Effects of Dietary Vitamin B6 Restriction on Hepatic Gene Expression Profile of Non-Obese and Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Serum and Hepatic Biochemical Analysis
2.3. Tissue Histologic Examination
2.4. Microarray Analysis
2.5. Statistical Analysis
3. Results
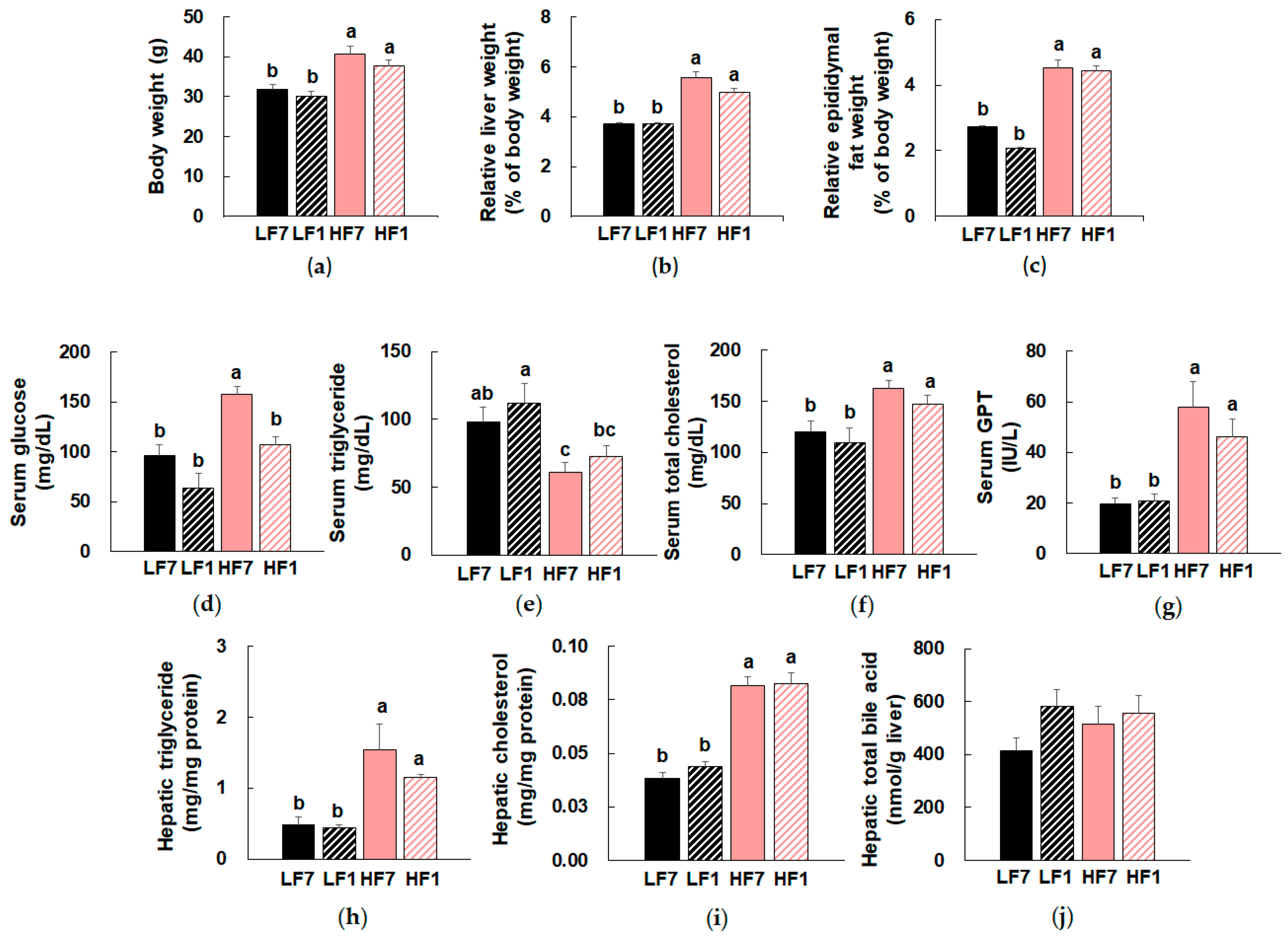
3.1. Effects of Dietary Vitamin B6 Restriction on Serum and Hepatic Biochemical Parameters of Mice
3.2. Effects of Dietary Vitamin B6 Restriction on Hepatic Transcriptome of Mice
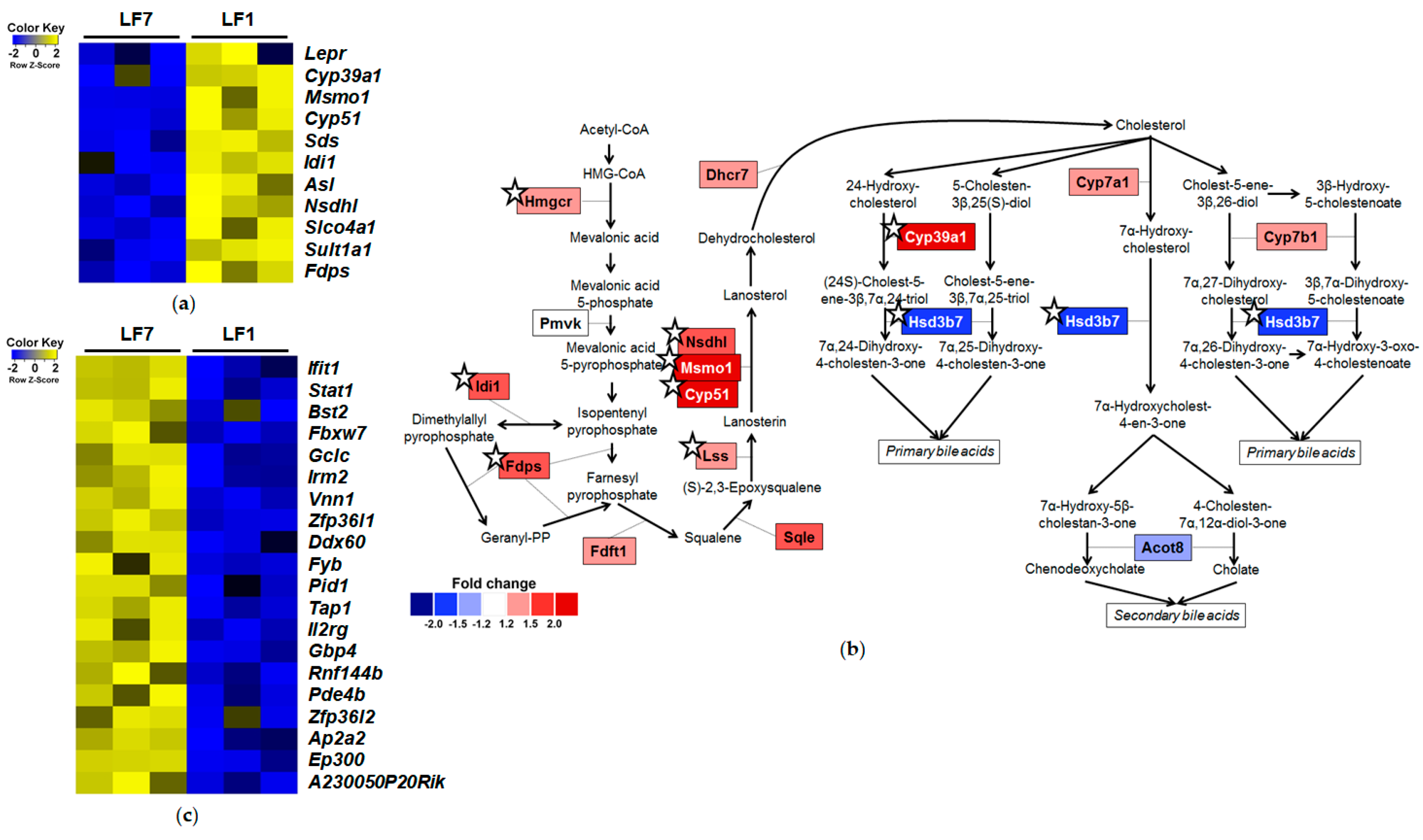
3.3. Effects of Dietary Vitamin B6 Restriction on Hepatic Transcriptome of Mice Fed a Low-Fat Diet
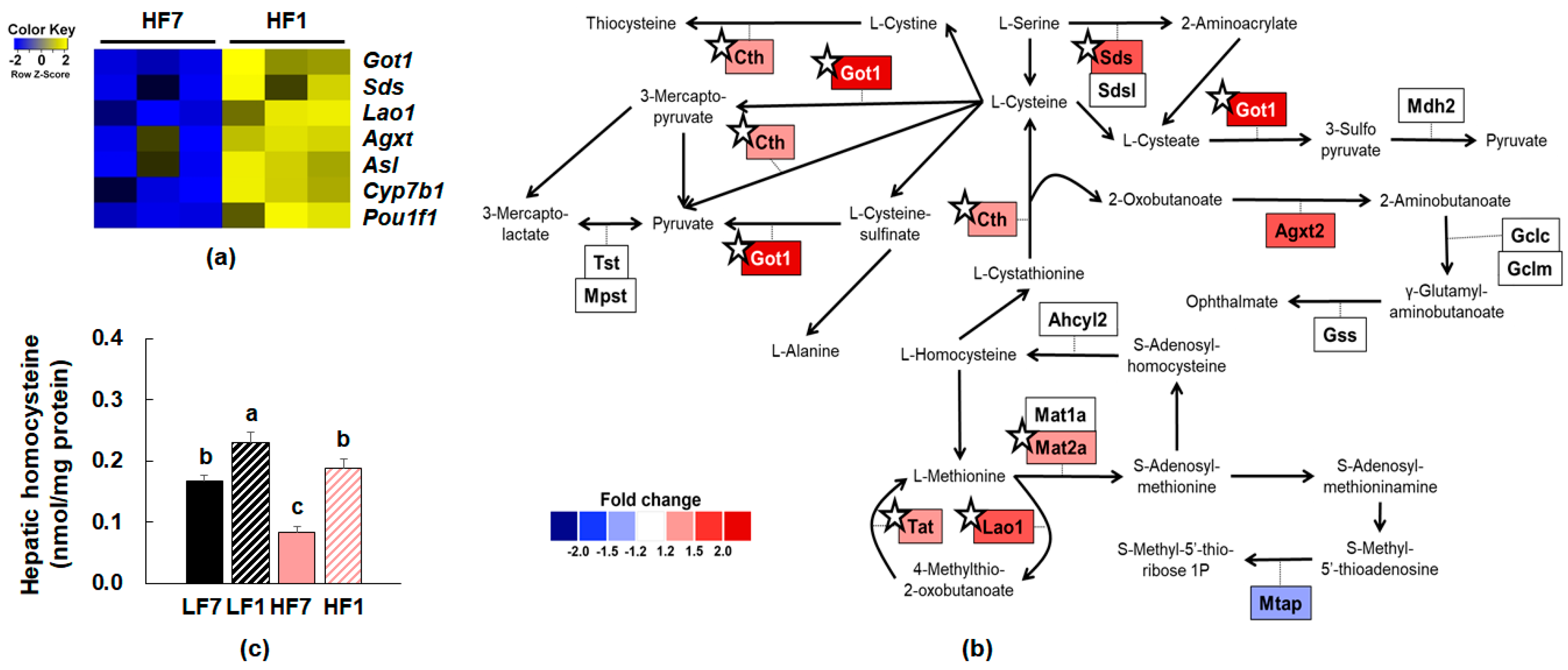
3.4. Effects of Dietary Vitamin B6 Restriction on Hepatic Transcriptome of Mice Fed a High-Fat Diet
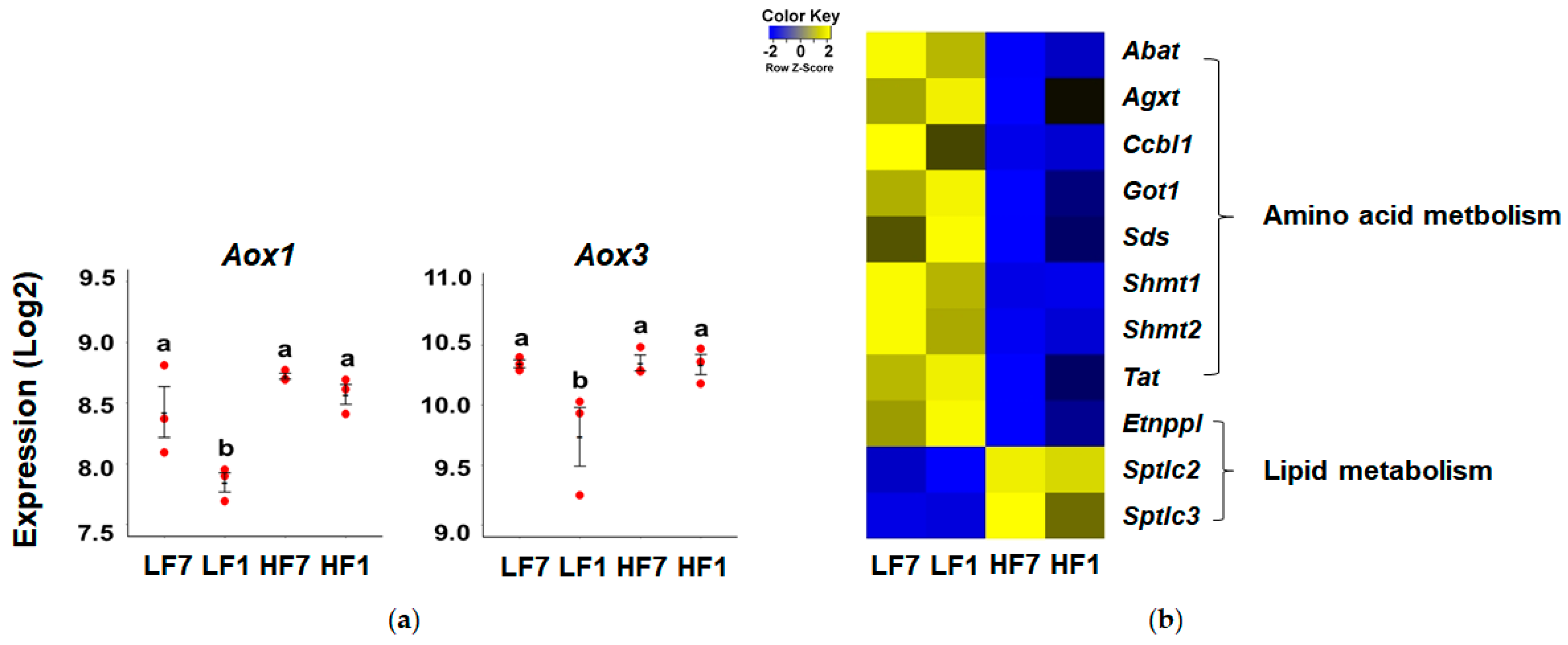
3.5. Effects of High-Fat Diet on Hepatic Vitamin B6 Metabolism and Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aasheim, E.T.; Hofsø, D.; Hjelmesæth, J.; Birkeland, K.I.; Bøhmer, T. Vitamin status in morbidly obese patients: A cross-sectional study. Am. J. Clin. Nutr. 2008, 87, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Nix, W.A.; Zirwes, R.; Bangert, V.; Kaiser, R.P.; Schilling, M.; Hostalek, U.; Obeid, R. Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes Res. Clin. Pract. 2015, 107, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotto, V.; Choi, S.-W.; Friso, S. Vitamin B6: A challenging link between nutrition and inflammation in CVD. Br. J. Nutr. 2011, 106, 183–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riggs, K.M.; Spiro, A.; Tucker, K.; Rush, D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am. J. Clin. Nutr. 1996, 63, 306–314. [Google Scholar] [CrossRef]
- Meydani, S.N.; Ribaya-Mercado, J.D.; Russell, R.M.; Sahyoun, N.; Morrow, F.D.; Gershoff, S.N. Vitamin B−6 deficiency impairs interleukin 2 production and lymphocyte proliferation in elderly adults. Am. J. Clin. Nutr. 1991, 53, 1275–1280. [Google Scholar] [CrossRef]
- Qian, B.; Shen, S.; Zhang, J.; Jing, P. Effects of Vitamin B6 Deficiency on the Composition and Functional Potential of T Cell Populations. J. Immunol. Res. 2017, 2017, 2197975. [Google Scholar] [CrossRef] [Green Version]
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Asp. Med. 2017, 53, 10–27. [Google Scholar] [CrossRef]
- Dalto, D.B.; Matte, J.J. Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation. Nutrients 2017, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Paul, L.; Ueland, P.M.; Selhub, J. Mechanistic perspective on the relationship between pyridoxal 5′-phosphate and inflammation. Nutr. Rev. 2013, 71, 239–244. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Cho, Y.-O. Evaluation of vitamin B6intake and status of 20- to 64-year-old Koreans. Nutr. Res. Pract. 2014, 8, 688–694. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Maras, J.E.; Bakun, P.; Tucker, K.L. Dietary Intake of Vitamin B-6, Plasma Pyridoxal 5′-Phosphate, and Homocysteine in Puerto Rican Adults. J. Am. Diet. Assoc. 2010, 110, 1660–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.S.; Picciano, M.F.; Jacques, P.F.; Selhub, J. Plasma pyridoxal 5′-phosphate in the US population: The National Health and Nutrition Examination Survey, 2003–2004. Am. J. Clin. Nutr. 2008, 87, 1446–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, S.-C.; Khor, G.-L.; Loh, S.-P. Association between Dietary Folate Intake and Blood Status of Folate and Homocysteine in Malaysian Adults. J. Nutr. Sci. Vitaminol. 2011, 57, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Mayengbam, S.; Raposo, S.; Aliani, M.; House, J.D. Oral exposure to the anti-pyridoxine compound 1-amino d-proline further perturbs homocysteine metabolism through the transsulfuration pathway in moderately vitamin B6 deficient rats. J. Nutr. Biochem. 2015, 26, 241–249. [Google Scholar] [CrossRef]
- Leklem, J.E. Vitamin B-6: A Status Report. J. Nutr. 1990, 120, 1503–1507. [Google Scholar] [CrossRef]
- Cabrini, L.; Bochicchio, D.; Bordoni, A.; Sassi, S.; Marchetti, M.; Maranesi, M. Correlation between dietary polyunsaturated fatty acids and plasma homocysteine concentration in vitamin B6-deficient rats. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 94–99. [Google Scholar] [CrossRef]
- Mayengbam, S.; House, J.D.; Aliani, M. Investigation of vitamin B6 inadequacy, induced by exposure to the anti-B6 factor 1-amino d-proline, on plasma lipophilic metabolites of rats: A metabolomics approach. Eur. J. Nutr. 2015, 55, 1213–1223. [Google Scholar] [CrossRef]
- Davis, S.R.; Scheer, J.B.; Quinlivan, E.P.; Coats, B.S.; Stacpoole, P.W.; Gregory, J.F. Dietary vitamin B-6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am. J. Clin. Nutr. 2005, 81, 648–655. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.R.; Quinlivan, E.P.; Stacpoole, P.W.; Gregory, J.F. Plasma Glutathione and Cystathionine Concentrations Are Elevated but Cysteine Flux Is Unchanged by Dietary Vitamin B-6 Restriction in Young Men and Women. J. Nutr. 2006, 136, 373–378. [Google Scholar] [CrossRef]
- Gregory, J.F.; Park, Y.; Lamers, Y.; Bandyopadhyay, N.; Chi, Y.-Y.; Lee, K.; Kim, S.; Da Silva, V.; Hove, N.; Ranka, S.; et al. Metabolomic Analysis Reveals Extended Metabolic Consequences of Marginal Vitamin B-6 Deficiency in Healthy Human Subjects. PLoS ONE 2013, 8, e63544. [Google Scholar] [CrossRef] [Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, J.G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Coburn, S.P. A critical review of minimal vitamin B6 requirements for growth in various species with a proposed method of calculation. Vitam. Horm. 1994, 48, 259–300. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Ruiz, J.I.; Ochoa, B. Quantification in the subnanomolar range of phospholipids and neutral lipids by monodimensional thin-layer chromatography and image analysis. J. Lipid Res. 1997, 38, 1482–1489. [Google Scholar] [PubMed]
- Minniti, G.; Piana, A.; Armani, U.; Cerone, R. Determination of plasma and serum homocysteine by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A 1998, 828, 401–405. [Google Scholar] [CrossRef]
- Kim, J.; Choi, A.; Kwon, Y.H. Maternal Protein Restriction Altered Insulin Resistance and Inflammation-Associated Gene Expression in Adipose Tissue of Young Adult Mouse Offspring in Response to a High-Fat Diet. Nutrients 2020, 12, 1103. [Google Scholar] [CrossRef]
- Hardy, T.; Oakley, F.; Anstee, Q.M.; Day, C.P. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu. Rev. Pathol. 2016, 11, 451–496. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Mocellin, S.; Briarava, M.; Pilati, P. Vitamin B6 and Cancer Risk: A Field Synopsis and Meta-Analysis. J. Natl. Cancer Inst. 2016, 109, 1–9. [Google Scholar] [CrossRef]
- Shah, S.N.; Johnston, P.V.; Kummerow, F.A. The Effect of Pyridoxine on Cholesterol Metabolism. J. Nutr. 1960, 72, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Okada, M.; Iwami, T. Effect of pyridoxine deficiency on cholesterogenesis in rats fed different levels of protein. J. Nutr. Sci. Vitaminol. 1977, 23, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Lupien, P.J.; Hinse, C.M.; Avery, M. Cholesterol metabolism and vitamin B6. I. Hepatic cholesterogenesis and pyridoxine deficiency. Can. J. Biochem. 1969, 47, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Hinse, C.M.; Lupien, P.J. Cholesterol Metabolism and Vitamin B6. III. The Stimulation of Hepatic Cholesterogenesis in the Vitamin B6-Deficient Rat. Can. J. Biochem. 1971, 49, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, E.; Yamamoto, T.; Yamamoto, K.; Nakagawa, T.; Hayakawa, T. Accumulation of lipid in rat liver was induced by vitamin B6deficiency and was ameliorated by supplemental phosphatidylcholine in the diet. Biosci. Biotechnol. Biochem. 2015, 79, 1320–1326. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, E.; Yamamoto, T.; Fujishita, M.; Ota, Y.; Yamamoto, K.; Nakagawa, T.; Hayakawa, T. Choline and betaine ameliorate liver lipid accumulation induced by vitamin B6 deficiency in rats. Biosci. Biotechnol. Biochem. 2016, 81, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Doke, S.; Inagaki, N.; Hayakawa, T.; Tsuge, H. Effect of vitamin B6 deficiency on an antibody production in mice. Biosci. Biotechnol. Biochem. 1997, 61, 1331–1336. [Google Scholar] [CrossRef] [Green Version]
- Mayengbam, S.; Raposo, S.; Aliani, M.; House, J.D. A Vitamin B-6 Antagonist from Flaxseed Perturbs Amino Acid Metabolism in Moderately Vitamin B-6–Deficient Male Rats. J. Nutr. 2015, 146, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Tully, D.B.; Allgood, V.E.; Cidlowski, J.A. Modulation of steroid receptor-mediated gene expression by vitamin B 6. FASEB J. 1994, 8, 343–349. [Google Scholar] [CrossRef]
- Marzio, A.; Merigliano, C.; Gatti, M.; Verni, F. Sugar and Chromosome Stability: Clastogenic Effects of Sugars in Vitamin B6-Deficient Cells. PLoS Genet. 2014, 10, e1004199. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Um, H.-J.; Ko, J.W.; Won, S.B.; Kwon, Y.H. Effects of Dietary Vitamin B6 Restriction on Hepatic Gene Expression Profile of Non-Obese and Obese Mice. Nutrients 2020, 12, 3821. https://doi.org/10.3390/nu12123821
Um H-J, Ko JW, Won SB, Kwon YH. Effects of Dietary Vitamin B6 Restriction on Hepatic Gene Expression Profile of Non-Obese and Obese Mice. Nutrients. 2020; 12(12):3821. https://doi.org/10.3390/nu12123821
Chicago/Turabian StyleUm, Hyun-Jee, Je Won Ko, Sae Bom Won, and Young Hye Kwon. 2020. "Effects of Dietary Vitamin B6 Restriction on Hepatic Gene Expression Profile of Non-Obese and Obese Mice" Nutrients 12, no. 12: 3821. https://doi.org/10.3390/nu12123821





