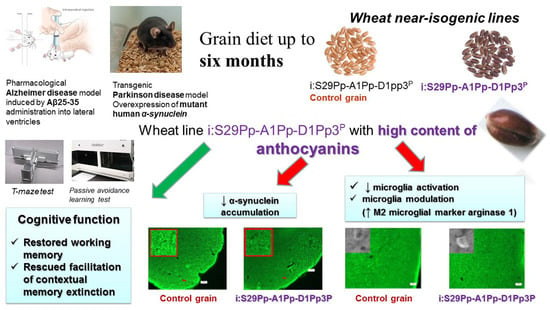
Evaluating the Effects of Grain of Isogenic Wheat Lines Differing in the Content of Anthocyanins in Mouse Models of Neurodegenerative Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Procedures Involving Animals
2.1.1. Experimental Design and Treatment (Diets)
2.1.2. The Model of AD
2.2. Behavioral Tests
2.2.1. The Open Field Test
2.2.2. The Passive Avoidance Test
2.2.3. The T-Maze Test
2.2.4. Barnes Maze Test
2.3. IHC Analysis
2.4. Biochemical Assays
2.5. Data Analysis
3. Results
3.1. Effects of Grain Diet on Body Weight Gain
3.2. Effects on Biochemical Parameters of Serum
3.3. Behavioral Effects
3.3.1. The Open Field Test
3.3.2. The T-Maze Test
3.3.3. Barnes Test
3.3.4. The Passive Avoidance Test
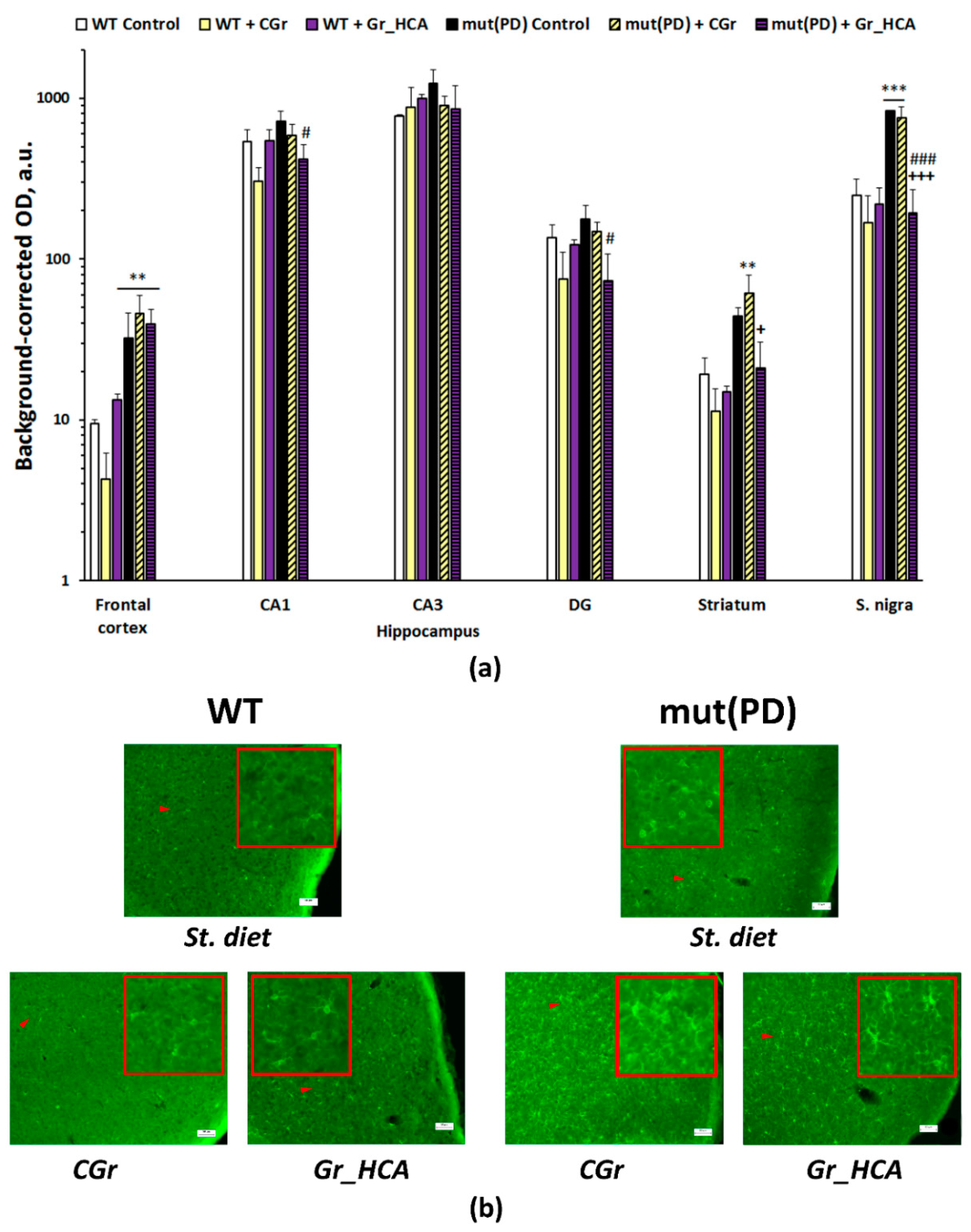
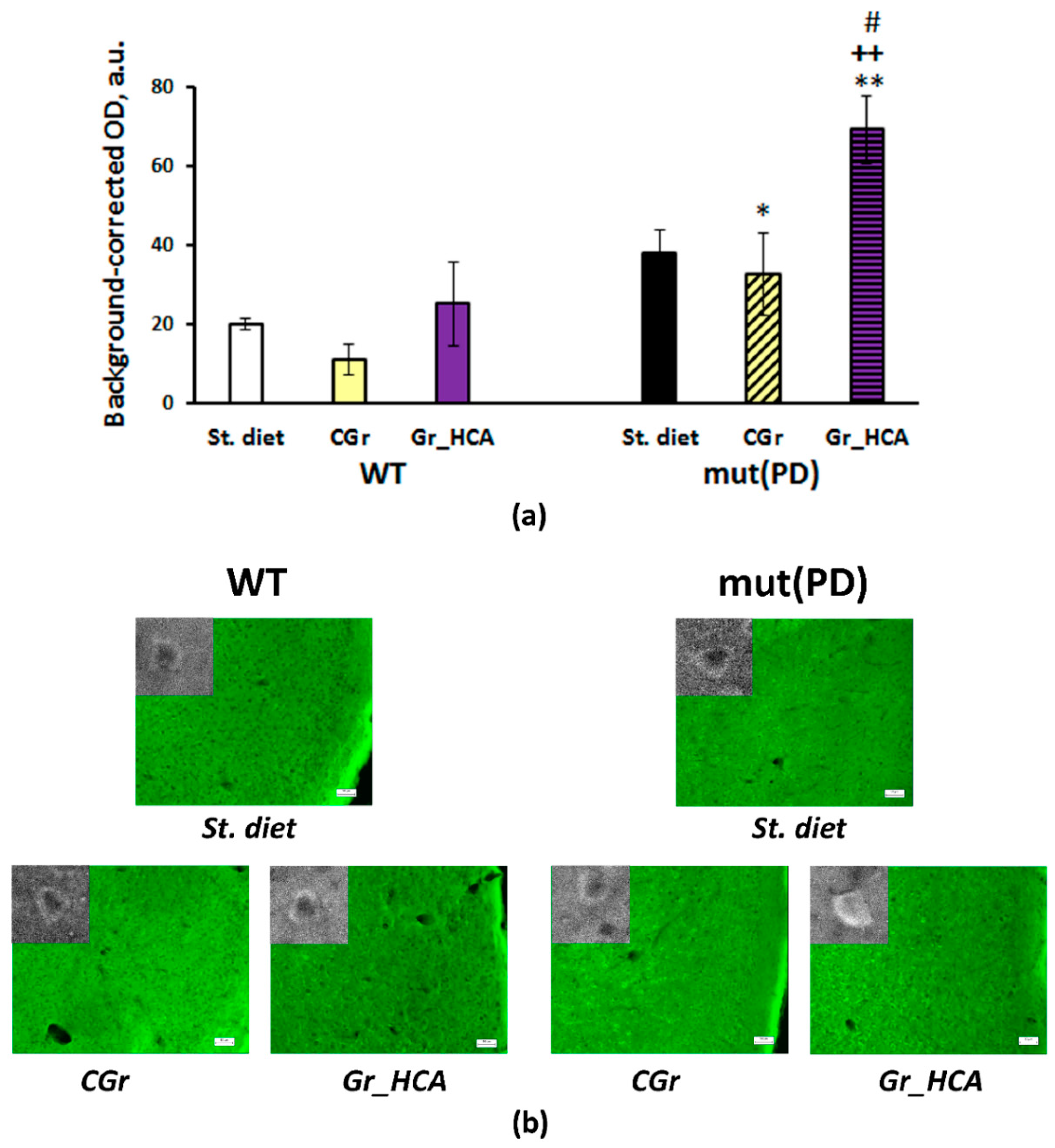
3.4. IHC Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, E.G.; Tanner, C.M. Impaired Cognition and the Risk of Parkinson Disease: Trouble in Mind. JAMA Neurol. 2017, 74, 1398–1400. [Google Scholar] [CrossRef]
- Jellinger, K.A. Dementia with Lewy bodies and Parkinson’s disease-dementia: Current concepts and controversies. J. Neural Transm. 2018, 125, 615–650. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharm. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Antonini, A.; Zijlmans, J.C.; Burkhard, P.R.; Vingerhoets, F. Levodopa in the treatment of Parkinson’s disease: An old drug still going strong. Clin. Interv. Aging 2010, 5, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.K.; Dandapat, J.; Dash, U.C.; Kanhar, S. Features and outcomes of drugs for combination therapy as multi-targets strategy to combat Alzheimer’s disease. J. Ethnopharmacol. 2018, 215, 42–73. [Google Scholar] [CrossRef] [PubMed]
- Frozza, R.L.; Lourenco, M.V.; De Felice, F.G. Challenges for Alzheimer’s Disease Therapy: Insights from Novel Mechanisms Beyond Memory Defects. Front. Neurosci. 2018, 12, 37. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 316, 149–157. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural Antioxidant Anthocyanins—A Hidden Therapeutic Candidate in Metabolic Disorders with Major Focus in Neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Zia Ul Haq, M.; Saad, B. Anthocyanins and Human Health: Biomolecular and Therapeutic Aspects; Springer International Publishing: Madison, WI, USA, 2016; p. 138. [Google Scholar] [CrossRef]
- Ficco, D.B.; De Simone, V.; Colecchia, S.A.; Pecorella, I.; Platani, C.; Nigro, F.; Finocchiaro, F.; Papa, R.; De Vita, P. Genetic variability in anthocyanin composition and nutritional properties of blue, purple, and red bread (Triticum aestivum L.) and durum (Triticum turgidum L. ssp. turgidum convar. durum) wheats. J. Agric. Food Chem. 2014, 62, 8686–8695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, E.I.; Shoeva, O.Y.; Khlestkina, E.K. Marker-assisted development of bread wheat near-isogenic lines carrying various combinations of purple pericarp (Pp) alleles. Euphytica 2015, 203, 469–476. [Google Scholar] [CrossRef]
- Morris, C.F.; McLean, D.; Engleson, J.A.; Fuerst, E.P.; Burgos, F.; Coburn, E. Some observations on the granivorous feeding behavior preferences of the house mouse (Mus musculus L.). Mammalia 2012, 76, 209–218. [Google Scholar] [CrossRef]
- Beloshapka, A.N.; Buff, P.R.; Fahey, G.C.; Swanson, K.S. Compositional Analysis of Whole Grains, Processed Grains, Grain Co-Products, and Other Carbohydrate Sources with Applicability to Pet Animal Nutrition. Foods 2016, 5, 23. [Google Scholar] [CrossRef]
- Morgounov, A.; Karaduman, Y.; Akin, B.; Aydogan, S.; Baenziger, P.S.; Bhatta, M.; Chudinov, V.; Dreisigacker, S.; Govindan, V.; Güler, S.; et al. Yield and quality in purple-grained wheat isogenic lines. Agronomy 2020, 10, 86. [Google Scholar] [CrossRef]
- Choi, J.Y.; Cho, E.J.; Lee, H.S.; Lee, J.M.; Yoon, Y.H.; Lee, S. Tartary buckwheat improves cognition and memory function in an in vivo amyloid-beta-induced Alzheimer model. Food Chem. Toxicol. 2013, 53, 105–111. [Google Scholar] [CrossRef]
- Lee, A.Y.; Choi, J.M.; Lee, Y.A.; Shin, S.H.; Cho, E.J. Beneficial effect of black rice (Oryza sativa L. var. japonica) extract on amyloid beta-induced cognitive dysfunction in a mouse model. Exp. Med. 2020, 20, 64. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.H.; Bae, S.S.; Hong, K.W.; Lee, D.S.; Leem, J.Y.; Choi, B.T.; Shin, H.K. Protective effect of the phosphodiesterase III inhibitor cilostazol on amyloid beta-induced cognitive deficits associated with decreased amyloid beta accumulation. Biochem. Biophys. Res. Commun. 2011, 408, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, 4th ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Boston, MI, USA, 2013; p. 360. [Google Scholar]
- Pupyshev, A.B.; Tikhonova, M.A.; Akopyan, A.A.; Tenditnik, M.V.; Dubrovina, N.I.; Korolenko, T.A. Therapeutic activation of autophagy by combined treatment with rapamycin and trehalose in a mouse MPTP-induced model of Parkinson’s disease. Pharm. Biochem. Behav. 2019, 177, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.; Rawlins, J.N. T-maze alternation in the rodent. Nat. Protoc. 2006, 1, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.M.; Magda, G.; Abel, S. Spatial memory: Theoretical basis and comparative review on experimental methods in rodents. Behav. Brain Res. 2009, 203, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, P.A. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004, 28, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Lipina, T.V.; Prasad, T.; Yokomaku, D.; Luo, L.; Connor, S.A.; Kawabe, H.; Wang, Y.T.; Brose, N.; Roder, J.C.; Craig, A.M. Cognitive Deficits in Calsyntenin-2-deficient Mice Associated with Reduced GABAergic Transmission. Neuropsychopharmacology 2016, 41, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, M.A.; Ho, S.C.; Akopyan, A.A.; Kolosova, N.G.; Weng, J.C.; Meng, W.Y.; Lin, C.L.; Amstislavskaya, T.G.; Ho, Y.J. Neuroprotective effects of ceftriaxone treatment on cognitive and neuronal deficits in a rat model of accelerated senescence. Behav. Brain Res. 2017, 330, 8–16. [Google Scholar] [CrossRef]
- Garelick, M.G.; Storm, D.R. The relationship between memory retrieval and memory extinction. Proc. Natl. Acad. Sci. USA 2005, 102, 9091–9092. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13)C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S. A theoretical physiologically based pharmacokinetic approach for modeling the fate of anthocyanins in vivo. Crit. Rev. Food Sci. Nutr. 2017, 57, 3197–3207. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramirez, B.A.; Catalan, U.; Fernandez-Castillejo, S.; Rubio, L.; Macia, A.; Sola, R. Anthocyanin Tissue Bioavailability in Animals: Possible Implications for Human Health. A Systematic Review. J. Agric. Food Chem. 2018, 66, 11531–11543. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999, 19, 8114–8121. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef]
- Shih, P.H.; Chan, Y.C.; Liao, J.W.; Wang, M.F.; Yen, G.C. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J. Nutr. Biochem. 2010, 21, 598–605. [Google Scholar] [CrossRef]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.L.; Simon, J.E.; Lila, M.A.; Rochet, J.C. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinsons disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef]
- Ramos, P.; Herrera, R.; Moya-León, M.A. Anthocyanins: Food Sources and Benefits to Consumer‘s Health. In Handbook of Anthocyanins: Food Sources, Chemical Applications and Health Benefits; Warner, L.M., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2014; pp. 363–384. [Google Scholar]
- Strygina, K.V.; Khlestkina, E.K. Anthocyanins synthesis in potato (Solanum tuberosum L.): Genetic markers for smart breeding. Sel’skokhozyaistvennaya Biol. Agric. Biol. 2017, 52, 37–49. [Google Scholar] [CrossRef][Green Version]
- Bartl, P.; Albreht, A.; Skrt, M.; Tremlova, B.; Ostadalova, M.; Smejkal, K.; Vovk, I.; Ulrih, N.P. Anthocyanins in purple and blue wheat grains and in resulting bread: Quantity, composition, and thermal stability. Int. J. Food Sci. Nutr. 2015, 66, 514–519. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Usenko, N.I.; Gordeeva, E.I.; Stabrovskaya, O.I.; Sharfunova, I.B.; Otmakhova, Y.S. Evaluation of wheat products with high flavonoid content: Justification of importance of marker-assisted development and production of flavonoid-rich wheat cultivars. Vavilovskii Zhurnal Genet. I Sel. (Vavilov J. Genet. Breed.) 2017, 21, 545–553. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, J.; Li, Y.; Wang, C. Quality of noodles made from colour-grained wheat. Czech. J. Food Sci. 2018, 36, 314–320. [Google Scholar] [CrossRef]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F.; Blanco, A. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015, 180, 64–70. [Google Scholar] [CrossRef]
- Usenko, N.I.; Khlestkina, E.K.; Asavasanti, S.; Gordeeva, E.I.; Yudina, R.S.; Otmakhova, Y.S. Possibilities of enriching food products with anthocyanins by using new forms of cereals. Foods Raw Mater. 2018, 6, 128–135. [Google Scholar] [CrossRef]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar] [PubMed]
- Rozanska, D.; Regulska-Ilow, B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv. Clin. Exp. Med. 2018, 27, 135–142. [Google Scholar] [CrossRef]
- Farrell, K.F.; Krishnamachari, S.; Villanueva, E.; Lou, H.; Alerte, T.N.; Peet, E.; Drolet, R.E.; Perez, R.G. Non-motor parkinsonian pathology in aging A53T alpha-synuclein mice is associated with progressive synucleinopathy and altered enzymatic function. J. Neurochem. 2014, 128, 536–546. [Google Scholar] [CrossRef]
- Paumier, K.L.; Sukoff Rizzo, S.J.; Berger, Z.; Chen, Y.; Gonzales, C.; Kaftan, E.; Li, L.; Lotarski, S.; Monaghan, M.; Shen, W.; et al. Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson’s disease. PLoS ONE 2013, 8, e70274. [Google Scholar] [CrossRef]
- Unger, E.L.; Eve, D.J.; Perez, X.A.; Reichenbach, D.K.; Xu, Y.; Lee, M.K.; Andrews, A.M. Locomotor hyperactivity and alterations in dopamine neurotransmission are associated with overexpression of A53T mutant human alpha-synuclein in mice. Neurobiol. Dis. 2006, 21, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Selkoe, D.J. A beta oligomers-a decade of discovery. J. Neurochem. 2007, 101, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Mroczko, B.; Groblewska, M.; Litman-Zawadzka, A.; Kornhuber, J.; Lewczuk, P. Amyloid beta oligomers (AbetaOs) in Alzheimer’s disease. J. Neural Transm. 2018, 125, 177–191. [Google Scholar] [CrossRef]
- Pike, C.J.; Walencewicz-Wasserman, A.J.; Kosmoski, J.; Cribbs, D.H.; Glabe, C.G.; Cotman, C.W. Structure-activity analyses of beta-amyloid peptides: Contributions of the beta 25-35 region to aggregation and neurotoxicity. J. Neurochem. 1995, 64, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Yankner, B.A.; Duffy, L.K.; Kirschner, D.A. Neurotrophic and neurotoxic effects of amyloid beta protein: Reversal by tachykinin neuropeptides. Science 1990, 250, 279–282. [Google Scholar] [CrossRef] [PubMed]
- El Bitar, F.; Meunier, J.; Villard, V.; Almeras, M.; Krishnan, K.; Covey, D.F.; Maurice, T.; Akwa, Y. Neuroprotection by the synthetic neurosteroid enantiomers ent-PREGS and ent-DHEAS against Abeta(2)(5)(-)(3)(5) peptide-induced toxicity in vitro and in vivo in mice. Psychopharmacology 2014, 231, 3293–3312. [Google Scholar] [CrossRef]
- El-Shiekh, R.A.; Ashour, R.M.; Abd El-Haleim, E.A.; Ahmed, K.A.; Abdel-Sattar, E. Hibiscus sabdariffa L. A potent natural neuroprotective agent for the prevention of streptozotocin-induced Alzheimer’s disease in mice. Biomed. Pharm. 2020, 128, 110303. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Jiang, Y.; Xu, Y.; Zhao, H.; Lyu, X.; Wu, T. Bilberry anthocyanins improve neuroinflammation and cognitive dysfunction in APP/PSEN1 mice via the CD33/TREM2/TYROBP signaling pathway in microglia. Food Funct. 2020, 11, 1572–1584. [Google Scholar] [CrossRef]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Jagmag, S.A.; Tripathi, N.; Shukla, S.D.; Maiti, S.; Khurana, S. Evaluation of Models of Parkinson’s Disease. Front. Neurosci. 2015, 9, 503. [Google Scholar] [CrossRef]
- Oaks, A.W.; Frankfurt, M.; Finkelstein, D.I.; Sidhu, A. Age-dependent effects of A53T alpha-synuclein on behavior and dopaminergic function. PLoS ONE 2013, 8, e60378. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Tada, Y.; Muroi, Y.; Unno, T.; Ishii, T. Selective loss of dopaminergic neurons in the substantia nigra pars compacta after systemic administration of MPTP facilitates extinction learning. Life Sci. 2015, 137, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.I.; Muroi, Y.; Unno, T.; Ishii, T. Rolipram improves facilitation of contextual fear extinction in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. J. Pharm. Sci. 2017, 134, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Alvarez-Fernandez, M.A.; Cerezo, A.B.; Richard, T.; Troncoso, A.M.A.; Garcia-Parrilla, M.A.C. Protocatechuic Acid: Inhibition of Fibril Formation, Destabilization of Preformed Fibrils of Amyloid-beta and alpha-Synuclein, and Neuroprotection. J. Agric. Food Chem. 2016, 64, 7722–7732. [Google Scholar] [CrossRef] [PubMed]
- Pogacnik, L.; Pirc, K.; Palmela, I.; Skrt, M.; Kim, K.S.; Brites, D.; Brito, M.A.; Ulrih, N.P.; Silva, R.F. Potential for brain accessibility and analysis of stability of selected flavonoids in relation to neuroprotection in vitro. Brain Res. 2016, 1651, 17–26. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Meireles, M.; Marques, C.; Norberto, S.; Santos, P.; Fernandes, I.; Mateus, N.; Faria, A.; Calhau, C. Anthocyanin effects on microglia M1/M2 phenotype: Consequence on neuronal fractalkine expression. Behav. Brain Res. 2016, 305, 223–228. [Google Scholar] [CrossRef]
- Park, H.J.; Oh, S.H.; Kim, H.N.; Jung, Y.J.; Lee, P.H. Mesenchymal stem cells enhance alpha-synuclein clearance via M2 microglia polarization in experimental and human parkinsonian disorder. Acta Neuropathol. 2016, 132, 685–701. [Google Scholar] [CrossRef]




| Parameter | Group | F, p | ||
|---|---|---|---|---|
| St. diet | CGr | Gr_HCA | ||
| Indices of liver function | ||||
| ALT, U/L | 52.2 ± 7.68 | 41.6 ± 7.0 | 54.8 ± 8.6 | F(2, 12) < 1 |
| AST, U/L | 272.4 ± 15.7 | 286.5 ± 25.8 | 326.1 ± 8.1 | F(2, 12) = 1.5, p > 0.05 |
| Total bilirubin, mmol/L | 1.86 ± 0.82 | 2.04 ± 0.63 | 1.20 ± 1.05 | F(2, 12) < 1 |
| Indices of kidney function and protein metabolism | ||||
| Creatinine, μmol/L | 56.0 ± 3.9 | 55.3 ± 3.6 | 65.4 ± 0.6 | F(2, 12) = 1.9, p > 0.05 |
| Uric acid, mmol/L | 191.2 ± 15.4 | 132.2 ± 11.9 (#) | 189.1 ± 14.2 (+) | F(2, 12) = 5.95, p < 0.05 |
| Indices of lipid metabolism | ||||
| Total cholesterol, mmol/L | 2.12 ± 0.09 | 4.51 ± 0.19 (###) | 4.98 ± 0.34 (###) | F(2, 12) = 66.0, p < 0.001 |
| Triglycerides, mmol/L | 0.91 ± 0.09 | 0.73 ± 0.05 | 0.82 ± 0.02 | F(2, 12) = 2.0, p > 0.05 |
| LDL-C, mmol/L | 0.43 ± 0.01 | 0.89 ± 0.04 (###) | 1.04 ± 0.06 (###, +) | F(2, 12) = 73.8, p < 0.001 |
| HDL, mmol/L (α-cholesterol) | 0.95 ± 0.06 | 2.27 ± 0.12 (###) | 2.51 ± 0.23 (###) | F(2, 12) = 47.1, p < 0.001 |
| Atherogenic coefficient | 1.24 ± 0.07 | 0.99 ± 0.03 (##) | 0.99 ± 0.06 (#) | F(2, 12) = 7.1, p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhonova, M.A.; Shoeva, O.Y.; Tenditnik, M.V.; Ovsyukova, M.V.; Akopyan, A.A.; Dubrovina, N.I.; Amstislavskaya, T.G.; Khlestkina, E.K. Evaluating the Effects of Grain of Isogenic Wheat Lines Differing in the Content of Anthocyanins in Mouse Models of Neurodegenerative Disorders. Nutrients 2020, 12, 3877. https://doi.org/10.3390/nu12123877
Tikhonova MA, Shoeva OY, Tenditnik MV, Ovsyukova MV, Akopyan AA, Dubrovina NI, Amstislavskaya TG, Khlestkina EK. Evaluating the Effects of Grain of Isogenic Wheat Lines Differing in the Content of Anthocyanins in Mouse Models of Neurodegenerative Disorders. Nutrients. 2020; 12(12):3877. https://doi.org/10.3390/nu12123877
Chicago/Turabian StyleTikhonova, Maria A., Olesya Yu. Shoeva, Michael V. Tenditnik, Marina V. Ovsyukova, Anna A. Akopyan, Nina I. Dubrovina, Tamara G. Amstislavskaya, and Elena K. Khlestkina. 2020. "Evaluating the Effects of Grain of Isogenic Wheat Lines Differing in the Content of Anthocyanins in Mouse Models of Neurodegenerative Disorders" Nutrients 12, no. 12: 3877. https://doi.org/10.3390/nu12123877
APA StyleTikhonova, M. A., Shoeva, O. Y., Tenditnik, M. V., Ovsyukova, M. V., Akopyan, A. A., Dubrovina, N. I., Amstislavskaya, T. G., & Khlestkina, E. K. (2020). Evaluating the Effects of Grain of Isogenic Wheat Lines Differing in the Content of Anthocyanins in Mouse Models of Neurodegenerative Disorders. Nutrients, 12(12), 3877. https://doi.org/10.3390/nu12123877