Possible Reduction of Cardiac Risk after Supplementation with Epigallocatechin Gallate and Increase of Ketone Bodies in the Blood in Patients with Multiple Sclerosis. A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Procedure
2.3. Intervention
2.4. Measurements
2.5. Ethical Concerns
2.6. Statistical Analysis
3. Results
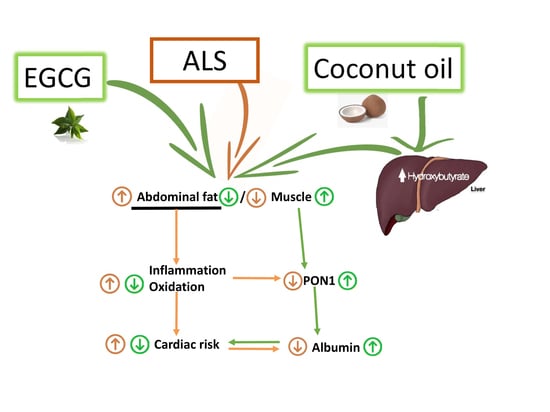
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kutzelnigg, A.; Lassmann, H. Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Int. Neuroradiol. 2014, 122, 15–58. [Google Scholar] [CrossRef]
- Demir, S. Multiple Sclerosis Functional Composite. Arch. Neuropsychiatry 2018, 55, S66–S68. [Google Scholar] [CrossRef]
- Wens, I.; Dalgas, U.; Vandenabeele, F.; Krekels, M.; Grevendonk, L.; Eijnde, B.O. Multiple Sclerosis Affects Skeletal Muscle Characteristics. PLoS ONE 2014, 9, e108158. [Google Scholar] [CrossRef] [PubMed]
- Tettey, P.; Simpson, S.; Taylor, B.; Ponsonby, A.-L.; Lucas, R.M.; Dwyer, T.; Kostner, K.; Van Der Mei, I.A. AUSLONG Investigators Group An adverse lipid profile and increased levels of adiposity significantly predict clinical course after a first demyelinating event. J. Neurol. Neurosurg. Psychiatry 2017, 88, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Heber, D. An integrative view of obesity. Am. J. Clin. Nutr. 2010, 91, 280S–283S. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’Ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med Sci. 2017, 4, 851–863. [Google Scholar] [CrossRef]
- Katarina, V.; Gordana, T.; Svetlana, M.D.; Milica, B. Oxidative stress and neuroinflammation should be both considered in the occurrence of fatigue and depression in multiple sclerosis. Acta Neurol. Belg. 2018, 120, 853–861. [Google Scholar] [CrossRef]
- Jia, X.-J.; Liu, L.-X.; Tian, Y.-M.; Wang, R.; Lu, Q. The correlation between oxidative stress level and intra-abdominal fat in obese males. Medicine 2019, 98, e14469. [Google Scholar] [CrossRef]
- Efrat, M.; Aviram, M. Paraoxonase 1 Interactions with HDL, Antioxidants and Macrophages Regulate Atherogenesis—A Protective Role for HDL Phospholipids. Paraoxonases Inflamm. Infect. Toxicol. 2009, 660, 153–166. [Google Scholar] [CrossRef]
- Kappelle, P.J.W.H.; De Boer, J.F.; Perton, F.G.; Annema, W.; De Vries, R.; Dullaart, R.P.F.; Tietge, U.J.F. Increased LCAT activity and hyperglycaemia decrease the antioxidative functionality of HDL. Eur. J. Clin. Investig. 2011, 42, 487–495. [Google Scholar] [CrossRef]
- Watson, A.D.; Berliner, J.A.; Hama, S.Y.; La Du, B.N.; Faull, K.F.; Fogelman, A.M.; Navab, M. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J. Clin. Investig. 1995, 96, 2882–2891. [Google Scholar] [CrossRef] [PubMed]
- Menini, T.; Gugliucci, A. Paraoxonase 1 in neurological disorders. Redox Rep. 2014, 19, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, M.; Trentini, A.; Romani, A.; Valacchi, G.; Bellini, T.; Bonaccorsi, G.; Fainardi, E.; Cavicchio, C.; Passaro, A.; Zuliani, G.; et al. Decreased arylesterase activity of paraoxonase-1 (PON-1) might be a common denominator of neuroinflammatory and neurodegenerative diseases. Int. J. Biochem. Cell Biol. 2016, 81, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Baynard, T.; Hilgenkamp, T.I.; Schroeder, E.C.; Motl, R.W.; Fernhall, B. Measures of adiposity differentially correlate with C-reactive protein among persons with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 25, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vella, C.A.; Nelson, M.C.; Unkart, J.T.; Miljkovic, I.; Allison, M.A. Skeletal muscle area and density are associated with lipid and lipoprotein cholesterol levels: The Multi-Ethnic Study of Atherosclerosis. J. Clin. Lipidol. 2020, 14, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Korenfeld, Y.; Boarin, S.; Korinek, J.; Jensen, M.D.; Parati, G.; Lopez-Jimenez, F. Normal weight obesity: A risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur. Heart J. 2010, 31, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Riera-Fortuny, C.; Real, J.T.; Chaves, F.J.; Morales-Suárez-Varela, M.; Martínez-Triguero, M.L.; Morillas-Ariño, C.; Mijares, A.H. The relation between obesity, abdominal fat deposit and the angiotensin-converting enzyme gene I/D polymorphism and its association with coronary heart disease. Int. J. Obes. 2004, 29, 78–84. [Google Scholar] [CrossRef]
- Chaves, G.S.S.; Ghisi, G.L.M.; Grace, S.L.; Oh, P.; Ribeiro, A.L.; Britto, R.R. Effects of comprehensive cardiac rehabilitation on functional capacity and cardiovascular risk factors in Brazilians assisted by public health care: Protocol for a randomized controlled trial. Braz. J. Phys. Ther. 2016, 20, 592–600. [Google Scholar] [CrossRef][Green Version]
- Palavra, F.; Marado, D.; Mascarenhas-Melo, F.; Sereno, J.; Teixeira, F.; Nunes, C.C.; Gonçalves, G.; Teixeira, F.; Reis, F. New Markers of Early Cardiovascular Risk in Multiple Sclerosis Patients: Oxidized-LDL Correlates with Clinical Staging. Dis. Markers 2013, 34, 341–348. [Google Scholar] [CrossRef]
- Karmon, Y.; Ramanathan, M.; Minagar, A.; Zivadinov, R.; Weinstock-Guttman, B. Arterial, venous and other vascular risk factors in multiple sclerosis. Neurol. Res. 2012, 34, 754–760. [Google Scholar] [CrossRef]
- Jamroz-Wiśniewska, A.; Bełtowski, J.; Stelmasiak, Z.; Bartosik-Psujek, H. Paraoxonase 1 activity in different types of multiple sclerosis. Mult. Scler. J. 2009, 15, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Hossain, J.; Morandi, E.; Tanasescu, R.; Frakich, N.; Caldano, M.; Onion, D.; Faraj, T.A.; Erridge, C.; Gran, B. The Soluble Form of Toll-Like Receptor 2 Is Elevated in Serum of Multiple Sclerosis Patients: A Novel Potential Disease Biomarker. Front. Immunol. 2018, 9, 457. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.M.; Popolo, A.; Trifa, A.P.; Stanciu, L.A. Phytochemicals in Cardiovascular and Respiratory Diseases: Evidence in Oxidative Stress and Inflammation. Oxidative Med. Cell. Longev. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Kritchevsky, S.B.; Newman, A.B.; Goodpaster, B.H.; Tylavsky, F.A.; Nevitt, M.C.; Harris, T.B. Lower serum albumin concentration and change in muscle mass: The Health, Aging and Body Composition Study. Am. J. Clin. Nutr. 2005, 82, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Liou, S.-Y.; Kuo, W.-W.; Wu, H.-C.; Chang, Y.-L.; Chen, T. Chemiluminescence analysis of antioxidant capacity for serum albumin isolated from healthy or uremic volunteers. Luminescence 2016, 31, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Arques, S. Human serum albumin in cardiovascular diseases. Eur. J. Intern. Med. 2018, 52, 8–12. [Google Scholar] [CrossRef]
- Arques, S. Serum albumin and cardiovascular diseases: A comprehensive review of the literature. Ann. Cardiol. Angéiol. 2018, 67, 82–90. [Google Scholar] [CrossRef]
- Ulivelli, M.; Priora, R.; Di Giuseppe, D.; Coppo, L.; Summa, D.; Margaritis, A.; Frosali, S.; Bartalini, S.; Martini, G.; Cerase, A.; et al. Homocysteinemia control by cysteine in cerebral vascular patients after methionine loading test: Evidences in physiological and pathological conditions in cerebro-vascular and multiple sclerosis patients. Amino Acids 2016, 48, 1477–1489. [Google Scholar] [CrossRef]
- Kim, D.Y.; Hao, J.; Liu, R.; Turner, G.; Shi, F.-D.; Rho, J.M. Inflammation-Mediated Memory Dysfunction and Effects of a Ketogenic Diet in a Murine Model of Multiple Sclerosis. PLoS ONE 2012, 7, e35476. [Google Scholar] [CrossRef]
- Gershuni, V.M.; Yan, S.L.; Medici, V. Nutritional Ketosis for Weight Management and Reversal of Metabolic Syndrome. Curr. Nutr. Rep. 2018, 7, 97–106. [Google Scholar] [CrossRef]
- Bezard, J.; Bugaut, M.; Clement, G. Triglyceride composition of coconut oil. J. Am. Oil Chem. Soc. 1971, 48, 134–139. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Z.; Xu, Y.; Xiao, S.; Meydani, S.N.; Wu, D. Epigallocatechin-3-Gallate Ameliorates Experimental Autoimmune Encephalomyelitis by Altering Balance among CD4+ T-Cell Subsets. Am. J. Pathol. 2012, 180, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, J.; Pae, M.; Meydani, S.N. Green tea EGCG, T cells, and T cell-mediated autoimmune diseases. Mol. Asp. Med. 2012, 33, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-J.; Hsieh, M.-T.; Wu, C.-R.; Wood, W.G.; Chen, Y.-F. Green Tea Extract Ameliorates Learning and Memory Deficits in Ischemic Rats via Its Active Component Polyphenol Epigallocatechin-3-gallate by Modulation of Oxidative Stress and Neuroinflammation. Evid. Based Complement Altern. Med. 2012, 2012, 163106. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-Y.; Zhao, C.-N.; Gan, R.-Y.; Xu, X.-Y.; Wei, X.-L.; Corke, H.; Atanasov, A.G.; Li, H.-B. Effects and Mechanisms of Tea and Its Bioactive Compounds for the Prevention and Treatment of Cardiovascular Diseases: An Updated Review. Antioxidants 2019, 8, 166. [Google Scholar] [CrossRef]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2009, 101, 886–894. [Google Scholar] [CrossRef]
- Wu, A.H.; Spicer, D.; Stanczyk, F.Z.; Tseng, C.-C.; Yang, C.S.; Pike, M.C. Effect of 2-Month Controlled Green Tea Intervention on Lipoprotein Cholesterol, Glucose, and Hormone Levels in Healthy Postmenopausal Women. Cancer Prev. Res. 2012, 5, 393–402. [Google Scholar] [CrossRef]
- Dapcich, V.; Salvador, G.; Ribas, L.; Pérez, C.; Aranceta, J.; Serra, L.L. Guía de Alimentación Saludable; Sociedad Española de Nutrición Comunitaria: Barcelona, Spain, 2004. [Google Scholar]
- Feng, W.Y. Metabolism of Green Tea Catechins: An Overview. Curr. Drug Metab. 2006, 7, 755–809. [Google Scholar] [CrossRef]
- Gibson, A.A.; Seimon, R.V.; Lee, C.M.Y.; Ayre, J.; Franklin, J.; Markovic, T.P.; Caterson, I.D.; Sainsbury, A. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes. Rev. 2014, 16, 64–76. [Google Scholar] [CrossRef]
- Fernández-Juan, A.; Ramírez-Gil, C.; van der Werf, L. La valoración antropométrica en el contexto de la escuela como medida para detectar y prevenir efectos a largo plazo de la obesidad y del sobrepeso en niños en edad escolar. Rev. Colomb. Cardiol. 2016, 23, 435–442. [Google Scholar] [CrossRef]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.L.N.; Chan, J. Body mass index, waist circumference and waist: Hip ratio as predictors of cardiovascular risk—A review of the literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L.; et al. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: A case-control study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef]
- Browning, L.M.; Hsieh, S.D.; Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr. Res. Rev. 2010, 23, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.A. Physiology of Swimming and Diving. Exercise Physiology; Academic Press: Baltimore, MD, USA, 1968. [Google Scholar]
- Rocha, M.S.L. Peso ósseo do brasileiro de ambos os sexos de 17 a 25 años. Arq. Anatomía Antropol. 1975, 1, 445–451. [Google Scholar]
- Matiegka, J. The testing of physical efficiency. Am. J. Phys. Anthr. 1921, 4, 223–230. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef]
- Ceron, J.J.; Tecles, F.; Tvarijonaviciute, A. Serum paraoxonase 1 (PON1) measurement: An update. BMC Vet. Res. 2014, 10, 74. [Google Scholar] [CrossRef]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Amirabdollahian, F.; Haghighatdoost, F. Anthropometric Indicators of Adiposity Related to Body Weight and Body Shape as Cardiometabolic Risk Predictors in British Young Adults: Superiority of Waist-to-Height Ratio. J. Obes. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Park, J.; Kim, N.H.; Kim, S.H.; Kim, J.-S.; Kim, Y.H.; Lim, H.E.; Kim, E.J.; Na, J.O.; Cho, G.-Y.; Baik, I.; et al. Visceral adiposity and skeletal muscle mass are independently and synergistically associated with left ventricular structure and function: The Korean Genome and Epidemiology Study. Int. J. Cardiol. 2014, 176, 951–955. [Google Scholar] [CrossRef]
- Dayrit, F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Sackner-Bernstein, J.; Kanter, D.; Kaul, S. Dietary Intervention for Overweight and Obese Adults: Comparison of Low-Carbohydrate and Low-Fat Diets. A Meta-Analysis. PLoS ONE 2015, 10, e0139817. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Juliá, S.; Moreno, M.L.; Barrios, C.; Ortí, J.E.D.L.R.; Drehmer, E. Antioxidant Alternatives in the Treatment of Amyotrophic Lateral Sclerosis: A Comprehensive Review. Front. Physiol. 2020, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Eng, Q.Y.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J. Ethnopharmacol. 2018, 210, 296–310. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kim, C.-T.; Kim, Y. Green Tea (–)-Epigallocatechin-3-Gallate Reduces Body Weight with Regulation of Multiple Genes Expression in Adipose Tissue of Diet-Induced Obese Mice. Ann. Nutr. Metab. 2009, 54, 151–157. [Google Scholar] [CrossRef]
- Hill, A.M.; Coates, A.M.; Buckley, J.D.; Ross, R.; Thielecke, F.; Howe, P.R.C. Can EGCG Reduce Abdominal Fat in Obese Subjects? J. Am. Coll. Nutr. 2007, 26, 396S–402S. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Swain, J.F.; Feldman, H.A.; Wong, W.W.; Hachey, D.L.; Garcia-Lago, E.; Ludwig, D.S. Effects of Dietary Composition on Energy Expenditure During Weight-Loss Maintenance. JAMA 2012, 307, 2627–2634. [Google Scholar] [CrossRef]
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Low-carbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef]
- Cao, Q.; Yu, S.; Xiong, W.; Li, Y.; Li, H.; Li, J.; Li, F. Waist-hip ratio as a predictor of myocardial infarction risk: A systematic review and meta-analysis. Medicine 2018, 97, e11639. [Google Scholar] [CrossRef]
- Al-Shamiri, M.Q.; Habbab, F.M.A.; Al-Qahtani, S.S.; Alghalayini, K.A.; Al-Qattan, O.M.; El-Shaer, F. Waist-to-Height Ratio (WHtR) in Predicting Coronary Artery Disease Compared to Body Mass Index and Waist Circumference in a Single Center from Saudi Arabia. Cardiol. Res. Pract. 2020, 2020, 4250793. [Google Scholar] [CrossRef]
- Li, P.; Liu, A.; Liu, C.; Qu, Z.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Role and mechanism of catechin in skeletal muscle cell differentiation. J. Nutr. Biochem. 2019, 74, 108225. [Google Scholar] [CrossRef]
- Veldhorst, M.A.; Smeets, A.; Soenen, S.; Hochstenbach-Waelen, A.; Hursel, R.; Diepvens, K.; Lejeune, M.; Luscombe-Marsh, N.; Westerterp-Plantenga, M. Protein-induced satiety: Effects and mechanisms of different proteins. Physiol. Behav. 2008, 94, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.R.; Kallaur, A.P.; Reiche, E.M.V.; Kaimen-Maciel, D.R.; Panis, C.; Lozovoy, M.A.B.; Morimoto, H.K.; Maes, M.; Dichi, I.; Simão, A.N.C. Albumin and Protein Oxidation are Predictors that Differentiate Relapsing-Remitting from Progressive Clinical Forms of Multiple Sclerosis. Mol. Neurobiol. 2016, 54, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Ronit, A.; Kirkegaard-Klitbo, D.M.; Dohlmann, T.L.; Lundgren, J.; Sabin, C.A.; Phillips, A.N.; Nordestgaard, B.G.; Afzal, S. Plasma Albumin and Incident Cardiovascular Disease: Results from the CGPS and an Updated Meta-Analysis. Arter. Thromb. Vasc. Biol. 2020, 40, 473–482. [Google Scholar] [CrossRef]
- Sun, X.; Ferguson, H.N.; Hagerman, A.E. Sun Conformation and Aggregation of Human Serum Albumin in the Presence of Green Tea Polyphenol (EGCg) and/or Palmitic Acid. Biomolecules 2019, 9, 705. [Google Scholar] [CrossRef]
- Bae, M.-J.; Ishii, T.; Minoda, K.; Kawada, Y.; Ichikawa, T.; Mori, T.; Kamihira, M.; Nakayama, T. Albumin stabilizes (-)-epigallocatechin gallate in human serum: Binding capacity and antioxidant property. Mol. Nutr. Food Res. 2009, 53, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Do, G.-M.; Kwon, E.-Y.; Kim, H.-J.; Jeon, S.-M.; Ha, T.-Y.; Park, T.; Choi, M.-S. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem. Biophys. Res. Commun. 2008, 374, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-P.; Wu, M.-S.; Yang, C.-C.; Huang, K.-C.; Liou, S.-Y.; Hsu, S.-M.; Chien, C.-T. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am. J. Clin. Nutr. 2007, 86, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Arunima, S.; Rajamohan, T. Effect of virgin coconut oil enriched diet on the antioxidant status and paraoxonase 1 activity in ameliorating the oxidative stress in rats—A comparative study. Food Funct. 2013, 4, 1402. [Google Scholar] [CrossRef]
- Maki, K.C.; Hasse, W.; Dicklin, M.R.; Bell, M.; Buggia, M.A.; Cassens, M.E.; Eren, F. Corn Oil Lowers Plasma Cholesterol Compared with Coconut Oil in Adults with Above-Desirable Levels of Cholesterol in a Randomized Crossover Trial. J. Nutr. 2018, 148, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, J.; Pang, X.; Zhang, X.; Wang, S.; Wu, D. Epigallocatechin-3-gallate inhibits angiotensin II-induced C-reactive protein generation through interfering with the AT1-ROS-ERK1/2 signaling pathway in hepatocytes. Naunyn Schmiedeberg’s Arch. Pharmacol. 2016, 389, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Riegsecker, S.; Wiczynski, D.; Kaplan, M.J.; Ahmed, S. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 2013, 93, 307–312. [Google Scholar] [CrossRef] [PubMed]


| Control Group N = 17 | Intervention Group N = 16 | ||||||
| Freq | % | Freq | % | Chi2 | p | ||
| Sex | Women | 14 | 58.3% | 22 | 81.5% | 3.279 | 0.070 |
| Men | 10 | 41.7% | 5 | 18.5% | |||
| MS type | Relapsing-Remitting | 17 | 70.8% | 20 | 74.1% | 0.067 | 0.796 |
| Secondary-Progressive | 7 | 29.2% | 7 | 25.9% | |||
| Cardiac risk | Low | 5 | 29.4% | 5 | 31.3% | 0.696 | 0.706 |
| Moderate | 4 | 23.5% | 2 | 12.5% | |||
| High | 8 | 47.1% | 9 | 56.3% | |||
| Control group N = 21 | Intervention group N = 25 | ||||||
| Mean | SD | Mean | SD | Z | p | ||
| Age (years) | 49.83 | 12.42 | 44.56 | 11.27 | −1.558 | 0.119 | |
| EDSS | 3.80 | 2.00 | 3.37 | 2.03 | −0.780 | 0.435 | |
| Weight | 70.44 | 18,13 | 68,63 | 13,56 | −0.245 | 0.806 | |
| BMI | 25.72 | 6.01 | 25.92 | 5.29 | −0.142 | 0.887 | |
| PON1(UI/L) | 2.88 | 0.77 | 2.67 | 0.62 | −0.832 | 0.405 | |
| WHR | 0.95 | 0.08 | 0.89 | 0.10 | −1.625 | 0.104 | |
| WHtR | 0.60 | 0.08 | 0.57 | 0.08 | −0.721 | 0.471 | |
| Fat mass (%) | 18.85 | 5.00 | 19.53 | 3.78 | −0.764 | 0.445 | |
| Muscle mass (%) | 38.38 | 4.15 | 39.39 | 2.88 | −0.547 | 0.584 | |
| Albumin (g/dL) | 4.66 | 0.41 | 4.69 | 0.29 | −0.414 | 0.679 | |
| CRP (mg/L) | 5.03 | 4.39 | 3.59 | 2.08 | −0.799 | 0.424 | |
| BHB (Mmol/L) | 0.05 | 0.02 | 0.06 | 0.04 | −0.932 | 0.351 | |
| Control Group N = 17 | Intervention Group N = 16 | ||||||
| Freq | % | Freq | % | Chi2 | p | ||
| Cardiac risk | Low | 8 | 47.1% | 6 | 37.5% | 2.343 | 0.310 |
| Moderate | 1 | 5.9% | 4 | 25.0% | |||
| High | 8 | 47.1% | 6 | 37.5% | |||
| Control group N = 21 | Intervention group N = 25 | ||||||
| Mean | SD | Mean | SD | Z | p | ||
| BMI | 25.36 | 5.85 | 25.16 | 4.94 | −0.068 | 0.946 | |
| PON1(UI/L) | 2.49 | 0.79 | 2.97 | 0.65 | −0.369 | 0.001 | |
| WHR | 0.94 | 0.08 | 0.87 | 0.09 | −1.969 | 0.049 | |
| WHtR | 0.60 | 0.09 | 0.55 | 0.07 | −1.515 | 0.130 | |
| Fat mass (%) | 19.01 | 5.26 | 17.74 | 3.32 | −0.701 | 0.483 | |
| Muscle mass (%) | 38.01 | 4.02 | 41.10 | 2.81 | −2.115 | 0.023 | |
| Albumin (g/dL) | 4.55 | 0.44 | 4.83 | 0.19 | −2.316 | 0.021 | |
| CRP (mg/L) | 4.72 | 3.55 | 3.50 | 2.02 | −1.671 | 0.095 | |
| BHB (Mmol/L) | 0.04 | 0.04 | 0.10 | 0.10 | −2.655 | 0.008 | |
| Control Group N = 21 | Intervention Group N = 25 | |||||
|---|---|---|---|---|---|---|
| Mean * | SD | Mean * | SD | F | p | |
| BMI | −0.5182 | 0.89418 | −0.7629 | 1.03918 | 0.782 | 0.381 |
| PON1 Pre-Post (UI/L) | −0.0047 | 0.40128 | 0.2427 | 0.35522 | 4.501 | 0.040 |
| WHR Pre-Post | −0.0029 | 0.03016 | −0.0156 | 0.02421 | 1.761 | 0.194 |
| WHtR Pre-Post | 0.0006 | 0.02045 | −0.0238 | 0.01857 | 12.752 | 0.001 |
| Fat mass Pre-Post (%) | 0.2152 | 1.54251 | −1.7870 | 1.51905 | 21.275 | 0.000 |
| Muscle mass Pre-Post (%) | −0.5958 | 1.50057 | 0.8307 | 2.02952 | 7.975 | 0.007 |
| Albumin Pre-Post (g/dL) | −0.1353 | 0.36632 | 0.1435 | 0.28599 | 8.222 | 0.006 |
| CRP Pre-Post (mg/L) | −0.3230 | 5.72099 | −0.0889 | 1.85873 | 0.040 | 0.843 |
| BHB Pre-Post (Mmol/L) | −0.0024 | 0.04309 | 0.0431 | 0.09899 | 3.167 | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benlloch, M.; Cuerda Ballester, M.; Drehmer, E.; Platero, J.L.; Carrera-Juliá, S.; López-Rodríguez, M.M.; Ceron, J.J.; Tvarijonaviciute, A.; Navarro, M.Á.; Moreno, M.L.; et al. Possible Reduction of Cardiac Risk after Supplementation with Epigallocatechin Gallate and Increase of Ketone Bodies in the Blood in Patients with Multiple Sclerosis. A Pilot Study. Nutrients 2020, 12, 3792. https://doi.org/10.3390/nu12123792
Benlloch M, Cuerda Ballester M, Drehmer E, Platero JL, Carrera-Juliá S, López-Rodríguez MM, Ceron JJ, Tvarijonaviciute A, Navarro MÁ, Moreno ML, et al. Possible Reduction of Cardiac Risk after Supplementation with Epigallocatechin Gallate and Increase of Ketone Bodies in the Blood in Patients with Multiple Sclerosis. A Pilot Study. Nutrients. 2020; 12(12):3792. https://doi.org/10.3390/nu12123792
Chicago/Turabian StyleBenlloch, María, María Cuerda Ballester, Eraci Drehmer, Jose Luis Platero, Sandra Carrera-Juliá, María Mar López-Rodríguez, Jose Joaquin Ceron, Asta Tvarijonaviciute, Marí Ángeles Navarro, Mari Luz Moreno, and et al. 2020. "Possible Reduction of Cardiac Risk after Supplementation with Epigallocatechin Gallate and Increase of Ketone Bodies in the Blood in Patients with Multiple Sclerosis. A Pilot Study" Nutrients 12, no. 12: 3792. https://doi.org/10.3390/nu12123792
APA StyleBenlloch, M., Cuerda Ballester, M., Drehmer, E., Platero, J. L., Carrera-Juliá, S., López-Rodríguez, M. M., Ceron, J. J., Tvarijonaviciute, A., Navarro, M. Á., Moreno, M. L., & de la Rubia Ortí, J. E. (2020). Possible Reduction of Cardiac Risk after Supplementation with Epigallocatechin Gallate and Increase of Ketone Bodies in the Blood in Patients with Multiple Sclerosis. A Pilot Study. Nutrients, 12(12), 3792. https://doi.org/10.3390/nu12123792