Modulation of Food Intake by Differential TAS2R Stimulation in Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Ex Vivo Treatment of Intestinal Segments
2.4. Studies of Food Intake
2.5. Enterohormone Quantification
2.6. Statistical Analysis
3. Results
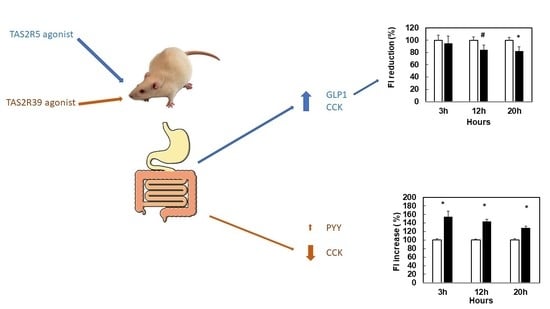
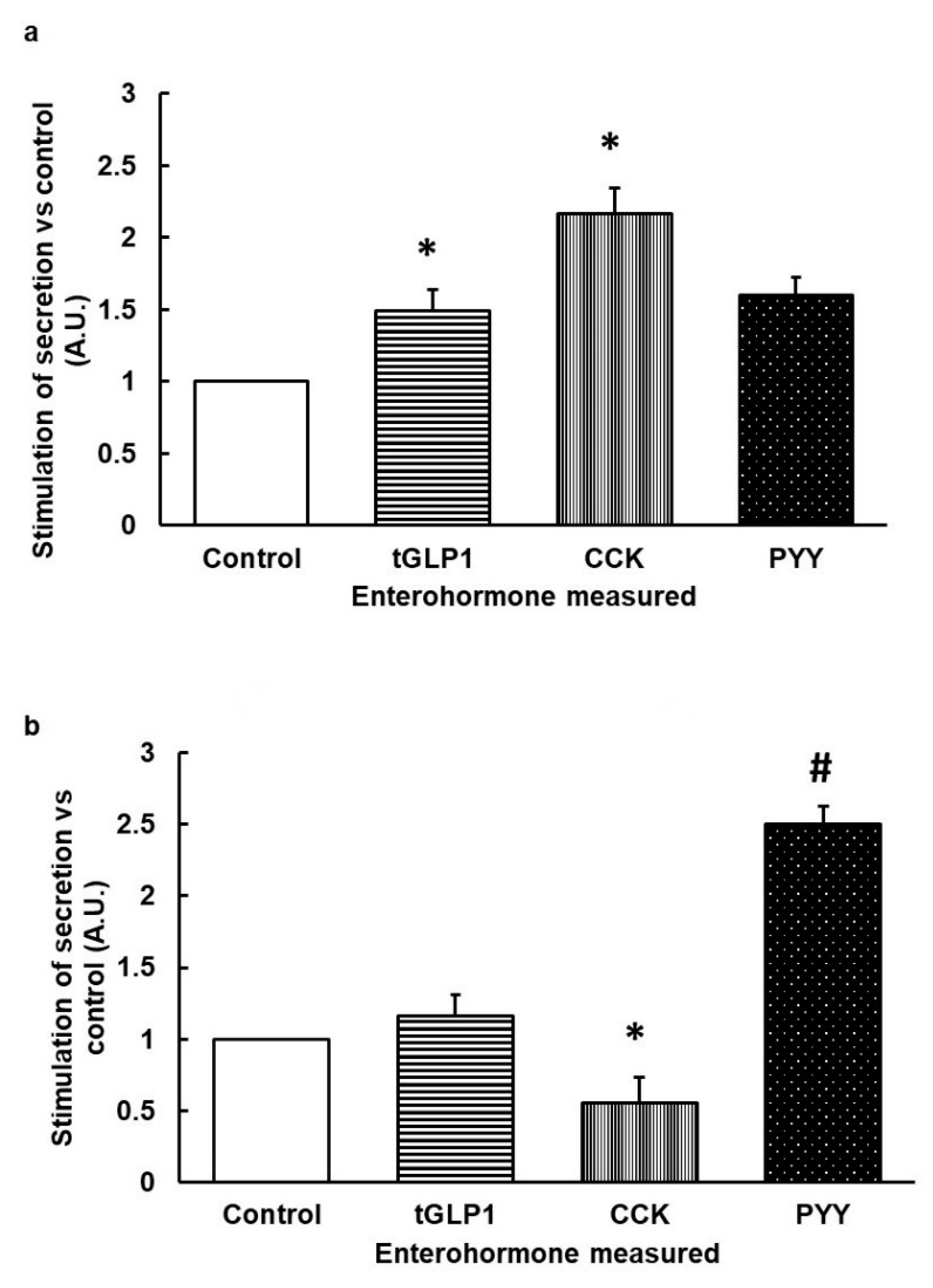
3.1. Stimulation with Specific Agonists of hTA2R5 Increases GLP1 and CCK Secretions, While Stimulation with Specific Agonists of hTA2R39 Tends to Increase PYY and Decrease CCK
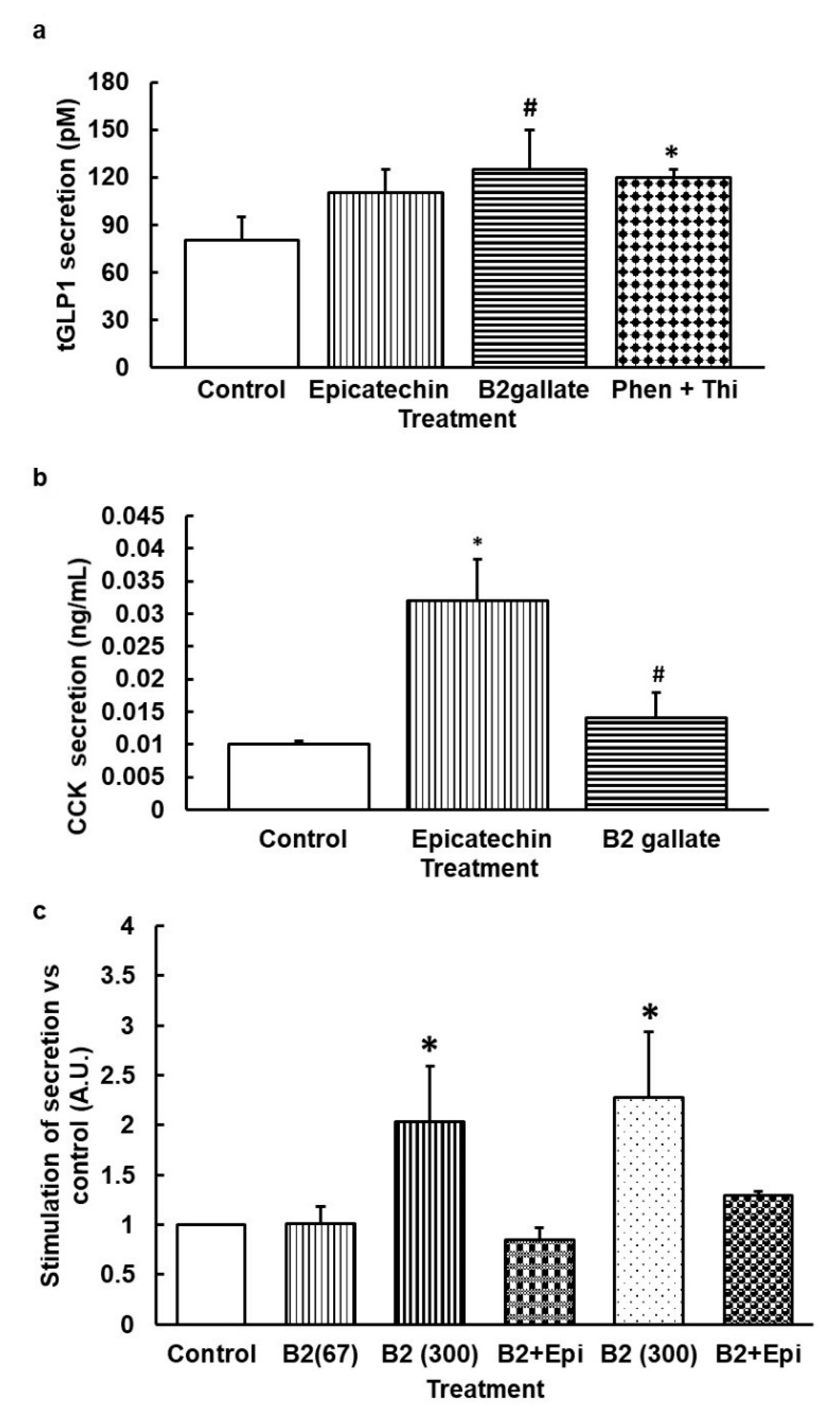
3.2. When Bitter TAS2Rs Are Subjected to Simultaneous Stimulation, the Effect on Secretome Is Similar to the Effect on the Receptor with Lower EC50 Only
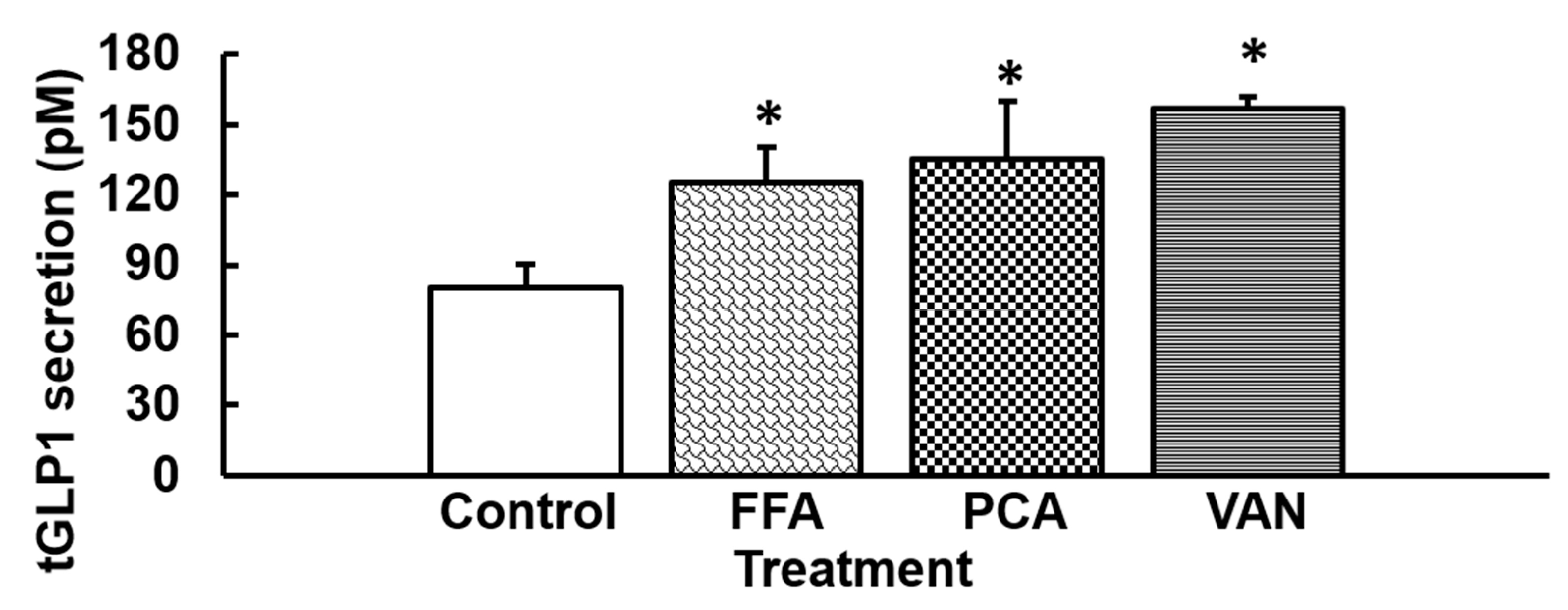
3.3. Stimulation with Agonists of hTAS2R14 Increases GLP1 Secretion
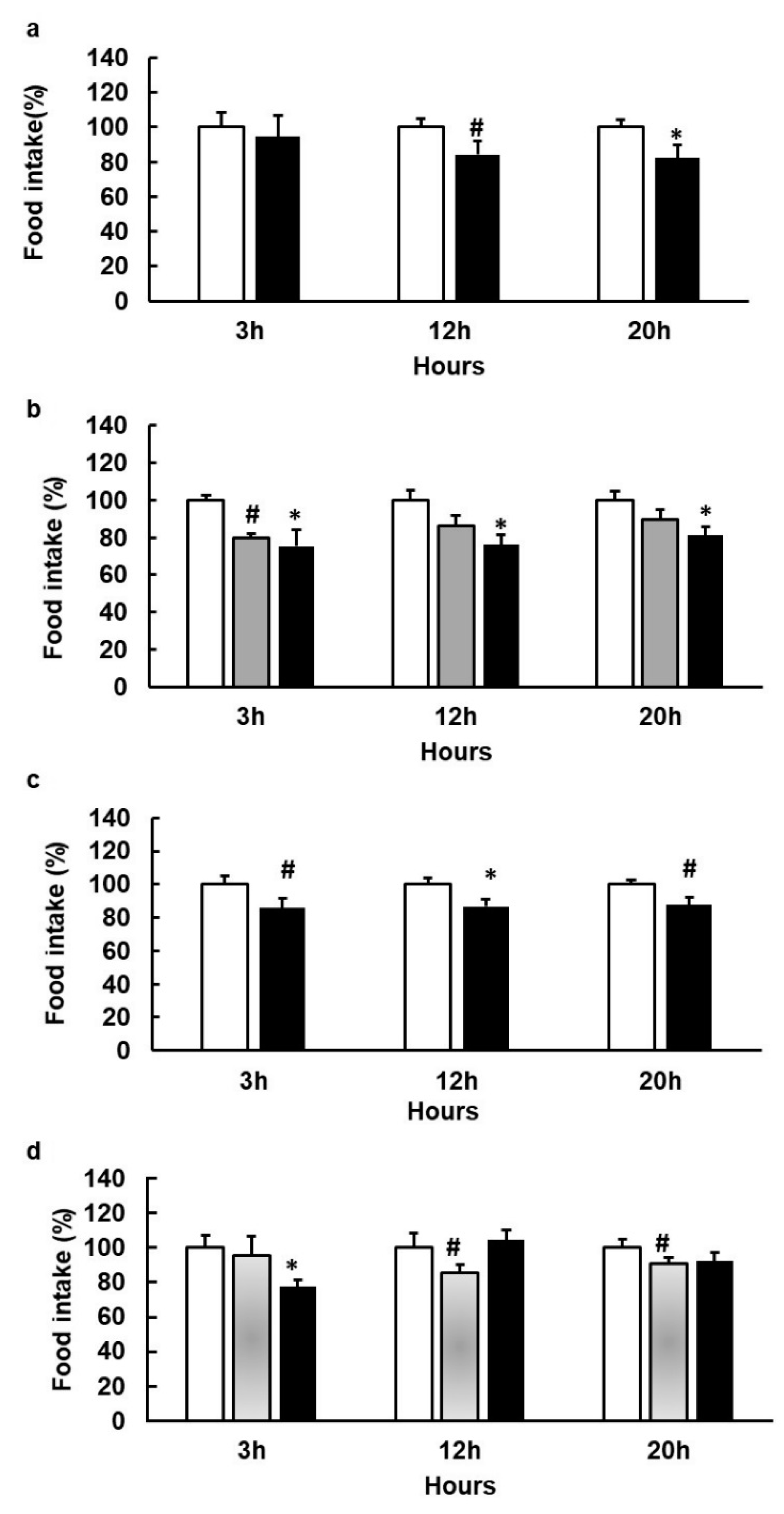
3.4. Agonists That Increase GLP1 and CCK Are More Effective in Limiting Food Intake
3.5. Stronger Agonism of hTAS2R39 Than hTAS2R5 Can Stimulate Food Intake
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blundell, J.E.; Finlayson, G.; Gibbons, C.; Caudwell, P.; Hopkins, M. The biology of appetite control: Do resting metabolic rate and fat-free mass drive energy intake? Physiol. Behav. 2015, 152, 473–478. [Google Scholar] [CrossRef]
- Mulla, C.M.; Middelbeek, R.J.W.; Patti, M.E. Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann. N. Y. Acad. Sci. 2018, 1411, 53–64. [Google Scholar] [CrossRef]
- Gissey, L.C.; Mariolo, J.C.; Mingrone, G. Intestinal peptide changes after bariatric and minimally invasive surgery: Relation to diabetes remission. Peptides 2018, 100, 114–122. [Google Scholar] [CrossRef]
- Sweeney, T.E.; Resident, S.; Morton, J.M.; Bariatric, C. Metabolic surgery: Action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 727–740. [Google Scholar] [CrossRef] [Green Version]
- Serrano, J.; Casanova-Martí, À.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A. Strategy for limiting food intake using food components aimed at multiple targets in the gastrointestinal tract. Trends Food Sci. Technol. 2017, 68, 113–129. [Google Scholar] [CrossRef]
- Glendinning, J.I. Is the bitter rejection response always adaptive? Physiol. Behav. 1994, 56, 1217–1227. [Google Scholar] [CrossRef]
- Nissim, I.; Dagan-Wiener, A.; Niv, M.Y. The taste of toxicity: A quantitative analysis of bitter and toxic molecules. IUBMB Life 2017, 69, 938–946. [Google Scholar] [CrossRef] [Green Version]
- Duffy, V.B.; Hayes, J.E.; Davidson, A.C.; Kidd, J.R.; Kidd, K.K.; Bartoshuk, L.M. Vegetable Intake in College-Aged Adults Is Explained by Oral Sensory Phenotypes and TAS2R38 Genotype. Chemosens. Percept. 2010, 3, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Jeruzal-Świątecka, J.; Fendler, W.; Pietruszewska, W. Clinical role of extraoral bitter taste receptors. Int. J. Mol. Sci. 2020, 21, 5156. [Google Scholar] [CrossRef]
- Foster, S.R.S.R.; Roura, E.; Thomas, W.G.W.G. Extrasensory perception: Odorant and taste receptors beyond the nose and mouth. Pharmacol. Ther. 2014, 142, 41–61. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Di Pizio, A.; Niv, M.Y. Bioorganic & Medicinal Chemistry Promiscuity and selectivity of bitter molecules and their receptors. Bioorg. Med. Chem. 2015, 23, 4082–4091. [Google Scholar] [CrossRef]
- Lossow, K.; Hübner, S.; Roudnitzky, N.; Slack, J.P.; Pollastro, F.; Behrens, M.; Meyerhof, W. Comprehensive analysis of mouse bitter taste receptors reveals different molecular receptive ranges for orthologous receptors in mice and humans. J. Biol. Chem. 2016, 291, 15358–15377. [Google Scholar] [CrossRef] [Green Version]
- Grassin-Delyle, S.; Salvator, H.; Mantov, N.; Abrial, C.; Brollo, M.; Faisy, C.; Naline, E.; Couderc, L.J.L.-J.; Devillier, P. Bitter Taste Receptors (TAS2Rs) in Human Lung Macrophages: Receptor Expression and Inhibitory Effects of TAS2R Agonists. Front. Physiol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Serrano, J.; Casanova-Martí, À.; Depoortere, I.; Blay, M.T.M.T.; Terra, X.; Pinent, M.; Ardévol, A. Subchronic treatment with grape-seed phenolics inhibits ghrelin production despite a short-term stimulation of ghrelin secretion produced by bitter-sensing flavanols. Mol. Nutr. Food Res. 2016, 60, 2554–2564. [Google Scholar] [CrossRef]
- Wang, Q.; Liszt, K.I.K.I.K.I.; Deloose, E.; Canovai, E.; Thijs, T.; Farré, R.; Ceulemans, L.J.L.J.; Lannoo, M.; Tack, J.; Depoortere, I. Obesity alters adrenergic and chemosensory signaling pathways that regulate ghrelin secretion in the human gut. FASEB J. 2019, 33, 4907–4920. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Silva, M.S.M.S.; García-Estevez, I.; Groβmann, P.; Brás, N.; Brandão, E.; Mateus, N.; De Freitas, V.; Behrens, M.; Meyerhof, W. Human Bitter Taste Receptors Are Activated by Different Classes of Polyphenols. J. Agric. Food Chem. 2018, 66, 8814–8823. [Google Scholar] [CrossRef] [PubMed]
- Wiener, A.; Shudler, M.; Levit, A.; Niv, M.Y. BitterDB: A database of bitter compounds. Nucleic Acids Res. 2012, 40, 413–419. [Google Scholar] [CrossRef]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different Phenolic Compounds Activate Distinct Human Bitter Taste Receptors. J. Agric. Food Chem. 2013, 61, 1525–1533. [Google Scholar] [CrossRef]
- Narukawa, M.; Noga, C.; Ueno, Y.; Sato, T.; Misaka, T.; Watanabe, T. Evaluation of the bitterness of green tea catechins by a cell-based assay with the human bitter taste receptor hTAS2R39. Biochem. Biophys. Res. Commun. 2011, 405, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, J.C.; Hofmann, T. Orosensory-Directed Identification of Astringent Mouthfeel and Bitter-Tasting Compounds in Red Wine. J. Agric. Food Chem. 2008, 56, 1376–1386. [Google Scholar] [CrossRef]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef]
- Srivastava, G.; Apovian, C.M. Current pharmacotherapy for obesity. Nat. Rev. Endocrinol. 2018, 14, 12–24. [Google Scholar] [CrossRef]
- Oswald, C.; Rappas, M.; Kean, J.; Doré, A.S.; Errey, J.C.; Bennett, K.; Deflorian, F.; Christopher, J.A.; Jazayeri, A.; Mason, J.S.; et al. Intracellular Allosteric Antagonism of the CCR9 Receptor. Nat. Publ. Gr. 2016, 540, 462–465. [Google Scholar] [CrossRef]
- Woo, J.A.; Castaño, M.; Goss, A.; Kim, D.; Lewandowski, E.M.; Chen, Y.; Liggett, S.B. Differential long-term regulation of TAS2R14 by structurally distinct agonists. FASEB J. 2019, 33, 12213–12225. [Google Scholar] [CrossRef]
- Kenakin, T.P. Chapter 4—Drug Antagonism: Orthosteric Drug Effects. In Pharmacology in Drug Discovery and Development, 2nd ed.; Academic Press: New York, NY, USA, 2017; pp. 65–100. ISBN 978-0-12-803752-2. [Google Scholar]
- Avau, B.; Rotondo, A.; Thijs, T.; Andrews, C.N.; Janssen, P.; Tack, J.; Depoortere, I. Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation. Sci. Rep. 2015, 5, 15985. [Google Scholar] [CrossRef]
- Hayes, J.E.J.E.; Wallace, M.R.; Knopik, V.S.V.S.; Herbstman, D.M.D.M.; Bartoshuk, L.M.L.M.; Duffy, V.B.V.B. Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chem. Senses 2011, 36, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Coltell, O.; Sorlí, J.V.; Asensio, E.M.; Fernández-Carrión, R.; Barragán, R.; Ortega-Azorín, C.; Estruch, R.; González, J.I.; Salas-Salvadó, J.; Lamon-Fava, S.; et al. Association between taste perception and adiposity in overweight or obese older subjects with metabolic syndrome and identification of novel taste-related genes. Am. J. Clin. Nutr. 2019, 109, 1709–1723. [Google Scholar] [CrossRef]
- Serrano, J.; Casanova-Martí, À.; Gil-Cardoso, K.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A. Acutely administered grape-seed proanthocyanidin extract acts as a satiating agent. Food Funct. 2016, 7, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Casanova-martí, À.; Serrano, J.; Portune, K.J.; Sanz, Y. Function microbiota and enteroendocrine secretions in. Food Funct. 2019, 10, 4062–4070. [Google Scholar] [CrossRef]
- González-Abuín, N.; Martínez-Micaelo, N.; Blay, M.; Ardévol, A.; Pinent, M. Grape-Seed Procyanidins Prevent the Cafeteria-Diet-Induced Decrease of Glucagon-Like Peptide-1 Production. J. Agric. Food Chem. 2014, 62, 1066–1072. [Google Scholar] [CrossRef]
- Serrano, J.; Casanova-Martí, À.; Blay, M.; Terra, X.; Ardévol, A.; Pinent, M. Defining conditions for optimal inhibition of food intake in rats by a grape-seed derived proanthocyanidin extract. Nutrients 2016, 8, 652. [Google Scholar] [CrossRef] [Green Version]

| Compound (hTAS2R) | EC50 1 (μM) | Effective Concentration 2 (μM) | Dose Administered to Rats | Dose for Treatment of Intestine Explants |
|---|---|---|---|---|
| 1,10-Phenantroline (hTAS2R5) [18] | Not defined | 100 | 290 μM | 150 μM |
| Thiamine(hTAS2R39) [11] | Not defined | 1000 | 7.5 mM | 1 mM |
| ECg (hTAS2R39) [19] | 88.2 | Not defined | 31 μM | - |
| Epicatechin (hTAS2R5) [19] | 3210 | 1000 | 0.84/1 mM | 1 mM |
| Epicatechin (hTAS2R39) [19] | 3800 | - | - | - |
| B2 gallate(hTAS2R5) [17] | 6.3 | Not defined | - | 20 μM |
| B2 gallate(hTAS2R39) [17] | 9.11 | Not defined | - | - |
| Epigallocatechin Gallate(EGCG) (hTAS2R5) [17] | 12.3 | - | - | - |
| EGCG(hTAS2R39) [17] | 8.5 | Not defined | 21/43 μM | 300 μM |
| EGCG(hTAS2R39) [20] | 181.6 | 10 | - | - |
| Flufenamic acid (hTAS2R14) [11] | Not defined | 10 | 50 μM | - |
| Protocatecuhic acid(hTAS2R14) [17] | 156 | Not defined | 300 μM | |
| Vanillic acid (hTAS2R14) [17] | 151 | Not defined | 1.5 mM | 300 μM |
| Procyanidin B2 [21] | Not defined | 485 μM 3 | 0.11 mM | 67/300 μM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grau-Bové, C.; Miguéns-Gómez, A.; González-Quilen, C.; Fernández-López, J.-A.; Remesar, X.; Torres-Fuentes, C.; Ávila-Román, J.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Blay, M.T.; et al. Modulation of Food Intake by Differential TAS2R Stimulation in Rat. Nutrients 2020, 12, 3784. https://doi.org/10.3390/nu12123784
Grau-Bové C, Miguéns-Gómez A, González-Quilen C, Fernández-López J-A, Remesar X, Torres-Fuentes C, Ávila-Román J, Rodríguez-Gallego E, Beltrán-Debón R, Blay MT, et al. Modulation of Food Intake by Differential TAS2R Stimulation in Rat. Nutrients. 2020; 12(12):3784. https://doi.org/10.3390/nu12123784
Chicago/Turabian StyleGrau-Bové, Carme, Alba Miguéns-Gómez, Carlos González-Quilen, José-Antonio Fernández-López, Xavier Remesar, Cristina Torres-Fuentes, Javier Ávila-Román, Esther Rodríguez-Gallego, Raúl Beltrán-Debón, M Teresa Blay, and et al. 2020. "Modulation of Food Intake by Differential TAS2R Stimulation in Rat" Nutrients 12, no. 12: 3784. https://doi.org/10.3390/nu12123784
APA StyleGrau-Bové, C., Miguéns-Gómez, A., González-Quilen, C., Fernández-López, J.-A., Remesar, X., Torres-Fuentes, C., Ávila-Román, J., Rodríguez-Gallego, E., Beltrán-Debón, R., Blay, M. T., Terra, X., Ardévol, A., & Pinent, M. (2020). Modulation of Food Intake by Differential TAS2R Stimulation in Rat. Nutrients, 12(12), 3784. https://doi.org/10.3390/nu12123784