Ketogenic Diet: A Dietary Modification as an Anxiolytic Approach?
Abstract
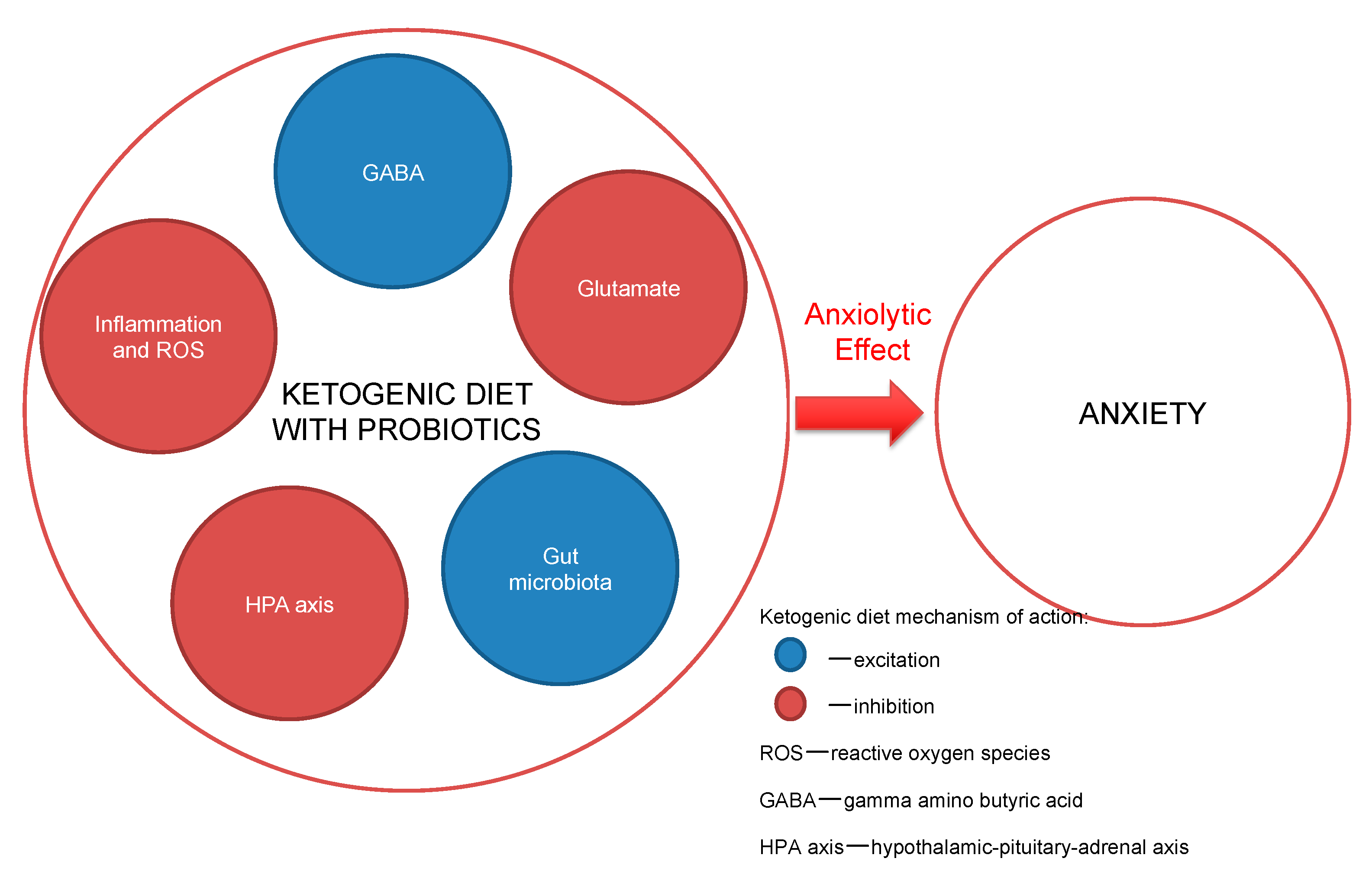
:1. Introduction
1.1. Neurotransmission and Gut-Microbiota Interplay in Anxiety
1.1.1. Monoamines
1.1.2. Hypothalamic-Pituitary-Adrenal Axis, Divalent Ions, Inflammation, and Reactive Oxygen Species in Anxiety
1.1.3. Excess Glutamate
1.1.4. GABA Deficiency
1.1.5. Gut Microbiota
1.2. Low Carbohydrate Diets and Their Hypothesized Impact on Anxiety Treatment
1.2.1. Low-Carbohydrate Diets
1.2.2. Gut Microbiota and the Steroid Pathway in the Potentiation of GABA Transmission in Low-Carbohydrate Diets
1.2.3. Anti-Inflammatory Effect of the Ketogenic Diet and Fatty Acids
1.2.4. The ‘Ketogenic Menu’
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bandelow, B.; Michaelis, S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [PubMed]
- World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: https://apps.who.int/iris/handle/10665/42980 (accessed on 20 September 2020).
- Kessler, R.C.; Petukhova, M.; Sampson, N.A.; Zaslavsky, A.M.; Wittchen, H.-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatry Res. 2012, 21, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.C.; Portera, L.; Weissman, M.M. The social costs of anxiety disorders. Br. J. Psychiatry Suppl. 1995, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.J.; Vos, T.; Scott, K.M.; Ferrari, A.J.; Whiteford, H.A. The global burden of anxiety disorders in 2010. Psychol. Med. 2014, 44, 2363–2374. [Google Scholar] [CrossRef]
- Stein, D.J.; Scott, K.M.; de Jonge, P.; Kessler, R.C. Epidemiology of anxiety disorders: From surveys to nosology and back. Dialogues Clin. Neurosci. 2017, 19, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Andlin-Sobocki, P.; Wittchen, H.U. Cost of anxiety disorders in Europe. Eur. J. Neurol. 2005, 12, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Maron, E.; Nutt, D. Biological markers of generalized anxiety disorder. Dialogues Clin. Neurosci. 2017, 19, 147–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoya, A.; Bruins, R.; 1 Katzman, M.A.; Blier, P. The noradrenergic paradox: Implications in the management of depression and anxiety. Neuropsychiatr. Dis. Treat. 2016, 12, 541–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickels, K.; Mangano, R.; Khan, A. A double-blind, placebo-controlled study of a flexible dose of venlafaxine ER in adult outpatients with generalized social anxiety disorder. J. Clin. Psychopharmacol. 2004, 24, 488–496. [Google Scholar] [CrossRef]
- Rickels, K.; Zaninelli, R.; McCafferty, J.; Bellew, K.; Iyengar, M.; Sheehan, D. Paroxetine treatment of generalized anxiety disorder: A double-blind, placebo-controlled study. Am. J. Psychiatry 2003, 160, 749–756. [Google Scholar] [CrossRef]
- Jakuszkowiak-Wojten, K.; Landowski, J.; Wiglusz, M.S.; Cubała, W.J. Cortisol as an indicator of hypothalmic-pitituary-adrenal axis dysregulation in patients with panic disorder: A literature review. Psychiatr. Danub. 2015, 27, S445–S451. [Google Scholar] [PubMed]
- Singewald, N.; Sinner, C.; Hetzenauer, A.; Sartori, S.B.; Murck, H. Magnesium deficient diet alter depression-and anxiety-related behavior in mice—Influence of desipramine and Hypericum perforatum extract. Neuropharmacology 2004, 47, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A. Probiotics supplementation in prophylaxis and treatment of depressive and anxiety disorders - a review of current research. Psychiatr. Pol. 2019, 53, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Overland, S.; Stewart, R.; Tell, G.S.; Bjelland, I.; Mykletun, A. Association between magnesium intake and depression and anxiety in community-dwelling adults: The Hordaland Health Study. Aust. N. Z. J. Psychiatry 2009, 43, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kuloglu, M.; Atmaca, M.; Tezcan, E.; Ustundag, B.; Bulut, S. Antioxidant enzyme and malondialdehyde levels in patients with panic disorder. Neuropsychobiology 2002, 46, 186–189. [Google Scholar] [CrossRef]
- Kuloglu, M.; Atmaca, M.; Tezcan, E.; Gecici, O.; Tunckol, H.; Ustundag, B. Antioxidant enzyme activities and malondialdehyde levels in patients with obsessive-compulsive disorder. Neuropsychobiology 2002, 46, 27–32. [Google Scholar] [CrossRef]
- Hassan, W.; Silva, C.E.; Mohammadzai, I.U.; da Rocha, J.B.; Landeira-Fernandez, J. Association of oxidative stress to the genesis of anxiety: Implications for possible therapeutic interventions. Curr. Neuropharmacol. 2014, 12, 120–139. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, S.G. Cognitive processes during fear acquisition and extinction in animals and humans: Implications for exposure therapy of anxiety disorders. Clin. Psychol. Rev. 2008, 28, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, S.G.; Sawyer, A.T.; Asnaani, A. d-cycloserine as an augmentation strategy for cognitive behavioral therapy of anxiety disorders: An update. Curr. Pharm. Des. 2012, 18, 5659–5662. [Google Scholar] [CrossRef]
- Möhler, H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 2012, 62, 42–53. [Google Scholar] [CrossRef]
- Pehrson, A.L.; Sanchez, C. Altered γ-aminobutyric acid neurotransmission in major depressive disorder: A critical review of the supporting evidence and the influence of serotonergic antidepressants. Drug Des. Dev. Ther. 2015, 9, 603–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.; Hen, R. The developmental origins of anxiety. Nat. Rev. Neurosci. 2004, 5, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Folsom, T.D. GABA receptor subunit distribution and FMRP-mGluR5 signaling abnormalities in the cerebellum of subjects with schizophrenia, mood disorders, and autism. Schizophr. Res. 2015, 167, 42–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lydiard, R.B. The role of GABA in anxiety disorders. J. Clin. Psychiatry 2003, 64, 21–27. [Google Scholar]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Banting, W. Letter on Corpulence Addressed to the Public. Obes. Res. 1993, 1, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, ketogenic diet and food intake control: A complex relationship. Front. Psychol. 2015, 2, 27. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, D.S. The Ketogenic Diet: Evidence for Optimism but High-Quality Research Needed. J. Nutr. 2020, 150, 1354–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einhorn, M. Lectures on Dietetics; Saunders: Philadelphia, PA, USA, 1922. [Google Scholar]
- Morgan, W. Diabetes Mellitus: Its History, Chemistry, Anatomy, Pathology, Physiology, and Treatment. Illustrated with Woodcuts, and Cases Successfully Treated; Homoeopathic Publishing Company: London, UK, 1877. [Google Scholar]
- Hippocrates. Epidemics 2; Smith, W.D., Ed.; Harvard University Press: Cambridge, MA, USA, 1994; Volume 7, p. 46. [Google Scholar]
- Atkins, R.C. Dr. Atkins’ New Diet Revolution; M. Evans & Company: New York, NY, USA, 2002. [Google Scholar]
- Rezaei, S.; Harsini, S.; Kavoosi, M.; Badv, R.S.; Mahmoudi, M. Efficacy of low glycemic index treatment in epileptic patients: A systematic review. Acta Neurol. Belg. 2018, 118, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-M.; Wang, H.-S. Medium-chain triglyceride ketogenic diet, an effective treatment for drug-resistant epilepsy and a comparison with other ketogenic diets. Biomed. J. 2013, 36, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A. The regulation of the release of ketone bodies by the liver. Adv. Enzyme Regul. 1966, 4, 339–354. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Fukuda, T.; Oyabu, C.; Tanaka, M.; Asano, M.; Yamazaki, M.; Fukui, M. Impact of low-carbohydrate diet on body composition: Meta-analysis of randomized controlled studies. Obes. Rev. 2016, 17, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, A.; Wiglusz, M.S.; Cubała, W.J. Ketogenic diet for schizophrenia: Nutritional approach to antipsychotic treatment. Med. Hypotheses 2018, 118, 74–77. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 2018, 174, 497. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Carrio, J.; Salazar, N.; Margolles, A.; González, S.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Suárez, A. Free Fatty Acids Profiles Are Related to Gut Microbiota Signatures and Short-Chain Fatty Acids. Front. Immunol. 2017, 8, 823. [Google Scholar] [CrossRef]
- Calderón, N.; Betancourt, L.; Hernández, L.; Rada, P. A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: A microdialysis study. Neurosci. Lett. 2017, 642, 158–162. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, N.; Medrihan, L.; Cappetti, B.; Baldelli, P.; Benfenati, F. 2-Deoxy-d-glucose enhances tonic inhibition through the neurosteroid-mediated activation of extrasynaptic GABAA receptors. Epilepsia 2016, 57, 1987–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, A.L.; Gasior, M.; Vining, E.P.; Rogawski, M.A. The neuropharmacology of the ketogenic diet. Pediatr. Neurol. 2007, 36, 281–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carver, C.M.; Reddy, D.S. Neurosteroid interactions with synaptic and extrasynaptic GABAA receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology 2013, 230, 151–188. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.S.; Vargas, F.; Dorrestein, P.C.; Chichlowski, M.; Berg, B.M.; Fleshner, M. Dietary prebiotics alter novel microbial dependent fecal metabolites that improve sleep. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millichap, J.G.; Yee, M.M. The diet factor in attention-deficit/hyperactivity disorder. Pediatrics 2012, 129, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindefeldt, M.; Eng, A.; Darban, H.; Bjerkner, A.; Zetterstrom, C.K.; Allander, T.; Andersson, B.; Borenstein, E.; Dahlin, M.; Prast-Nielsen, S. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes 2019, 5. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, S.; Zhou, Y.; Yu, L.; Zhang, L.; Wang, Y. Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res. 2018, 145, 163–168. [Google Scholar] [CrossRef]
- Xie, G.; Zhou, Q.; Qiu, C.Z.; Dai, W.K.; Wang, H.P.; Li, Y.H.; Liao, J.X.; Lu, X.G.; Lin, S.F.; Ye, J.H.; et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J. Gastroenterol. 2017, 23, 6164–6171. [Google Scholar] [CrossRef]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol. Autism 2016, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Swidsinski, A.; Dorffel, Y.; Loening-Baucke, V.; Gille, C.; Goktas, O.; Reisshauer, A.; Neuhaus, J.; Weylandt, K.H.; Guschin, A.; Bock, M. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front. Microbiol. 2017, 8, 1141. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, N.; Burke, L.M.; Vlahovich, N.; Charlesson, B.; O’Neill, H.; Ross, M.L.; Campbell, K.L.; Krause, L.; Morrison, M. The effects of dietary pattern during intensified training on stool microbiota of elite race walkers. Nutrients 2019, 11, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Wang, A.C.; Parikh, I.; Green, S.J.; Hoffman, J.D.; Chlipala, G.; Murphy, M.P.; Sokola, B.S.; Bauer, B.; Hartz, A.M.S.; et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci. Rep. 2018, 8, 6670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, P.G.; Rippy, N.A.; Dorenbos, K.; Conception, R.C.; Agarwal, A.K.; Rho, J.M. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004, 55, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Vieira, K.F. Nutritional therapies for mental disorders. Nutr. J. 2008, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.Y.; Kim, E.J.; Kim, A.; Lee, H.J.; Choi, H.J.; Yang, S.J. Nutritional factors affecting mental health. Clin. Nutr. Res. 2016, 5, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Freeman, M.P.; Hibbeln, J.R.; Wisner, K.L.; Davis, J.M.; Mischoulon, D.; Peet, M.; Keck, P.E.; Marangell, L.B., Jr.; Richardson, A.J.; Lake, J.; et al. Omega-3 fatty acids: Evidence basis for treatment and future research in psychiatry. J. Clin. Psychiatry 2006, 67, 1954–1967. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Cengotitabengoa, M.; González-Pinto, A. Nutritional supplements in depressive disorders. Actas Esp. Psiquiatr. 2017, 45, 8–15. [Google Scholar]
- Bodnar, L.M.; Wisner, K.L. Nutrition and Depression: Implications for Improving Mental Health Among Childbearing-Aged Women. Biol. Psychiatry 2005, 58, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Schweren, L.J.S.; Larsson, H.; Vinke, P.C.; Li, L.; Kvalvik, L.G.; Arias-Vasquez, A.; Haavik, J.; Hartman, C.A. Diet quality, stress and common mental health problems: A cohort study of 121,008 adults. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Mercer, J.G. Diet-Regulated Anxiety. Int. J. Endocrinol. 2013, 2013, 701967. [Google Scholar] [CrossRef] [PubMed]
- Bot, M.; Brouwer, I.A.; Roca, M.; Kohls, E.; Penninx, B.W.J.H.; Watkins, E.; van Grootheest, G.; Cabout, M.; Hegerl, U.; Gili, M.; et al. Effect of Multinutrient Supplementation and Food-Related Behavioral Activation Therapy on Prevention of Major Depressive Disorder Among Overweight or Obese Adults with Subsyndromal Depressive Symptoms. The MooDFOOD Randomized Clinical Trial. JAMA 2019, 321, 858–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacka, F.N.; Pasco, J.A.; Mykletun, A.; Williams, L.J.; Hodge, A.M.; O’Reilly, S.L.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Association of Western and traditional diets with depression and anxiety in women. Am. J. Psychiatry 2010, 167, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davison, K.M.; Kaplan, B.J. Food intake and blood cholesterol levels of community-based adults with mood disorders. Davison Kaplan BMC Psychiatry 2012, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Geijer, L.; Ekelund, M. Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: A case report. J. Med. Case Rep. 2015, 9, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, A.; Cubała, W.J.; Wielewicka, A. Ketogenic Diet: A Dietary Modification as an Anxiolytic Approach? Nutrients 2020, 12, 3822. https://doi.org/10.3390/nu12123822
Włodarczyk A, Cubała WJ, Wielewicka A. Ketogenic Diet: A Dietary Modification as an Anxiolytic Approach? Nutrients. 2020; 12(12):3822. https://doi.org/10.3390/nu12123822
Chicago/Turabian StyleWłodarczyk, Adam, Wiesław Jerzy Cubała, and Aleksandra Wielewicka. 2020. "Ketogenic Diet: A Dietary Modification as an Anxiolytic Approach?" Nutrients 12, no. 12: 3822. https://doi.org/10.3390/nu12123822
APA StyleWłodarczyk, A., Cubała, W. J., & Wielewicka, A. (2020). Ketogenic Diet: A Dietary Modification as an Anxiolytic Approach? Nutrients, 12(12), 3822. https://doi.org/10.3390/nu12123822




