Propolis Suppresses UV-Induced Photoaging in Human Skin through Directly Targeting Phosphoinositide 3-Kinase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Extraction of Propolis
2.3. Cell Culture and UV Irradiation
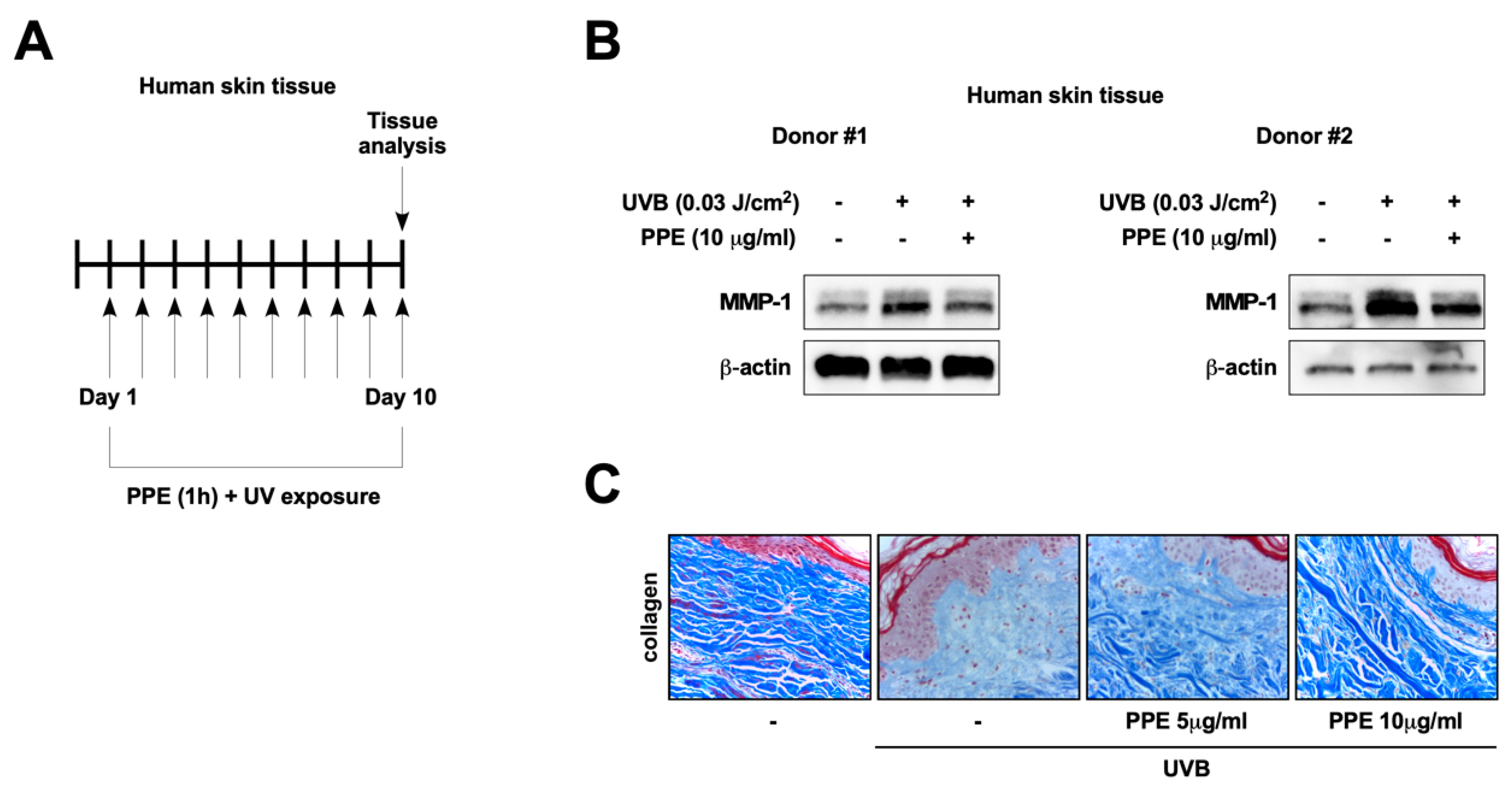
2.4. Excised Human Skin and UV Irradiation
2.5. Cell Cytotoxicity Assay
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Quantitative Real-Time PCR
2.8. Histological Analysis
2.9. Immunoblot Assay
2.10. High-Performance Liquid Chromatography (HPLC) Analysis
2.11. Kinase Assay
2.12. Modeling the Structures of Chemical Compounds on PI3K
3. Results
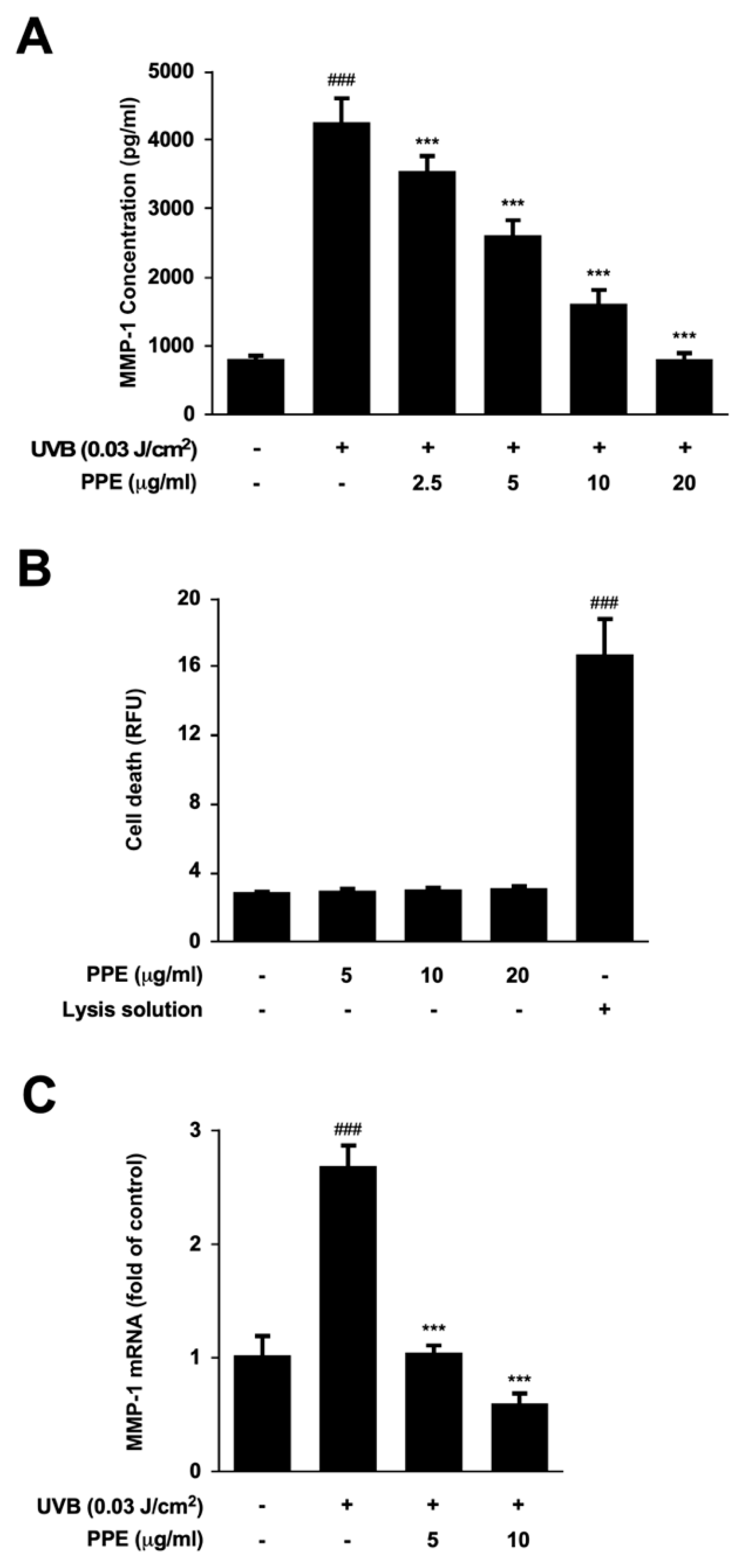
3.1. Propolis Extract Suppresses UV-Mediated MMP-1 Expression and Collagen Degradation in Human Skin
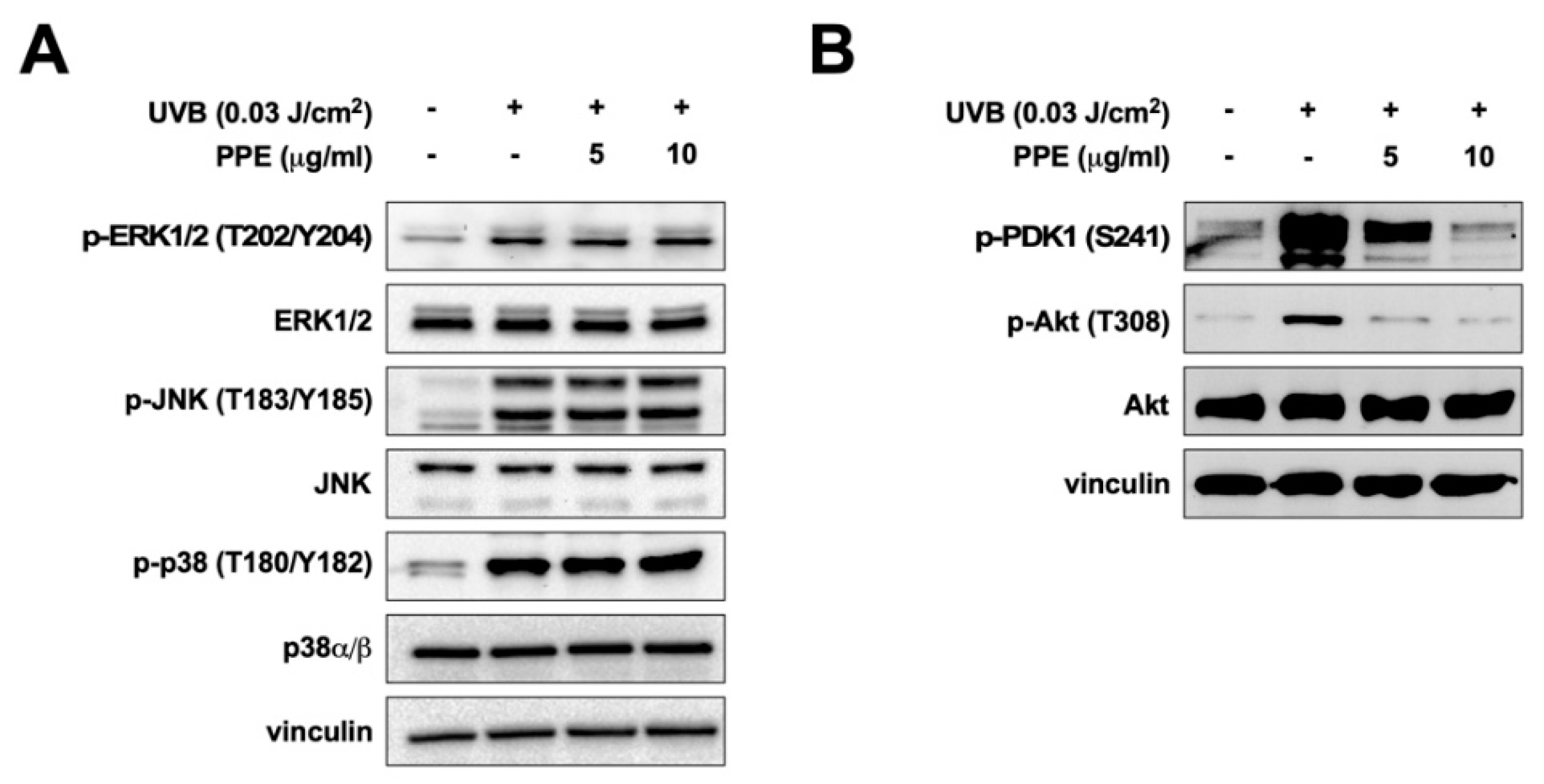
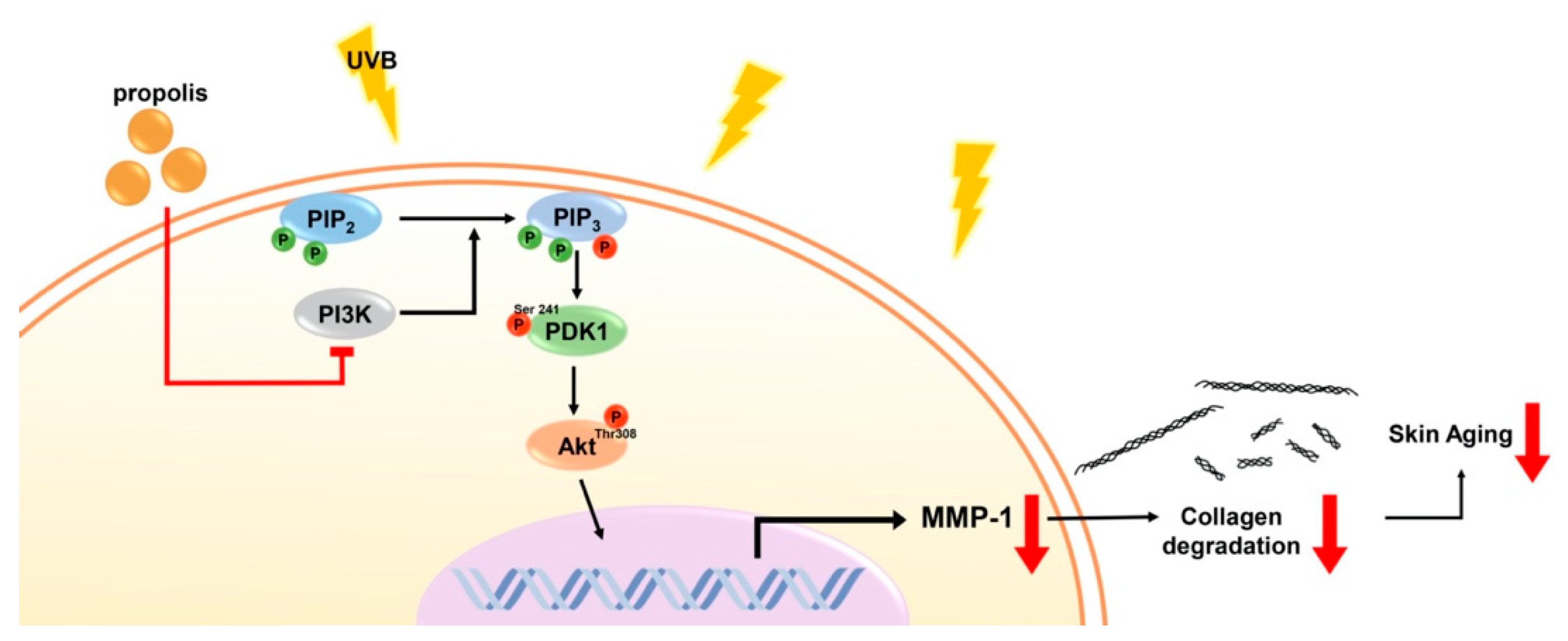
3.2. Propolis Specifically Downregulates Akt and PDK1 Signaling Pathways
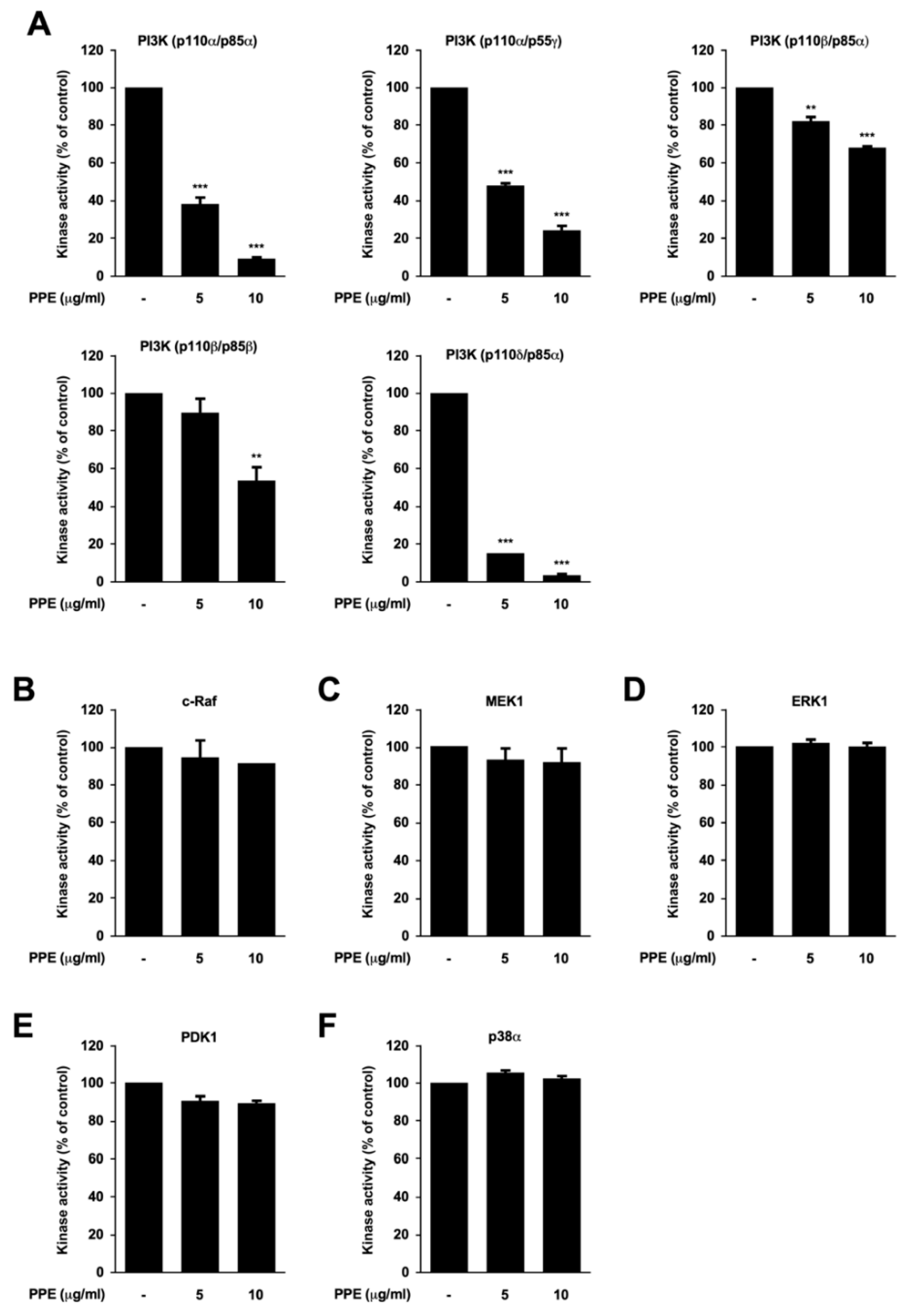
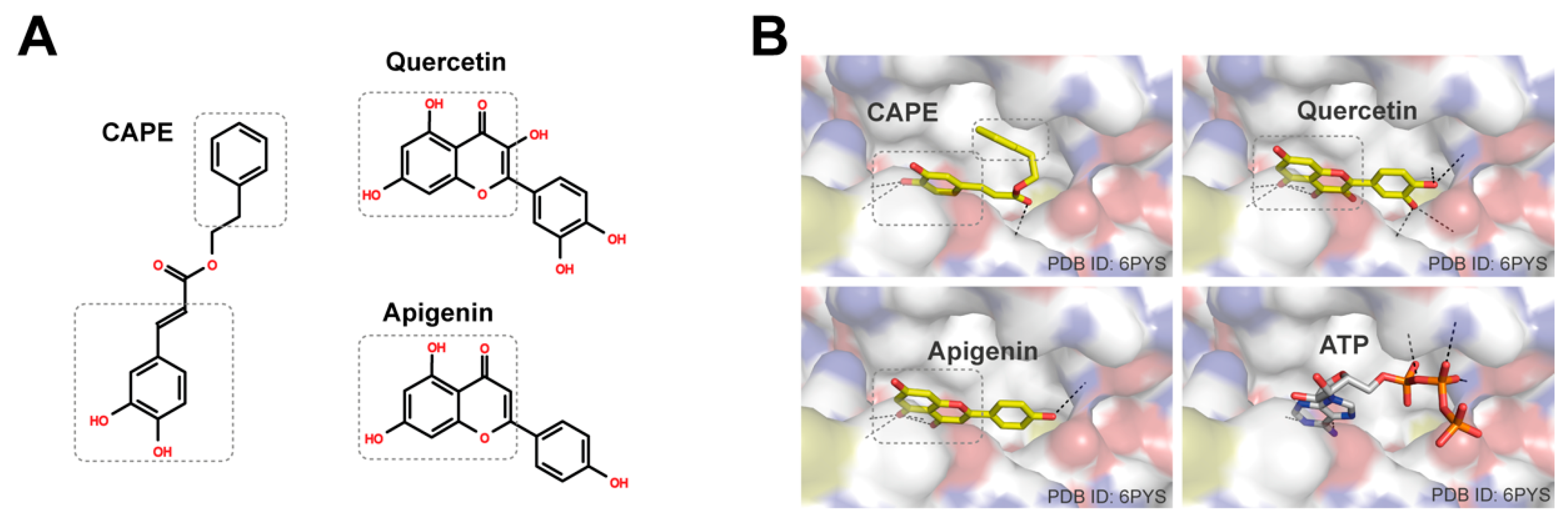
3.3. Propolis Directly Targets Phosphoinositide 3-Kinase (PI3K)
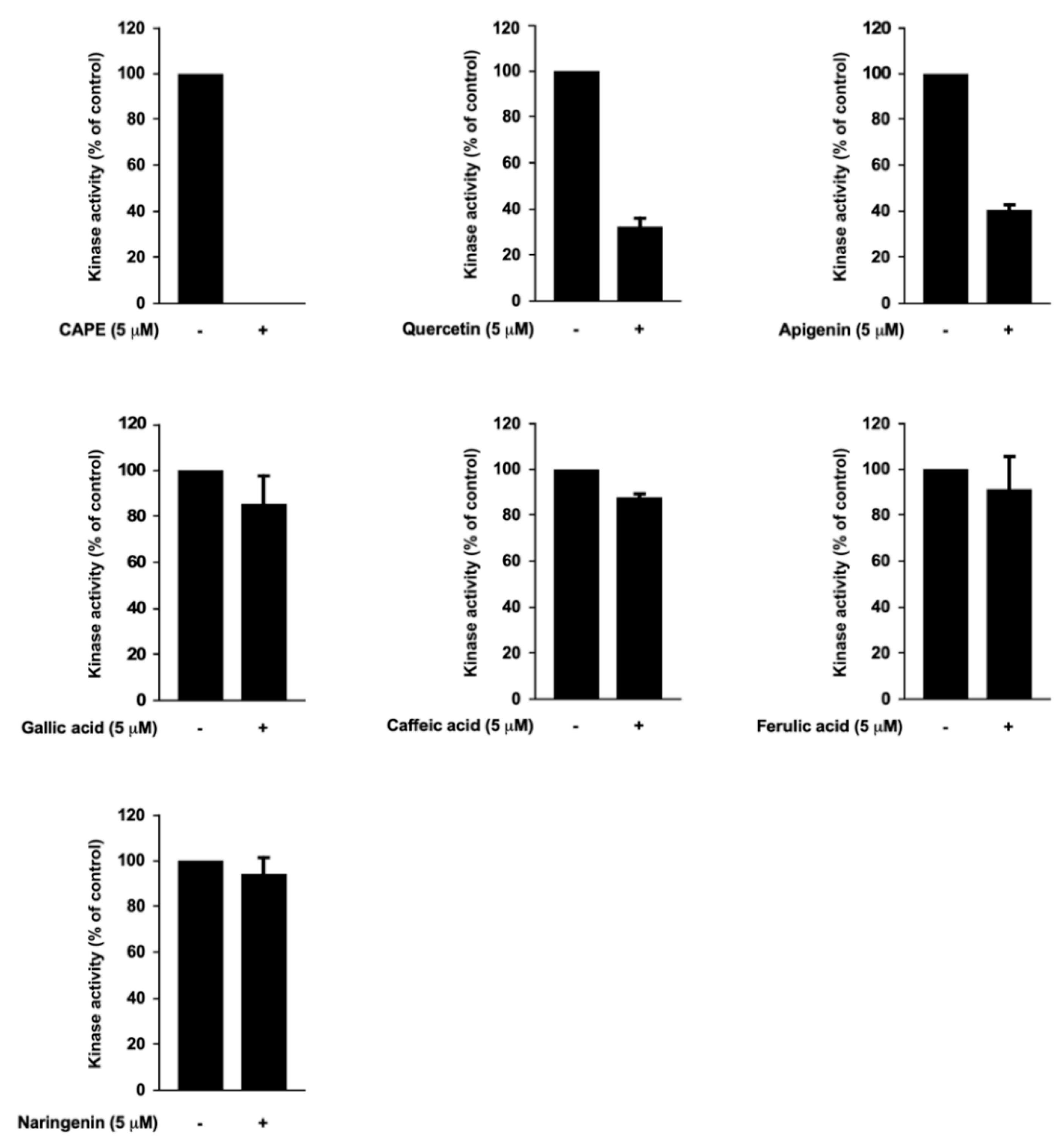
3.4. Caffeic Acid Phenethyl Ester (CAPE), Quercetin, and Apigenin Are the Bioactive Components of Propolis in Targeting PI3K
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Philips, N.; Auler, S.; Hugo, R.; Gonzalez, S. Beneficial Regulation of Matrix Metalloproteinases for Skin Health. Enzym. Res. 2011, 2011, 427285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Talwar, H.S.; Griffiths, C.E.; Fisher, G.J.; Hamilton, T.A.; Voorhees, J.J. Reduced type I and type III procollagens in photodamaged adult human skin. J. Investig. Dermatol. 1995, 105, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Amzel, L.M.; Huang, C.H.; Mandelker, D.; Lengauer, C.; Gabelli, S.B.; Vogelstein, B. Structural comparisons of class I phosphoinositide 3-kinases. Nat. Rev. Cancer 2008, 8, 665–669. [Google Scholar] [CrossRef] [Green Version]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [Green Version]
- Noh, E.M.; Park, J.; Song, H.R.; Kim, J.M.; Lee, M.; Song, H.K.; Hong, O.Y.; Whang, P.H.; Han, M.K.; Kwon, K.B.; et al. Skin Aging-Dependent Activation of the PI3K Signaling Pathway via Downregulation of PTEN Increases Intracellular ROS in Human Dermal Fibroblasts. Oxid. Med. Cell. Longev. 2016, 2016, 6354261. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, A.; Park, J.M.; Kim, S.H.; Chung, A.S. Ultraviolet B-induced matrix metalloproteinase-1 and -3 secretions are mediated via PTEN/Akt pathway in human dermal fibroblasts. J. Cell. Physiol. 2006, 209, 775–785. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [Green Version]
- Ambi, A.; Bryan, J.; Borbon, K.; Centeno, D.; Liu, T.; Chen, T.P.; Cattabiani, T.; Traba, C. Are Russian propolis ethanol extracts the future for the prevention of medical and biomedical implant contaminations? Phytomedicine 2017, 30, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Chien, Y.W.; Chang, M.L.; Hou, C.C.; Chan, C.H.; Tang, H.W.; Huang, H.Y. Taiwanese Green Propolis Ethanol Extract Delays the Progression of Type 2 Diabetes Mellitus in Rats Treated with Streptozotocin/High-Fat Diet. Nutrients 2018, 10, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Bode, A.M.; Dong, Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE 2003, 2003, re2. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [Green Version]
- Matheny, R.W., Jr.; Adamo, M.L. PI3K p110 alpha and p110 beta have differential effects on Akt activation and protection against oxidative stress-induced apoptosis in myoblasts. Cell Death Differ. 2010, 17, 677–688. [Google Scholar] [CrossRef] [Green Version]
- Utermark, T.; Rao, T.; Cheng, H.; Wang, Q.; Lee, S.H.; Wang, Z.C.; Iglehart, J.D.; Roberts, T.M.; Muller, W.J.; Zhao, J.J. The p110α and p110β isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes Dev. 2012, 26, 1573–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondonno, N.P.; Lewis, J.R.; Blekkenhorst, L.C.; Bondonno, C.P.; Shin, J.H.; Croft, K.D.; Woodman, R.J.; Wong, G.; Lim, W.H.; Gopinath, B.; et al. Association of flavonoids and flavonoid-rich foods with all-cause mortality: The Blue Mountains Eye Study. Clin. Nutr. 2020, 39, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrand, L.; Byun, S. Induction of Synthetic Lethality by Natural Compounds Targeting Cancer Signaling. Curr. Pharm. Des. 2017, 23, 4311–4320. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Oh, K.N.; Yun, H.J.; Jeong, H.G. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J. Dermatol. Sci. 2011, 61, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Cheng, X.; Yi, B.; Zhang, X.; Li, Q. Apigenin induces dermal collagen synthesis via smad2/3 signaling pathway. Eur. J. Histochem. 2015, 59, 2467. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Jo, S.; Choi, H.K.; Choi, S.; Byun, S.; Lim, T.G. Caffeic Acid Phenethyl Ester Inhibits UV-Induced MMP-1 Expression by Targeting Histone Acetyltransferases in Human Skin. Int. J. Mol. Sci. 2019, 20, 3055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.G.; Byun, S. Quercetin Directly Targets JAK2 and PKCdelta and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Chen, W.K.; Wang, C.J.; Lin, W.L.; Tseng, T.H. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 2008, 226, 178–191. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. PI-103 and Quercetin Attenuate PI3K-AKT Signaling Pathway in T- Cell Lymphoma Exposed to Hydrogen Peroxide. PLoS ONE 2016, 11, e0160686. [Google Scholar] [CrossRef]
| No. | Compound | mg/kg of Extract |
|---|---|---|
| 1 | Gallic acid | 290.88 |
| 2 | Protocatechuic acid | 69.61 |
| 3 | Catechin | 248.37 |
| 4 | Caffeic acid | 868.34 |
| 5 | 4-Coumaric acid | 2170.78 |
| 6 | Ferulic acid | 691.84 |
| 7 | Quercetin | 1070.33 |
| 8 | Naringenin | 504.65 |
| 9 | Apigenin | 2597.77 |
| 10 | Caffeic acid phenethyl ester (CAPE) | 9153 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Auh, J.-H.; Oh, J.; Hong, S.; Choi, S.; Shin, E.J.; Woo, S.O.; Lim, T.-G.; Byun, S. Propolis Suppresses UV-Induced Photoaging in Human Skin through Directly Targeting Phosphoinositide 3-Kinase. Nutrients 2020, 12, 3790. https://doi.org/10.3390/nu12123790
Kim DH, Auh J-H, Oh J, Hong S, Choi S, Shin EJ, Woo SO, Lim T-G, Byun S. Propolis Suppresses UV-Induced Photoaging in Human Skin through Directly Targeting Phosphoinositide 3-Kinase. Nutrients. 2020; 12(12):3790. https://doi.org/10.3390/nu12123790
Chicago/Turabian StyleKim, Da Hyun, Joong-Hyuck Auh, Jeongyeon Oh, Seungpyo Hong, Sungbin Choi, Eun Ju Shin, Soon Ok Woo, Tae-Gyu Lim, and Sanguine Byun. 2020. "Propolis Suppresses UV-Induced Photoaging in Human Skin through Directly Targeting Phosphoinositide 3-Kinase" Nutrients 12, no. 12: 3790. https://doi.org/10.3390/nu12123790
APA StyleKim, D. H., Auh, J.-H., Oh, J., Hong, S., Choi, S., Shin, E. J., Woo, S. O., Lim, T.-G., & Byun, S. (2020). Propolis Suppresses UV-Induced Photoaging in Human Skin through Directly Targeting Phosphoinositide 3-Kinase. Nutrients, 12(12), 3790. https://doi.org/10.3390/nu12123790







