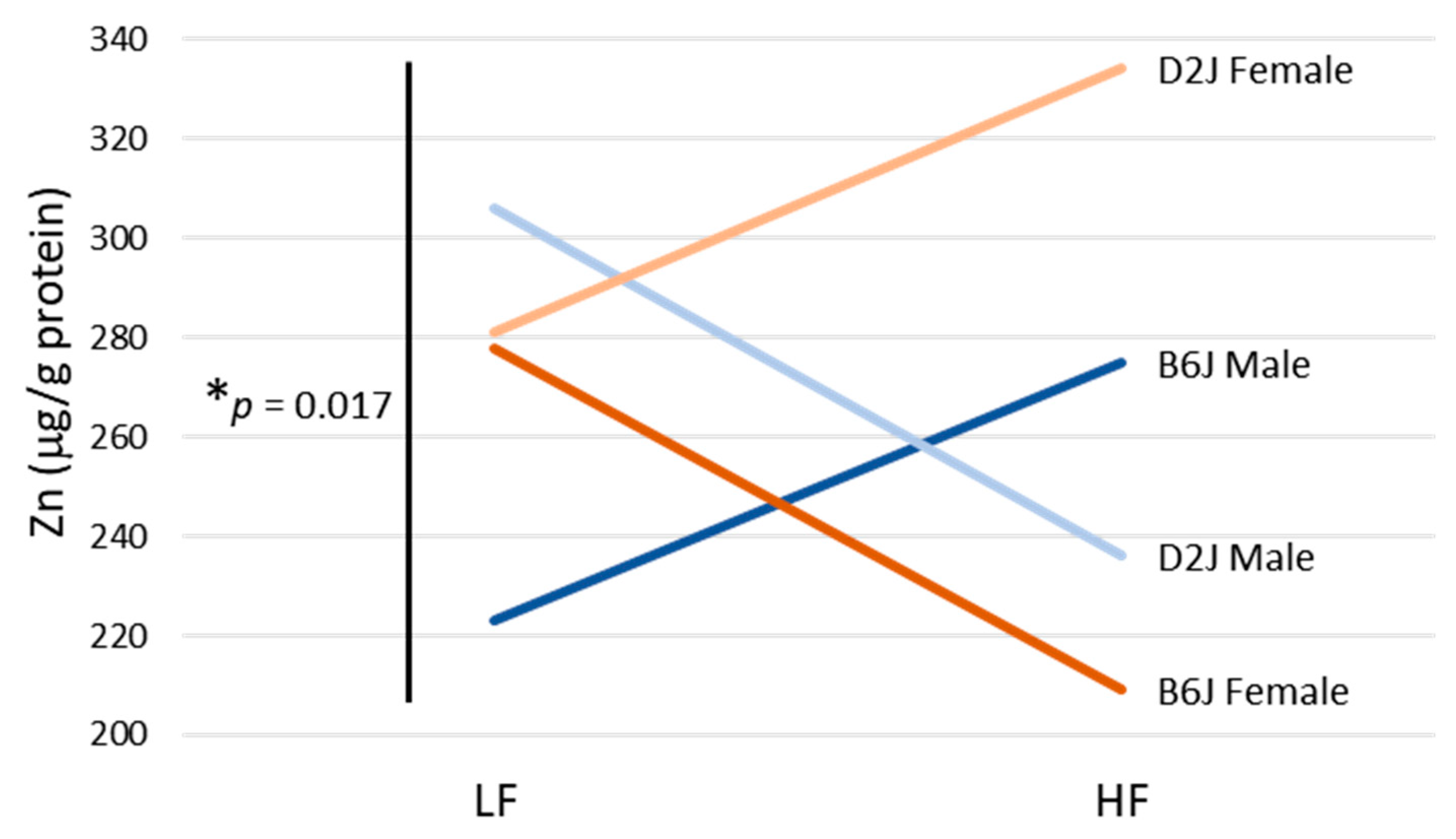
3.2. Impact of DIO on Trace Elements in the Olfactory Bulb
3.3. Impact of DIO on Gene Expression in the Olfactory Bulb
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hales, C.; Carroll, M.; Fryar, C.; Ogden, C. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018; National Center for Health Statistics: Hyattsville, MD, USA, 2020; p. 8. [Google Scholar]
- Martin-Jiménez, C.A.; Gaitán-Vaca, D.M.; Echeverria, V.; González, J.; Barreto, G.E. Relationship Between Obesity, Alzheimer’s Disease, and Parkinson’s Disease: An Astrocentric View. Mol. Neurobiol. 2017, 54, 7096–7115. [Google Scholar] [CrossRef]
- Mazon, J.N.; de Mello, A.H.; Ferreira, G.K.; Rezin, G.T. The impact of obesity on neurodegenerative diseases. Life Sci. 2017, 182, 22–28. [Google Scholar] [CrossRef]
- Brown, R.C.; Lockwood, A.H.; Sonawane, B.R. Neurodegenerative Diseases: An Overview of Environmental Risk Factors. Environ. Health Perspect. 2005, 113, 1250–1256. [Google Scholar] [CrossRef] [Green Version]
- Cristóvão, J.S.; Santos, R.; Gomes, C.M. Metals and Neuronal Metal Binding Proteins Implicated in Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Aschner, M.; Erikson, K.M.; Hernández, E.H.; Tjalkens, R. Manganese and its Role in Parkinson’s Disease: From Transport to Neuropathology. Neuromol. Med. 2009, 11, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Aboud, A.A.; Tidball, A.M.; Kumar, K.K.; Neely, M.D.; Ess, K.C.; Erikson, K.M.; Bowman, A.B. Genetic risk for Parkinson’s disease correlates with alterations in neuronal manganese sensitivity between two human subjects. NeuroToxicology 2012, 33, 1443–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fordahl, S.C.; Anderson, J.G.; Cooney, P.T.; Weaver, T.L.; Colyer, C.L.; Erikson, K.M. Manganese exposure inhibits the clearance of extracellular GABA and influences taurine homeostasis in the striatum of developing rats. NeuroToxicology 2010, 31, 639–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fordahl, S.; Cooney, P.; Qiu, Y.; Xie, G.; Jia, W.; Erikson, K.M. Waterborne manganese exposure alters plasma, brain, and liver metabolites accompanied by changes in stereotypic behaviors. Neurotoxicology Teratol. 2012, 34, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Plummer, J.; Liu, L.; Byrd, A.; Aschner, M.; Erikson, K.M. The impact of obesity on brain iron levels and α-synuclein expression is regionally dependent. Nutr. Neurosci. 2019, 22, 335–343. [Google Scholar] [CrossRef]
- Liu, L.; Byrd, A.; Plummer, J.; Erikson, K.M.; Harrison, S.H.; Han, J. The Effects of Dietary Fat and Iron Interaction on Brain Regional Iron Contents and Stereotypical Behaviors in Male C57BL/6J Mice. Front. Nutr. 2016, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Totten, M.S.; Pierce, D.M.; Erikson, K.M. The influence of sex and strain on trace element dysregulation in the brain due to diet-induced obesity. J. Trace Elem. Med. Biol. 2021, 63, 126661. [Google Scholar] [CrossRef]
- Ubeda-Bañon, I.; Saiz-Sanchez, D.; de la Rosa-Prieto, C.; Martinez-Marcos, A. α-Synuclein in the olfactory system in Parkinson’s disease: Role of neural connections on spreading pathology. Brain Struct. Funct. 2013, 219, 1513–1526. [Google Scholar] [CrossRef]
- Dusek, P.; Roos, P.M.; Litwin, T.; Schneider, S.A.; Flaten, T.P.; Aaseth, J. The neurotoxicity of iron, copper and manganese in Parkinson’s and Wilson’s diseases. J. Trace Elem. Med. Biol. 2015, 31, 193–203. [Google Scholar] [CrossRef]
- Adler, C.H.; Beach, T.G. Neuropathological basis of nonmotor manifestations of Parkinson’s disease: Neuropathology of Nonmotor PD. Mov. Disord. 2016, 31, 1114–1119. [Google Scholar] [CrossRef]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol. 2014, 127, 459–475. [Google Scholar] [CrossRef]
- Fullard, M.E.; Morley, J.F.; Duda, J.E. Olfactory Dysfunction as an Early Biomarker in Parkinson’s Disease. Neurosci. Bull. 2017, 33, 515–525. [Google Scholar] [CrossRef]
- Braak, H.; Tredici, K.D.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Mazurskyy, A.; Howitt, J. Initiation and Transmission of α-Synuclein Pathology in Parkinson’s Disease. Neurochem. Res. 2019, 44, 2685–2694. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Samudralwar, D.L.; Diprete, C.C.; Ni, B.-F.; Ehmann, W.D.; Markesbery, W.R. Elemental imbalances in the olfactory pathway in Alzheimer’s disease. J. Neurol. Sci. 1995, 130, 139–145. [Google Scholar] [CrossRef]
- Gardner, B.; Dieriks, B.V.; Cameron, S.; Mendis, L.H.S.; Turner, C.; Faull, R.L.M.; Curtis, M.A. Metal concentrations and distributions in the human olfactory bulb in Parkinson’s disease. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Galazka-Friedman, J. The history of the research of iron in parkinsonian substantia nigra. J. Neural Transm. 2012, 119, 1507–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, J.A. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol. Behav. 2018, 187, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, L.C.; Zhang, C.; Wu, Y.; Hu, W.; Zhang, Q.; Wang, J.; Li, S.; Zhao, L. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Charradi, K.; Mahmoudi, M.; Bedhiafi, T.; Kadri, S.; Elkahoui, S.; Limam, F.; Aouani, E. Dietary supplementation of grape seed and skin flour mitigates brain oxidative damage induced by a high-fat diet in rat: Gender dependency. Biomed. Pharmacother. 2017, 87, 519–526. [Google Scholar] [CrossRef]
- Malpetti, M.; Sala, A.; Vanoli, E.G.; Gianolli, L.; Luzi, L.; Perani, D. Unfavourable gender effect of high body mass index on brain metabolism and connectivity. Sci. Rep. 2018, 8, 12584. [Google Scholar] [CrossRef]
- Palacios, N.; Gao, X.; McCullough, M.L.; Jacobs, E.J.; Patel, A.V.; Mayo, T.; Schwarzschild, M.A.; Ascherio, A. Obesity, diabetes, and risk of Parkinson’s disease. Mov. Disord. 2011, 26, 2253–2259. [Google Scholar] [CrossRef] [Green Version]
- Norris, K.M.; Okie, W.; Kim, W.K.; Adhikari, R.; Yoo, S.; King, S.; Pazdro, R. A high-fat diet differentially regulates glutathione phenotypes in the obesity-prone mouse strains DBA/2J, C57BL/6J, and AKR/J. Nutr. Res. 2016, 36, 1316–1324. [Google Scholar] [CrossRef]
- Mozhui, K.; Karlsson, R.M.; Kash, T.L.; Ihne, J.; Norcross, M.; Patel, S.; Farrell, M.R.; Hill, E.E.; Graybeal, C.; Martin, K.P.; et al. Strain Differences in Stress Responsivity Are Associated with Divergent Amygdala Gene Expression and Glutamate-Mediated Neuronal Excitability. J. Neurosci. 2010, 30, 5357–5367. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.; Chang, G.Q.; Dourmashkin, J.T.; Leibowitz, S.F. Distinct phenotypes of obesity-prone AKR/J, DBA2J and C57BL/6J mice compared to control strains. Int. J. Obes. 2006, 30, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, M.K.; Hallahan, N.L.; Brown, S.H.; Liu, M.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 2013, 56, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- West, D.B.; Boozer, C.N.; Moody, D.L.; Atkinson, R.L. Dietary obesity in nine inbred mouse strains. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992, 262, R1025–R1032. [Google Scholar] [CrossRef] [PubMed]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. NeuroToxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Bjørklund, G. The role of zinc and copper in autism spectrum disorders. Acta Neurobiol. Exp. 2013, 73, 225–236. [Google Scholar]
- Giacconi, R.; Costarelli, L.; Piacenza, F.; Basso, A.; Rink, L.; Mariani, E.; Fulop, T.; Dedoussis, G.; Herbein, G.; Provinciali, M.; et al. Main biomarkers associated with age-related plasma zinc decrease and copper/zinc ratio in healthy elderly from ZincAge study. Eur. J. Nutr. 2017, 56, 2457–2466. [Google Scholar] [CrossRef]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum copper to zinc ratio: Relationship with aging and health status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Serrano-Sierra, A.; Torres-Jardón, R.; Zhu, H.; Yuan, Y.; Smith, D.; Delgado-Chávez, R.; Cross, J.V.; Medina-Cortina, H.; Kavanaugh, M.; et al. The impact of environmental metals in young urbanites’ brains. Exp. Toxicol. Pathol. 2013, 65, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, B.; Takanaga, H.; Hubert, N.; Rolfs, A.; Hediger, M.A. Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1). Biochem. J. 2007, 403, 59–69. [Google Scholar] [CrossRef]
- Arredondo, M.; Muñoz, P.; Mura, C.V.; Núñez, M.T. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am. J. Physiol. Cell Physiol. 2003, 284, C1525–C1530. [Google Scholar] [CrossRef]
- Sonnweber, T.; Ress, C.; Nairz, M.; Theurl, I.; Schroll, A.; Murphy, A.T.; Wroblewski, V.; Witcher, D.R.; Moser, P.; Ebenbichler, C.F.; et al. High-fat diet causes iron deficiency via hepcidin-independent reduction of duodenal iron absorption. J. Nutr. Biochem. 2012, 23, 1600–1608. [Google Scholar] [CrossRef]
- Citelli, M.; Fonte-Faria, T.; Nascimento-Silva, V.; Renovato-Martins, M.; Silva, R.; Luna, A.; Silva, S.; Barja-Fidalgo, C. Obesity Promotes Alterations in Iron Recycling. Nutrients 2015, 7, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Gotardo, E.M.F.; dos Santos, A.N.; Miyashiro, R.A.; Gambero, S.; Rocha, T.; Ribeiro, M.L.; Gambero, A. Mice That Are Fed a High-Fat Diet Display Increased Hepcidin Expression in Adipose Tissue. J. Nutr. Sci. Vitam. 2013, 59, 454–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupic, F. Duodenal mRNA expression of iron related genes in response to iron loading and iron deficiency in four strains of mice. Gut 2002, 51, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, R.; Garavaglia, B.; Memo, M. DMT1 Expression and Iron Levels at the Crossroads between Aging and Neurodegeneration. Front. Neurosci. 2019, 13, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.-W.; Tai, Y.K.; Chai, B.-H.; Chew, K.C.M.; Ang, E.-T.; Tsang, F.; Tan, B.W.Q.; Hong, E.T.E.; Asad, A.B.A.; Chuang, K.-H.; et al. Transgenic Mice Overexpressing the Divalent Metal Transporter 1 Exhibit Iron Accumulation and Enhanced Parkin Expression in the Brain. Neuromol. Med. 2017, 19, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Totten, M.; Zhang, Z.; Bucinca, H.; Erikson, K.; Santamaría, A.; Bowman, A.B.; Aschner, M. Iron and manganese-related CNS toxicity: Mechanisms, diagnosis and treatment. Expert. Rev. Neurother. 2019, 19, 1–18. [Google Scholar] [CrossRef]
- Chen, B.; Wen, X.; Jiang, H.; Wang, J.; Song, N.; Xie, J. Interactions between iron and α-synuclein pathology in Parkinson’s disease. Free Radic. Biol. Med. 2019, 141, 253–260. [Google Scholar] [CrossRef]
- Lingor, P.; Carboni, E.; Koch, J.C. Alpha-synuclein and iron: Two keys unlocking Parkinson’s disease. J. Neural Transm. 2017, 124, 973–981. [Google Scholar] [CrossRef]
- Tagliafierro, L.; Chiba-Falek, O. Up-regulation of SNCA gene expression: Implications to synucleinopathies. Neurogenetics 2016, 17, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Febbraro, F.; Giorgi, M.; Caldarola, S.; Loreni, F.; Romero-Ramos, M. alpha-Synuclein expression is modulated at the translational level by iron. Neuroreport 2012, 23, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Tan, E.-K. Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol. Neurodegener. 2017, 12, 75. [Google Scholar] [CrossRef]
- Wang, S.; Bolós, M.; Clark, R.; Cullen, C.L.; Southam, K.A.; Foa, L.; Dickson, T.C.; Young, K.M. Amyloid β precursor protein regulates neuron survival and maturation in the adult mouse brain. Mol. Cell. Neurosci. 2016, 77, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Roher, A.E.; Kokjohn, T.A.; Clarke, S.G.; Sierks, M.R.; Maarouf, C.L.; Serrano, G.E.; Sabbagh, M.S.; Beach, T.G. APP/Aβ structural diversity and Alzheimer’s disease pathogenesis. Neurochem. Int. 2017, 110, 1–13. [Google Scholar] [CrossRef]
- Lachen-Montes, M.; González-Morales, A.; Palomino, M.; Ausin, K.; Gómez-Ochoa, M.; Zelaya, M.V.; Ferrer, I.; Pérez-Mediavilla, A.; Fernández-Irigoyen, J.; Santamaría, E. Early-Onset Molecular Derangements in the Olfactory Bulb of Tg2576 Mice: Novel Insights into the Stress-Responsive Olfactory Kinase Dynamics in Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsolakidou, A.; Czibere, L.; Pütz, B.; Trümbach, D.; Panhuysen, M.; Deussing, J.M.; Wurst, W.; Sillaber, I.; Landgraf, R.; Holsboer, F.; et al. Gene expression profiling in the stress control brain region hypothalamic paraventricular nucleus reveals a novel gene network including Amyloid beta Precursor Protein. BMC Genom. 2010, 11, 546. [Google Scholar] [CrossRef] [Green Version]
- Goldblum, D.; Kipfer-Kauer, A.; Sarra, G.-M.; Wolf, S.; Frueh, B.E. Distribution of Amyloid Precursor Protein and Amyloid-β Immunoreactivity in DBA/2J Glaucomatous Mouse Retinas. Invest. Ophthalmol. Vis. Sci. 2007, 48, 5085. [Google Scholar] [CrossRef] [Green Version]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-F.; Yu, Y.; Zavitsanou, K.; Han, M.; Storlien, L. Differential expression of dopamine D2 and D4 receptor and tyrosine hydroxylase mRNA in mice prone, or resistant, to chronic high-fat diet-induced obesity. Mol. Brain Res. 2005, 135, 150–161. [Google Scholar] [CrossRef]
- Lee, A.K.; Mojtahed-Jaberi, M.; Kyriakou, T.; Aldecoa-Otalora Astarloa, E.; Arno, M.; Marshall, N.J.; Brain, S.D.; O’Dell, S.D. Effect of high-fat feeding on expression of genes controlling availability of dopamine in mouse hypothalamus. Nutrition 2010, 26, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Gallo, E.F. Disentangling the diverse roles of dopamine D2 receptors in striatal function and behavior. Neurochem. Int. 2019, 125, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Singh, S.; Shukla, S. Physiological and Functional Basis of Dopamine Receptors and Their Role in Neurogenesis: Possible Implication for Parkinson’s disease. J. Exp. Neurosci. 2018, 12, 117906951877982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, G.; O’Dowd, B.; George, S. Genotypic Differences in Brain Dopamine Receptor Function in the DBA/2J and C57BL/6J Inbred Mouse Strains. Eur. J. Pharm. 1994, 269, 349–364. [Google Scholar] [CrossRef]
- Carlin, J.; Hill-Smith, T.E.; Lucki, I.; Reyes, T.M. Reversal of dopamine system dysfunction in response to high-fat diet: High-Fat Diet and Dopamine: Recovery. Obesity 2013, 21, 2513–2521. [Google Scholar] [CrossRef] [Green Version]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 6, 1164–1178. [Google Scholar] [CrossRef]
- Genzer, Y.; Dadon, M.; Burg, C.; Chapnik, N.; Froy, O. Effect of dietary fat and the circadian clock on the expression of brain-derived neurotrophic factor (BDNF). Mol. Cell. Endocrinol. 2016, 430, 49–55. [Google Scholar] [CrossRef]
- Gan, L.; England, E.; Yang, J.-Y.; Toulme, N.; Ambati, S.; Hartzell, D.L.; Meagher, R.B.; Baile, C.A. A 72-hour high fat diet increases transcript levels of the neuropeptide galanin in the dorsal hippocampus of the rat. BMC Neurosci. 2015, 16, 51. [Google Scholar] [CrossRef] [Green Version]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef] [Green Version]

| B6J | D2J | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| LFD | 9 | 9 | 9 | 9 |
| HFD | 9 | 9 | 9 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Totten, M.S.; Pierce, D.M.; Erikson, K.M. Diet-Induced Obesity Disrupts Trace Element Homeostasis and Gene Expression in the Olfactory Bulb. Nutrients 2020, 12, 3909. https://doi.org/10.3390/nu12123909
Totten MS, Pierce DM, Erikson KM. Diet-Induced Obesity Disrupts Trace Element Homeostasis and Gene Expression in the Olfactory Bulb. Nutrients. 2020; 12(12):3909. https://doi.org/10.3390/nu12123909
Chicago/Turabian StyleTotten, Melissa S., Derek M. Pierce, and Keith M. Erikson. 2020. "Diet-Induced Obesity Disrupts Trace Element Homeostasis and Gene Expression in the Olfactory Bulb" Nutrients 12, no. 12: 3909. https://doi.org/10.3390/nu12123909
APA StyleTotten, M. S., Pierce, D. M., & Erikson, K. M. (2020). Diet-Induced Obesity Disrupts Trace Element Homeostasis and Gene Expression in the Olfactory Bulb. Nutrients, 12(12), 3909. https://doi.org/10.3390/nu12123909






