Dietary Cameroonian Plants Exhibit Anti-Inflammatory Activity in Human Gastric Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Hydroethanolic Extracts
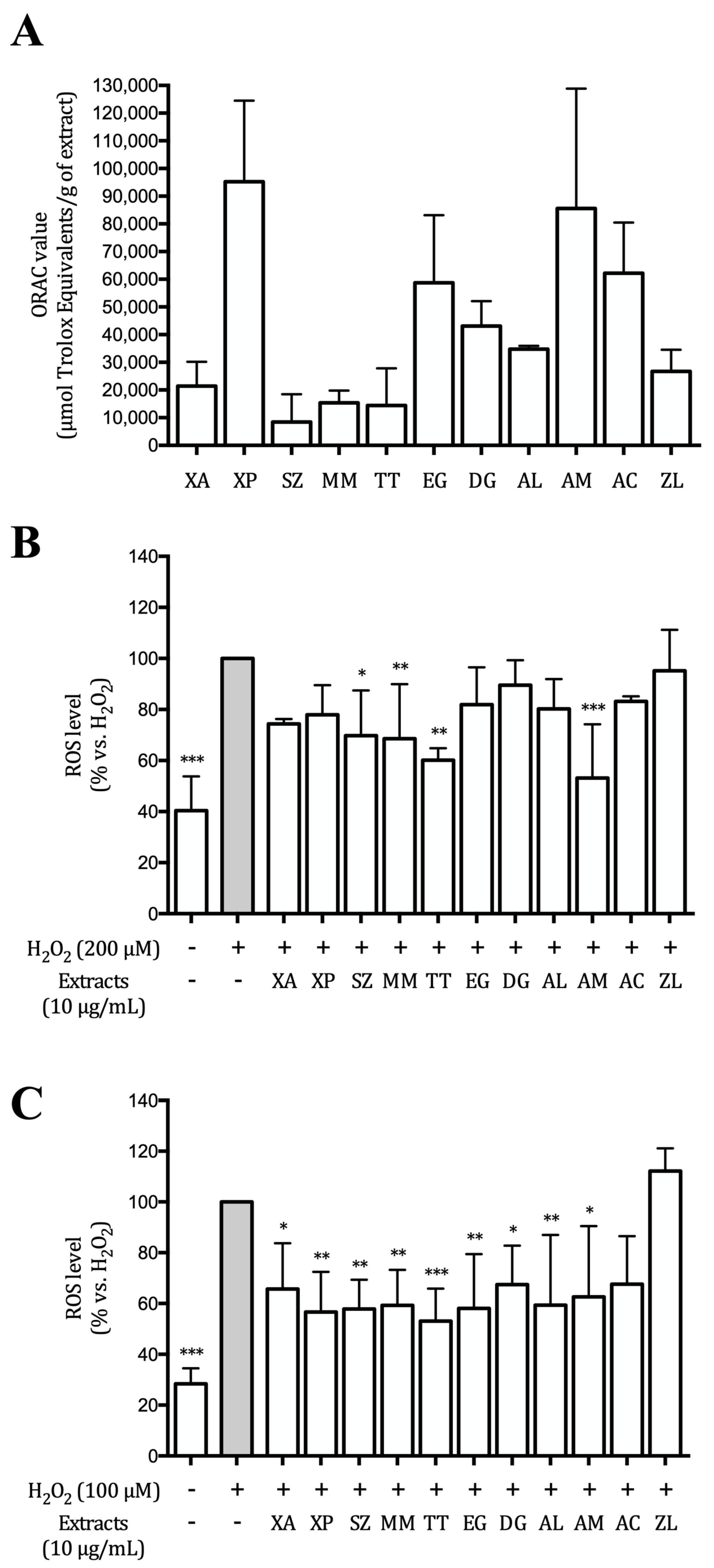
2.2. ORAC Assay
2.3. Cell Culture
2.4. Cytotoxicity Assay
2.5. Cell Treatment
2.6. ROS Production
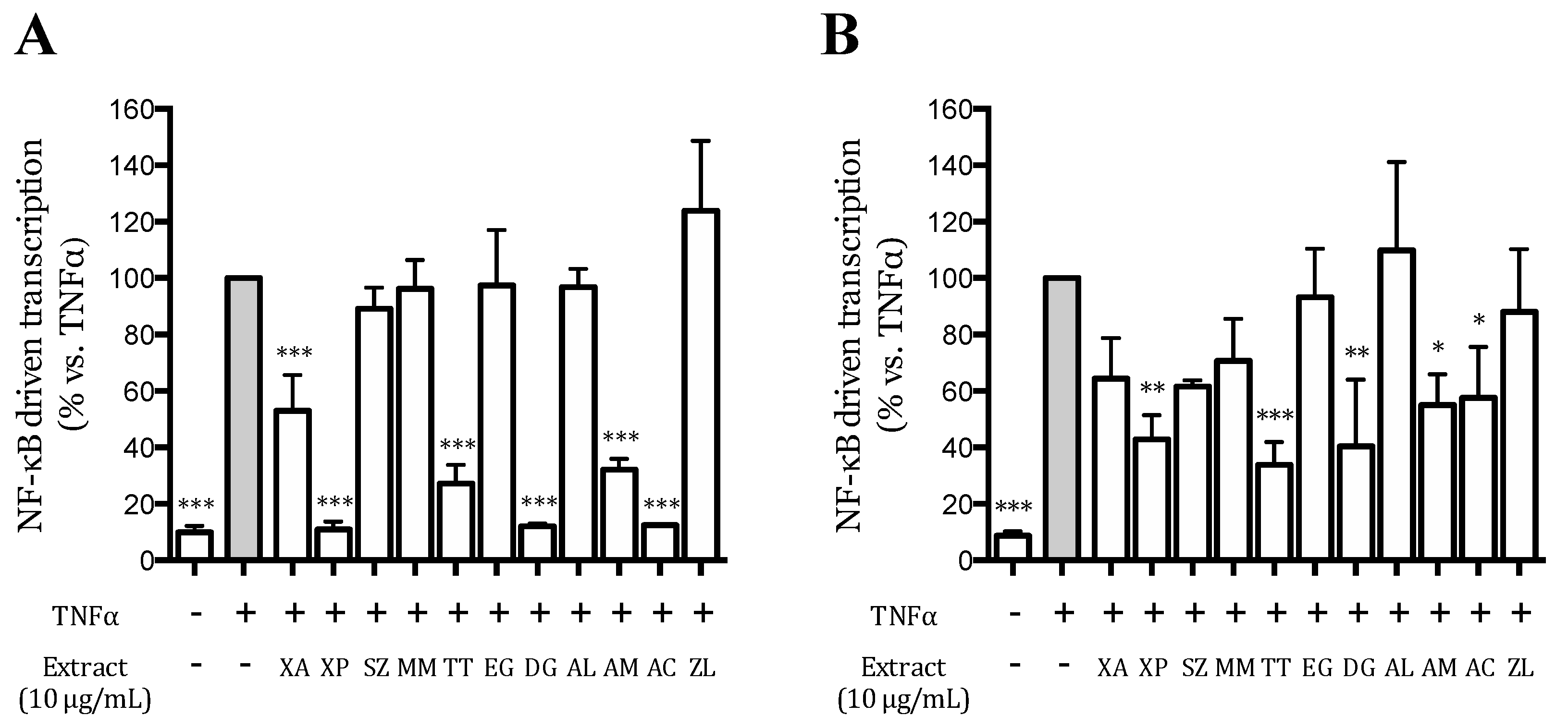
2.7. Transient Transfection Assays
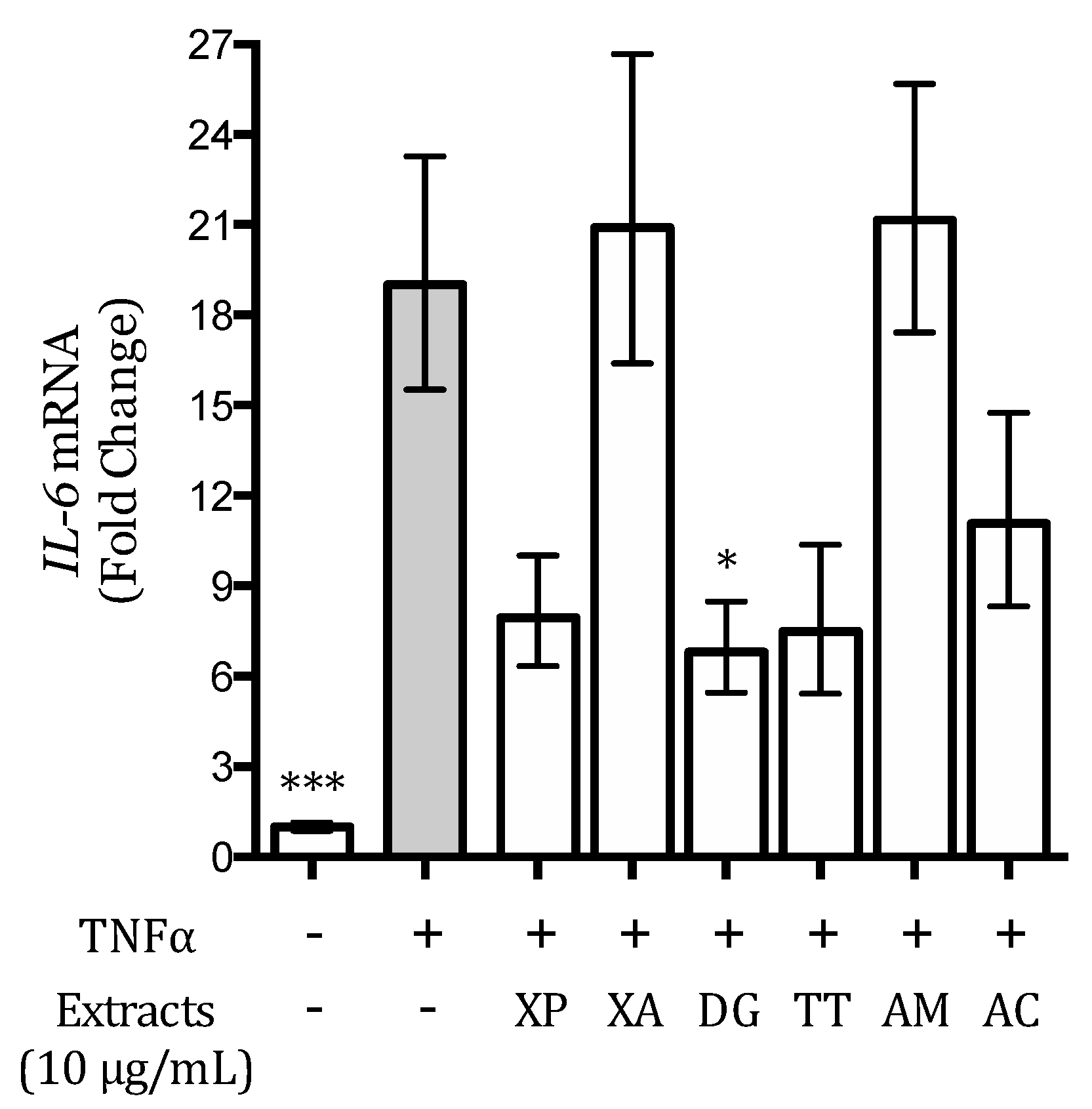
2.8. IL-8 and IL-6 Release
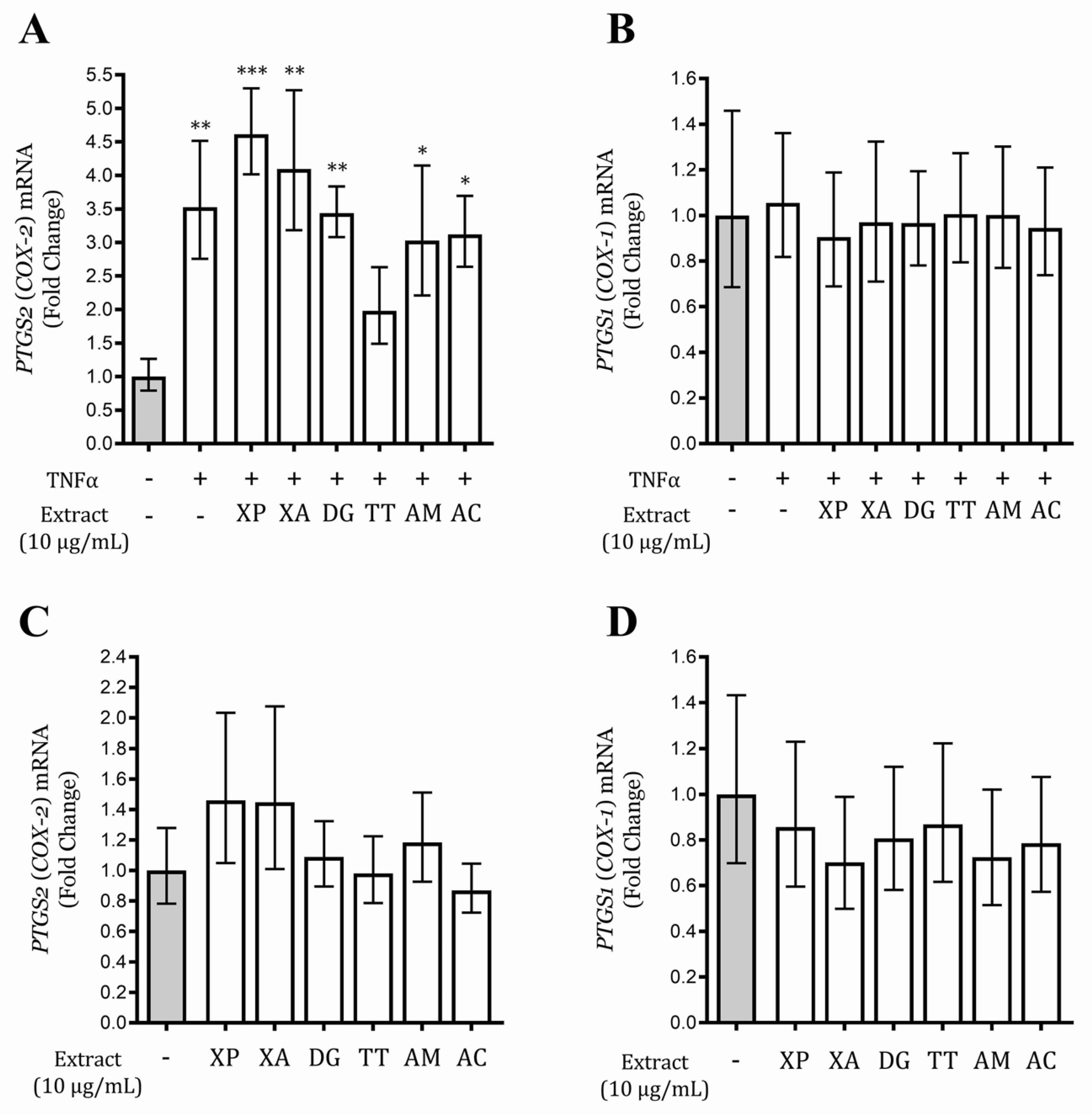
2.9. Gene Expression
2.10. Statistical Analysis
3. Results
3.1. Cytotoxicity of the Extracts in Gastric Epithelial Cells
3.2. Hydroalcoholic Extracts Inhibit ROS Production in AGS and GES-1 Cells
3.3. Effect of the Extracts on the TNFα-Induced NF-κB-Driven Transcription in AGS and GES-1 Cells
3.4. Plant Extracts Inhibit TNFα-Induced IL-8 Release and Expression in AGS and GES-1 Cells
3.5. Effect of the Extracts on the TNFα-Induced IL-6 Release and Expression in AGS and GES-1 Cells
3.6. Tetrapleura Tetraptera Extract Inhibits PTGS2 (COX-2) Gene Expression in AGS and GES-1 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peek, R.M., Jr.; Crabtree, J.E. Helicobacter infection and gastric neoplasia. J. Pathol. 2006, 208, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Noto, J.M.; Peek, R.M., Jr. Helicobacter pylori: An overview. Methods Mol. Biol. 2012, 921, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, H.; Mizuta, Y.; Miyazaki, M.; Takeshima, F.; Omagari, K.; Murase, K.; Nishiyama, T.; Inoue, K.; Murata, I.; Kohno, S. Implication of nf-kappab in helicobacter pylori-associated gastritis. Am. J. Gastroenterol. 2000, 95, 2768–2776. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Tsuchimoto, D.; Takizawa, H.; Azuma, A.; Wakebe, H.; Ohmoto, Y.; Imagawa, K.; Kikuchi, M.; Mukaida, N.; Matsushima, K. Mechanisms involved in helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, mkn45. Infect. Immun. 1997, 65, 3218–3224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasumoto, K.; Okamoto, S.; Mukaida, N.; Murakami, S.; Mai, M.; Matsushima, K. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on ap-1 and nf-kb-like binding sites of the interleukin 8 gene. J. Biol. Chem. 1992, 267, 22506–22511. [Google Scholar]
- Aggarwal, B.B. Nuclear factor-kappab: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.A.; Tummuru, M.K.; Blaser, M.J.; Kerr, L.D. Activation of il-8 gene expression by helicobacter pylori is regulated by transcription factor nuclear factor-kappa b in gastric epithelial cells. J. Immunol. 1998, 160, 2401–2407. [Google Scholar]
- Crabtree, J.E.; Wyatt, J.I.; Trejdosiewicz, L.K.; Peichl, P.; Nichols, P.H.; Ramsay, N.; Primrose, J.N.; Lindley, I.J. Interleukin-8 expression in helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J. Clin. Pathol. 1994, 47, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Crabtree, J.E.; Peichl, P.; Wyatt, J.I.; Stachl, U.; Lindley, I.J. Gastric interleukin-8 and iga il-8 autoantibodies in helicobacter pylori infection. Scand. J. Immunol. 1993, 37, 65–70. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T. Molecular and cellular mechanisms involved in helicobacter pylori-induced inflammation and oxidative stress. Free Radic. Biol. Med. 2002, 33, 323–336. [Google Scholar] [CrossRef]
- Kim, Y.; Seo, J.H.; Kim, H. Beta-carotene and lutein inhibit hydrogen peroxide-induced activation of nf-kappab and il-8 expression in gastric epithelial ags cells. J. Nutr. Sci. Vitaminol. 2011, 57, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Hawkey, C.J. Cox-1 and cox-2 inhibitors. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 801–820. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Arakawa, T.; Ueda, N.; Yamamoto, S. Transcriptional roles of nuclear factor kappa b and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in mc3t3-e1 cells. J. Biol. Chem. 1995, 270, 31315–31320. [Google Scholar] [CrossRef] [Green Version]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdou Bouba, A.; Njintang, Y.N.; Scher, J.; Mbofung, C.M.F. Phenolic compounds and radical scavenging potential of twenty cameroonian spices. Agric. Biol. J. N. Am. 2010, 1, 213–224. [Google Scholar] [CrossRef]
- Fokunang, C.N.; Ndikum, V.; Tabi, O.Y.; Jiofack, R.B.; Ngameni, B.; Guedje, N.M.; Tembe-Fokunang, E.A.; Tomkins, P.; Barkwan, S.; Kechia, F.; et al. Traditional medicine: Past, present and future research and development prospects and integration in the national health system of cameroon. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Tsabang, N.; Yedjou, C.G.; Tsambang, L.; Tchinda, A.T.; Donfagsiteli, N.; Agbor, G.A.; Tchounwou, P.; Nkongmeneck, B.A. Treatment of diabetes and/or hypertension using medicinal plants in cameroon. Med. Aromat. Plants 2015. [Google Scholar] [CrossRef] [Green Version]
- Noumi, E.; Dibakto, T.W. Medicinal plants used for peptic ulcer in the bangangte region, western cameroon. Fitoterapia 2000, 71, 406–412. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Tchuenguem, R.T.; Kuiate, J.R.; Teke, G.N.; Kechia, F.A.; Kuete, V. In vitro and in vivo antifungal activities of selected cameroonian dietary spices. BMC Complement. Altern. Med. 2014, 14, 58. [Google Scholar] [CrossRef] [Green Version]
- Kuete, V.; Krusche, B.; Youns, M.; Voukeng, I.; Fankam, A.G.; Tankeo, S.; Lacmata, S.; Efferth, T. Cytotoxicity of some cameroonian spices and selected medicinal plant extracts. J. Ethnopharmacol. 2011, 134, 803–812. [Google Scholar] [CrossRef]
- Fankam, A.G.; Kuete, V.; Voukeng, I.K.; Kuiate, J.R.; Pages, J.M. Antibacterial activities of selected cameroonian spices and their synergistic effects with antibiotics against multidrug-resistant phenotypes. BMC Complement. Altern. Med. 2011, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Ojewole, J.A.; Adewunmi, C.O. Anti-inflammatory and hypoglycaemic effects of tetrapleura tetraptera (taub) [fabaceae] fruit aqueous extract in rats. J. Ethnopharmacol. 2004, 95, 177–182. [Google Scholar] [CrossRef]
- Woguem, V.; Fogang, H.P.; Maggi, F.; Tapondjou, L.A.; Womeni, H.M.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Lupidi, G.; et al. Volatile oil from striped african pepper (xylopia parviflora, annonaceae) possesses notable chemopreventive, anti-inflammatory and antimicrobial potential. Food Chem. 2014, 149, 183–189. [Google Scholar] [CrossRef]
- Atchan Nwakiban, A.P.; Sokeng, A.J.; Dell’Agli, M.; Bossi, L.; Beretta, G.; Gelmini, F.; Deutou Tchamgoue, A.; Agbor Agbor, G.; Kuiate, J.R.; Daglia, M.; et al. Hydroethanolic plant extracts from cameroon positively modulate enzymes relevant to carbohydrate/lipid digestion and cardio-metabolic diseases. Food Funct. 2019, 10, 6533–6542. [Google Scholar] [CrossRef] [PubMed]
- Nwakiban, A.P.A.; Cicolari, S.; Piazza, S.; Gelmini, F.; Sangiovanni, E.; Martinelli, G.; Bossi, L.; Carpentier-Maguire, E.; Tchamgoue, A.D.; Agbor, G.; et al. Oxidative stress modulation by cameroonian spice extracts in hepg2 cells: Involvement of nrf2 and improvement of glucose uptake. Metabolites 2020, 10, 182. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.; Gomez-Cordoves, C.; Bartolome, B. Extending applicability of the oxygen radical absorbance capacity (orac-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Galli, G.V.; Bosisio, E.; D’Ambrosio, M. Inhibition of nf-kb and metalloproteinase-9 expression and secretion by parthenolide derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 1858–1860. [Google Scholar] [CrossRef]
- Bagheri, N.; Azadegan-Dehkordi, F.; Shirzad, H.; Rafieian-Kopaei, M.; Rahimian, G.; Razavi, A. The biological functions of il-17 in different clinical expressions of helicobacter pylori-infection. Microb. Pathog. 2015, 81, 33–38. [Google Scholar] [CrossRef]
- Tanahashi, T.; Kita, M.; Kodama, T.; Yamaoka, Y.; Sawai, N.; Ohno, T.; Mitsufuji, S.; Wei, Y.P.; Kashima, K.; Imanishi, J. Cytokine expression and production by purified helicobacter pylori urease in human gastric epithelial cells. Infect. Immun. 2000, 68, 664–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, Y.; Ning, T.; Wang, B. [establishment and characterization of a sv40 transformed human fetal gastric epithelial cell line-ges-1]. Zhonghua Zhong Liu Za Zhi 1994, 16, 7–10. [Google Scholar] [PubMed]
- Suzuki, M.; Miura, S.; Mori, M.; Kai, A.; Suzuki, H.; Fukumura, D.; Suematsu, M.; Tsuchiya, M. Rebamipide, a novel antiulcer agent, attenuates helicobacter pylori induced gastric mucosal cell injury associated with neutrophil derived oxidants. Gut 1994, 35, 1375–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butcher, L.D.; den Hartog, G.; Ernst, P.B.; Crowe, S.E. Oxidative stress resulting from helicobacter pylori infection contributes to gastric carcinogenesis. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Suh, S.J.; Kwak, C.H.; Chung, T.W.; Park, S.J.; Cheeeei, M.; Park, S.S.; Seo, C.S.; Son, J.K.; Chang, Y.C.; Park, Y.G.; et al. Pimaric acid from aralia cordata has an inhibitory effect on tnf-alpha-induced mmp-9 production and hasmc migration via down-regulated nf-kappab and ap-1. Chem. Biol. Interact. 2012, 199, 112–119. [Google Scholar] [CrossRef]
- Saha, A.; Blando, J.; Silver, E.; Beltran, L.; Sessler, J.; DiGiovanni, J. 6-shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of stat3 and nf-kappab signaling. Cancer Prev. Res. 2014, 7, 627–638. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.; Sang, Y.; Liu, W.; Yu, W.; Wang, X. Anti-inflammatory effects of gingerol on lipopolysaccharide-stimulated raw 264.7 cells by inhibiting nf-kappab signaling pathway. Inflammation 2018, 41, 835–845. [Google Scholar] [CrossRef]
- Ruifeng, G.; Yunhe, F.; Zhengkai, W.; Ershun, Z.; Yimeng, L.; Minjun, Y.; Xiaojing, S.; Zhengtao, Y.; Naisheng, Z. Chlorogenic acid attenuates lipopolysaccharide-induced mice mastitis by suppressing tlr4-mediated nf-kappab signaling pathway. Eur. J. Pharmacol. 2014, 729, 54–58. [Google Scholar] [CrossRef]
- Luo, K.W.; Wei, C.; Lung, W.Y.; Wei, X.Y.; Cheng, B.H.; Cai, Z.M.; Huang, W.R. Egcg inhibited bladder cancer sw780 cell proliferation and migration both in vitro and in vivo via down-regulation of nf-kappab and mmp-9. J. Nutr. Biochem. 2017, 41, 56–64. [Google Scholar] [CrossRef]
- Contreras, T.C.; Ricciardi, E.; Cremonini, E.; Oteiza, P.I. (-)-epicatechin in the prevention of tumor necrosis alpha-induced loss of caco-2 cell barrier integrity. Arch. Biochem. Biophys. 2015, 573, 84–91. [Google Scholar] [CrossRef]
- Kuate, D.; Kengne, A.P.; Biapa, C.P.; Azantsa, B.G.; Abdul Manan Bin Wan Muda, W. Tetrapleura tetraptera spice attenuates high-carbohydrate, high-fat diet-induced obese and type 2 diabetic rats with metabolic syndrome features. Lipids Health Dis. 2015, 14, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okokon, J.E.; Udokpoh, A.E.; Antia, B.S. Antimalaria activity of ethanolic extract of tetrapleura tetraptera fruit. J. Ethnopharmacol. 2007, 111, 537–540. [Google Scholar] [CrossRef] [PubMed]

| AGS Cells | GES-1 Cells | |
|---|---|---|
| IC50 (μg/mL) | ||
| Xylopia parviflora Spruce (XP) | 4.1 | >10 |
| Xylopia aethiopica (Dunal) A. Rich. (XA) | >10 | >10 |
| Tetrapleura tetraptera (Schum. and Thonn.) Taub (TT) | 9.7 | 5.9 |
| Dichrostachys glomerata (Forssk.) Chiov. (DG) | 2.1 | 8.8 |
| Aframomum melegueta K.Schum (AM) | 9.9 | >10 |
| Aframomum citratum (C.Pereira) K.Schum (AC) | 6.8 | >10 |
| AGS Cells | GES-1 Cells | |||
|---|---|---|---|---|
| IC50 (μg/mL) | ||||
| IL-8 release | IL-8 promoter activity | IL-8 release | IL-8 promoter activity | |
| Xylopia parviflora Spruce (XP) | 0.3 | 1.0 | 8.4 | >10 |
| Xylopia aethiopica (Dunal) A. Rich. (XA) | 8.1 | >10 | >10 | >10 |
| Tetrapleura tetraptera (Schum. and Thonn.) Taub (TT) | 1.4 | 1.4 | 5.7 | 4.6 |
| Dichrostachys glomerata (Forssk.) Chiov. (DG) | 0.2 | 0.3 | 4.2 | >10 |
| Aframomum melegueta K.Schum (AM) | 1.2 | 1.9 | >10 | >10 |
| Aframomum citratum (C.Pereira) K.Schum (AC) | 0.4 | 2.0 | 2.3 | >10 |
| GES-1 Cells IC50 (μg/mL) | |
|---|---|
| Xylopia parviflora Spruce (XP) | 3.5 |
| Xylopia aethiopica (Dunal) A. Rich. (XA) | >10 |
| Tetrapleura tetraptera (Schum. and Thonn.) Taub (TT) | 4.9 |
| Dichrostachys glomerata (Forssk.) Chiov. (DG) | 3.5 |
| Aframomum melegueta K.Schum (AM) | >10 |
| Aframomum citratum (C.Pereira) K.Schum (AC) | 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwakiban, A.P.A.; Fumagalli, M.; Piazza, S.; Magnavacca, A.; Martinelli, G.; Beretta, G.; Magni, P.; Tchamgoue, A.D.; Agbor, G.A.; Kuiaté, J.-R.; et al. Dietary Cameroonian Plants Exhibit Anti-Inflammatory Activity in Human Gastric Epithelial Cells. Nutrients 2020, 12, 3787. https://doi.org/10.3390/nu12123787
Nwakiban APA, Fumagalli M, Piazza S, Magnavacca A, Martinelli G, Beretta G, Magni P, Tchamgoue AD, Agbor GA, Kuiaté J-R, et al. Dietary Cameroonian Plants Exhibit Anti-Inflammatory Activity in Human Gastric Epithelial Cells. Nutrients. 2020; 12(12):3787. https://doi.org/10.3390/nu12123787
Chicago/Turabian StyleNwakiban, Achille Parfait Atchan, Marco Fumagalli, Stefano Piazza, Andrea Magnavacca, Giulia Martinelli, Giangiacomo Beretta, Paolo Magni, Armelle Deutou Tchamgoue, Gabriel Agbor Agbor, Jules-Roger Kuiaté, and et al. 2020. "Dietary Cameroonian Plants Exhibit Anti-Inflammatory Activity in Human Gastric Epithelial Cells" Nutrients 12, no. 12: 3787. https://doi.org/10.3390/nu12123787







