Effects of Chlorogenic Acids on Menopausal Symptoms in Healthy Women: A Randomized, Placebo-Controlled, Double-Blind, Parallel-Group Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Test Tablets
2.4. Assessment of Hot Flushes and Sweats
2.5. Assessment of Menopausal Symptoms, HRQOL, and Anxiety
2.6. Statistical Analysis
3. Results
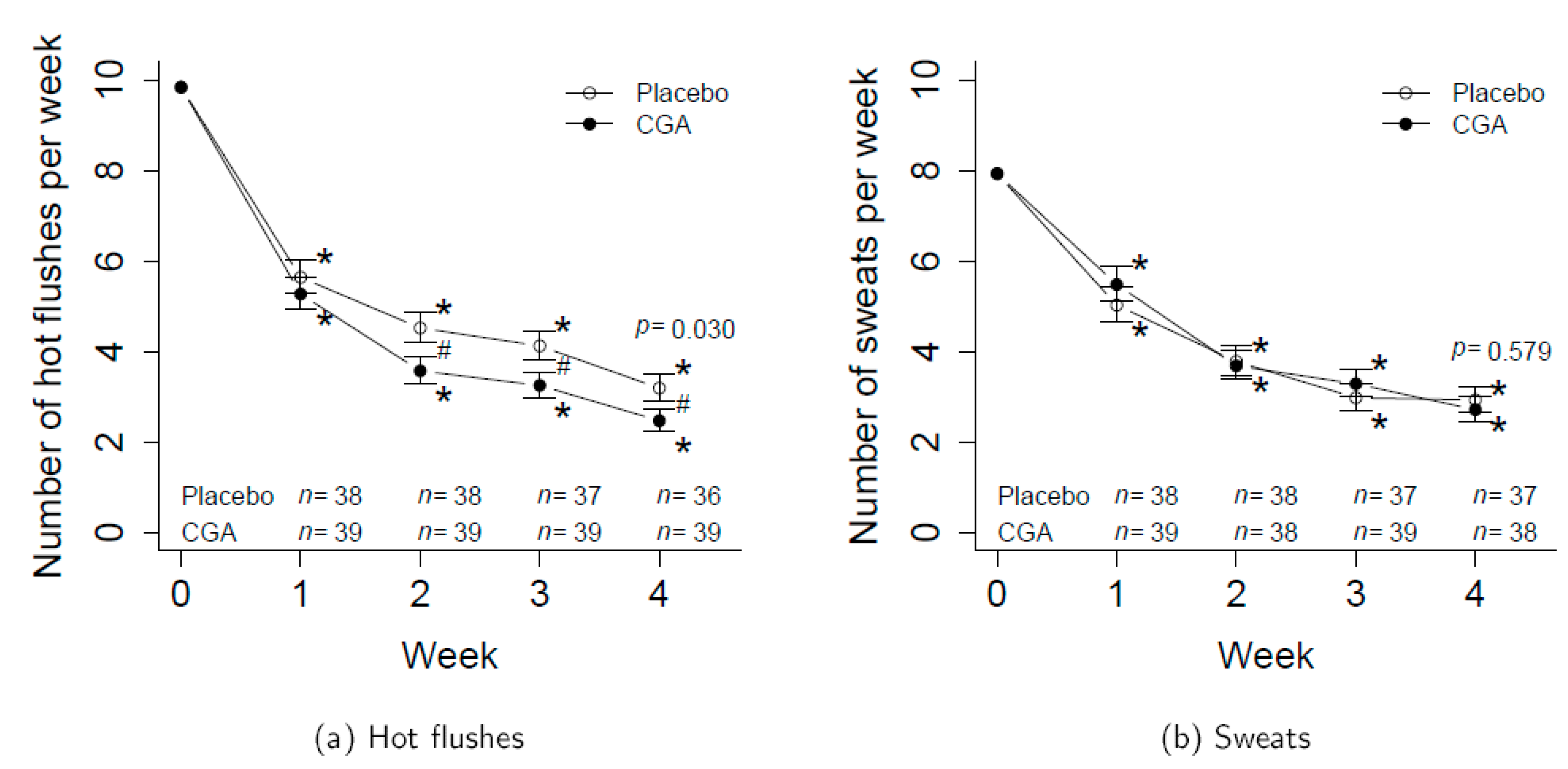
3.1. Number of Hot Flushes and Sweats
3.2. Severities of Hot Flushes and Sweats (VAS Assessment)
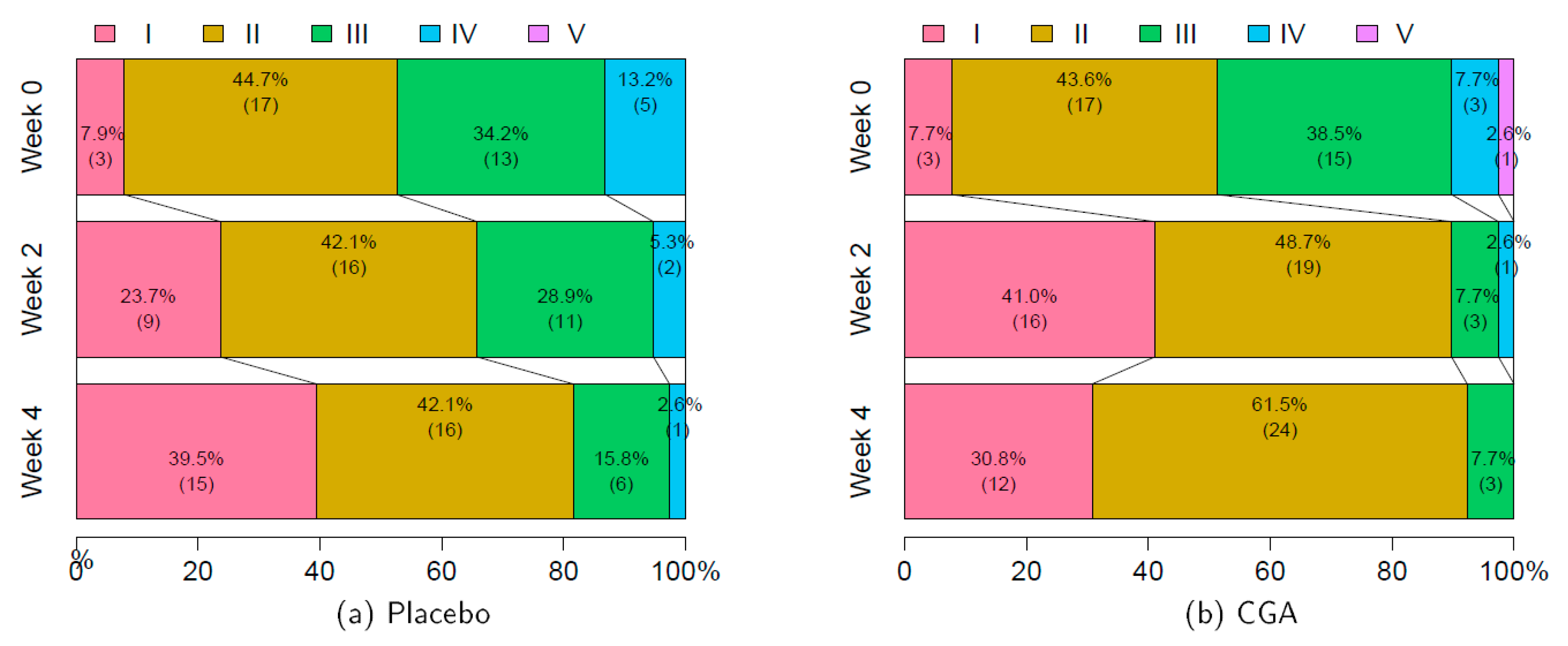
3.3. Menopausal Symptoms
3.4. HRQOL and Anxiety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Freedman, R.R. Hot flashes: Behavioral treatments, mechanisms, and relation to sleep. Am. J. Med. 2005, 118, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Mittelman-Smith, M.A.; Williams, H.; Krajewski-Hall, S.J.; McMullen, N.Y.; Rance, N.E. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc. Natl. Acad. Sci. USA 2012, 109, 19846–19851. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.L.; Poyner, D.R. Calcitonin gene-related peptide, adrenomedullin and flushing. Maturitas 2009, 64, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Thurston, R.C.; Chang, Y.; Barinas-mitchell, E.; Jennings, J.R.; von Känel, R.; Landsittel, D.P.; Matthews, K.A. Physiologically assessed hot flashes and endothelial function among midlife women. Menopause 2018, 25, 1354–1361. [Google Scholar] [CrossRef]
- Thurston, R.C.; Christie, I.C.; Matthews, K.A. Hot flashes and cardiac vagal control during women’s daily lives. Menopause 2012, 19, 406–412. [Google Scholar] [CrossRef]
- Moodithaya, S.S.; Avadhany, S.T. Comparison of cardiac autonomic activity between pre and post menopausal women using heart rate variability. Indian J. Physiol. Pharmacol. 2009, 53, 227–234. [Google Scholar]
- Eichling, P.S.; Sahni, J. Menopause related sleep disorders. J. Clin. Sleep Med. 2005, 1, 291–300. [Google Scholar] [CrossRef]
- Joffe, H.; Petrillo, L.; Viguera, A.; Koukopoulos, A.; Silver-Heilman, K.; Farrell, A.; Yu, G.; Silver, M.; Cohen, L.S. Eszopiclone improves insomnia and depressive and anxious symptoms in perimenopausal and postmenopausal women with hot flashes: A randomized, double-blinded, placebo-controlled crossover trial. Am. J. Obstet. Gynecol. 2010, 202, 171.e1–171.e11. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef]
- Sturdee, D.W.; Pines, A.; Archer, D.F.; Baber, R.J.; Barlow, D.; Birkhäuser, M.H.; Brincat, M.; Cardozo, L.; de Villiers, T.J.; Gambacciani, A.A.; et al. Updated IMS recommendations on postmenopausal hormone therapy and preventive strategies for midlife health. Climacteric 2011, 14, 302–320. [Google Scholar] [CrossRef]
- Chen, J.T.; Shiraki, M. Menopausal hot flash and calciotonin gene-related peptide; Effect of Keishi-bukuryo-gan, a kampo medicine, related to plasma calciotonin gene-related peptide level. Maturitas 2003, 45, 199–204. [Google Scholar] [CrossRef]
- Yasui, T.; Matsui, S.; Yamamoto, S.; Uemura, H.; Tsuchiya, N.; Noguchi, M.; Yuzurihara, M.; Kase, Y.; Irahara, M. Effects of Japanese traditional medicines on circulating cytokine levels in women with hot flashes. Menopause 2011, 18, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Jin, Y.; Huang, L.; Feng, S.; Hou, X.; Du, B.; Zheng, J.; Li, L. Black cohosh improves objective sleep in postmenopausal women with sleep disturbance. Climacteric 2015, 18, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Isoflavone supplements for menopausal women: A systematic review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef]
- Hirose, A.; Terauchi, M.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Low-dose isoflavone aglycone alleviates psychological symptoms of menopause in Japanese women: A randomized, double-blind, placebo-controlled study. Arch. Gynecol. Obstet. 2016, 293, 609–615. [Google Scholar] [CrossRef]
- Aso, T.; Uchiyama, S.; Matsumura, Y.; Taguchi, M.; Nozaki, M.; Takamatsu, K.; Ishizuka, B.; Kubota, T.; Mizunuma, H.; Ohta, H. A natural S-equol supplement alleviates hot flushes and other menopausal symptoms in equol nonproducing postmenopausal Japanese women. J. Womens Health 2012, 21, 92–100. [Google Scholar] [CrossRef]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: A randomized, double-blind, placebo-controlled pilot study. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Greendale, G.; Crawford, S.L.; Avis, N.E.; Brooks, M.M.; Thurston, R.C.; Karvonen-Gutierrez, C.; Waetjen, L.E.; Matthews, K. The menopause transition and women’s health at midlife: A progress report from the Study of Women’s Health across the Nation (SWAN). Menopause 2019, 26, 1213–1227. [Google Scholar] [CrossRef]
- Mori, H.; Tanaka, T.; Shima, H.; Kuniyasu, T.; Takahashi, M. Inhibitory effect of chlorogenic acid on methylazoxymethanol acetate-induced carcinogenesis in large intestine and liver of hamsters. Cancer Lett. 1986, 30, 49–54. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Giannetti, I.; Dattilo, C.; Scaccini, C. Coffee drinking influences plasma antioxidant capacity in humans. J. Agric. Food Chem. 2002, 50, 6211–6216. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamamoto, N.; Jokura, H.; Yamamoto, M.; Fujii, A.; Tokimitsu, I.; Saito, I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Soga, S.; Ota, N.; Shimotoyodome, A. Stimulation of postpransial fat utilization in healthy humans by daily consumption of chlorogenic acids. Biosci. Biotechnol. Biochem. 2013, 77, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, D.; Fujii, A.; Ohtsuka, M.; Murase, T. Ingestion of coffee polyphenols suppresses deterioration of skin barrier function after barrier disruption, concomitant with the modulation of autonomic nervous system activity in healthy subjects. Biosci. Biotechnol. Biochem. 2018, 82, 879–884. [Google Scholar] [CrossRef]
- Ochiai, R.; Tomonobu, K.; Ikushima, I. Effect of chlorogenic acids on fatigue and sleep in healthy males: A randomized, double-blind, placebo-controlled, crossover study. Food Sci. Nutr. 2018, 6, 2530–2536. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Yamaya, Y.; Suzuki, M.; Moritsuka, T. Statistical clustering of women complaining of climacteric syndrome by cluster analysis. Acta Obstet. Gynaecol. Jpn. 1979, 31, 607–614. [Google Scholar]
- Kupperman, H.S.; Blatt, M.H.; Wiesbader, H.; Filler, W. Comparative clinical evaluation of estrogenic preparations by the menopausal and amenorrheal indices. J. Clin. Endocrinol. Metab. 1953, 13, 688–703. [Google Scholar] [CrossRef]
- Fukuhara, S.; Suzukamo, Y. Manual of SF-8 Japanese Version; Institute for Health Outcomes & Process Evaluation Research: Kyoto, Japan, 2004. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Kansas City, MO, USA, 1983. [Google Scholar]
- Hunter, M.; Battersby, R.; Whitehead, M. Relationships between psychological symptoms, somatic complaints and menopausal status. Maturitas 1986, 8, 217–228. [Google Scholar] [CrossRef]
- Park, I.; Ochiai, R.; Ogata, H.; Kayaba, M.; Hari, S.; Hibi, M.; Katsuragi, Y.; Satoh, M.; Tokuyama, K. Effects of subacute ingestion of chlorogenic acids on sleep architecture and energy metabolism through activity of the autonomic nervous system: A randomised, placebo-controlled, double-blinded cross-over trial. Br. J. Nutr. 2017, 117, 979–984. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Chikama, A.; Mori, K.; Watanabe, T.; Shioya, Y.; Katsuragi, Y.; Tokimitsu, I. Hydroxyhydroquinone-free coffee: A double-blind, randomized controlled dose-response study of blood pressure. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Tanahashi, M.; Higaki, Y.; Iwata, K.; Sugiyama, Y. Ingestion of coffee polyphenols improves a scaly skin surface and the recovery rate of skin temperature after cold stress: A randomized, controlled trial. J. Nutr. Sci. Vitaminol. 2017, 63, 291–297. [Google Scholar] [CrossRef][Green Version]
- Suzuki, A.; Yamamoto, M.; Jokura, H.; Fujii, A.; Tokimitsu, I.; Hase, T.; Saito, I. Ferulic acid restores endothelium-dependent vasodilation in aortas of spontaneously hypertensive rats. Am. J. Hypertens. 2007, 20, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yang, J.; Zhao, G.; Yuan, Y. Ferulic acid inhibits endothelial cell proliferation through NO down-regulating ERK1/2 pathway. J. Cell Biochem. 2004, 93, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Fujii, A.; Yamamoto, N.; Yamamoto, M.; Ohminami, H.; Kameyama, A.; Shibuya, Y.; Nishizawa, Y.; Tokimitsu, I.; Saito, I. Improvement of hypertension and vascular dysfunction by hydroxyhydroquinone-free coffee in a genetic model of hypertension. FEBS Lett. 2006, 580, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. The effect of processing on chlorogenic acid content of commercially available coffee. Food Chem. 2013, 141, 3335–3340. [Google Scholar] [CrossRef]
- Faubion, S.S.; Sood, R.; Thielen, J.M.; Shuster, L.T. Caffeine and menopausal symptoms: What is the association? Menopause 2015, 22, 155–158. [Google Scholar] [CrossRef]


| Placebo | CGA | Test Statistic | |
|---|---|---|---|
| n = 38 | n = 39 | ||
| Age (years) | 48.2, 51.0, 55.0 (51.4 ± 4.3) | 48.0, 51.0, 54.5 (51.3 ± 4.6) | |
| Height (cm) | 155.0, 159.5, 162.0 (158.4 ± 5.0) | 155.5, 158.0, 162.0 (158.5 ± 4.7) | |
| Body weight (kg) | 49.2, 54.5, 60.0 (55.0 ± 7.2) | 51.0, 55.0, 59.0 (54.9 ± 6.9) | |
| BMI (kg/m2) | 19.6, 21.7, 23.7 (22.0 ± 3.1) | 19.9, 21.3, 23.2 (21.9 ± 2.8) | |
| mKMI total score | 17.2, 22.0, 27.8 (23.1 ± 8.0) | 16.0, 22.0, 27.5 (22.5 ± 8.0) | |
| Menopause Status | |||
| Premenopausal | 16% (6) | 15% (6) | |
| Perimenopausal | 32% (12) | 36% (14) | |
| Postmenopausal | 53% (20) | 46% (18) | |
| Missing | 0% (0) | 3% (1) |
| Baseline | Change from Baseline at Week 4 | Difference of Change | |||||
|---|---|---|---|---|---|---|---|
| Placebo | CGA | Placebo | p-Value | CGA | p-Value | p-Value | |
| n = 38 | n = 39 | n = 38 | Within Groups | n = 39 | Within Groups | Between Groups | |
| SF-8 | |||||||
| PCS-8 score | 43.4 ± 1.0 | 45.7 ± 1.1 | 2.6 ± 1.1 | 0.018 * | 2.4 ± 1.1 | 0.025 * | 0.712 |
| MCS-8 score | 44.6 ± 1.3 | 44.7 ± 1.3 | 4.7 ± 1.1 | <0.001 * | 3.5 ± 1.2 | 0.004 * | 0.308 |
| STAI | |||||||
| State anxiety | 45.7 ± 1.7 | 46.5 ± 1.6 | −4.8 ± 1.5 | 0.003 * | −4.5 ± 1.3 | 0.002 * | 0.560 |
| Trait anxiety | 50.2 ± 1.9 | 48.4 ± 1.7 | −4.6 ± 1.1 | <0.001 * | −4.0 ± 1.1 | 0.004 * | 0.465 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enokuchi, Y.; Suzuki, A.; Yamaguchi, T.; Ochiai, R.; Terauchi, M.; Kataoka, K. Effects of Chlorogenic Acids on Menopausal Symptoms in Healthy Women: A Randomized, Placebo-Controlled, Double-Blind, Parallel-Group Trial. Nutrients 2020, 12, 3757. https://doi.org/10.3390/nu12123757
Enokuchi Y, Suzuki A, Yamaguchi T, Ochiai R, Terauchi M, Kataoka K. Effects of Chlorogenic Acids on Menopausal Symptoms in Healthy Women: A Randomized, Placebo-Controlled, Double-Blind, Parallel-Group Trial. Nutrients. 2020; 12(12):3757. https://doi.org/10.3390/nu12123757
Chicago/Turabian StyleEnokuchi, Yuka, Atsushi Suzuki, Tohru Yamaguchi, Ryuji Ochiai, Masakazu Terauchi, and Kiyoshi Kataoka. 2020. "Effects of Chlorogenic Acids on Menopausal Symptoms in Healthy Women: A Randomized, Placebo-Controlled, Double-Blind, Parallel-Group Trial" Nutrients 12, no. 12: 3757. https://doi.org/10.3390/nu12123757
APA StyleEnokuchi, Y., Suzuki, A., Yamaguchi, T., Ochiai, R., Terauchi, M., & Kataoka, K. (2020). Effects of Chlorogenic Acids on Menopausal Symptoms in Healthy Women: A Randomized, Placebo-Controlled, Double-Blind, Parallel-Group Trial. Nutrients, 12(12), 3757. https://doi.org/10.3390/nu12123757






