Camphene Attenuates Skeletal Muscle Atrophy by Regulating Oxidative Stress and Lipid Metabolism in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Viability and Cell Morphology Observation
2.3. Immunocytochemistry
2.4. Real-Time Polymerase Chain Reaction (PCR)
2.5. Animal Care and Starvation Protocols for PET/CT Imaging
2.6. Small Animal PET-CT Imaging Protocol and Image Analysis
2.7. Histochemistry and Immunochemistry
2.8. Animal Care and Starvation Protocols for Energy Metabolism Experiments
2.9. Energy Metabolism Measurements
2.10. Statistical Analysis
3. Results
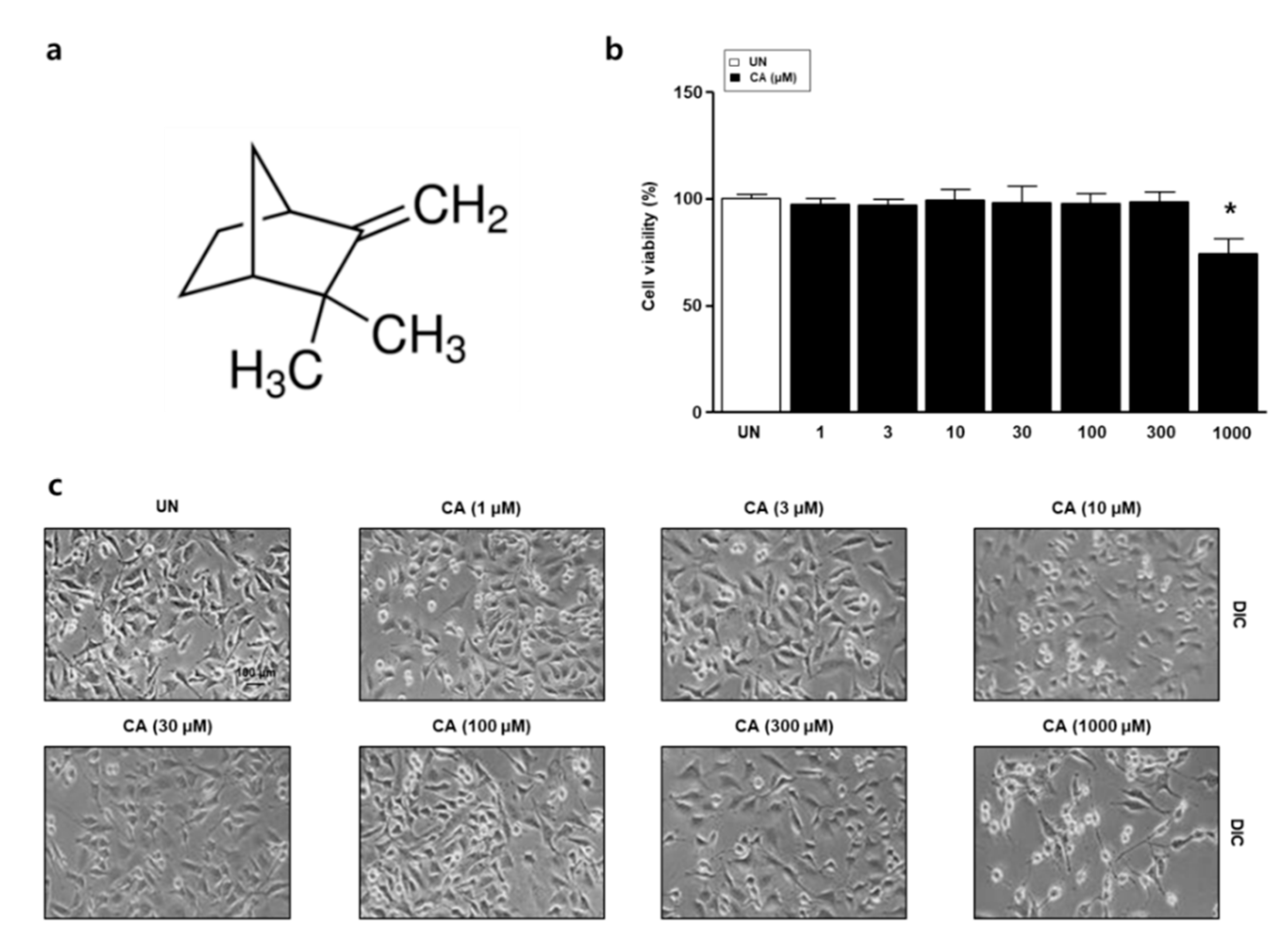
3.1. The Effect of Camphene on the Viability of L6 Skeletal Muscle Cells
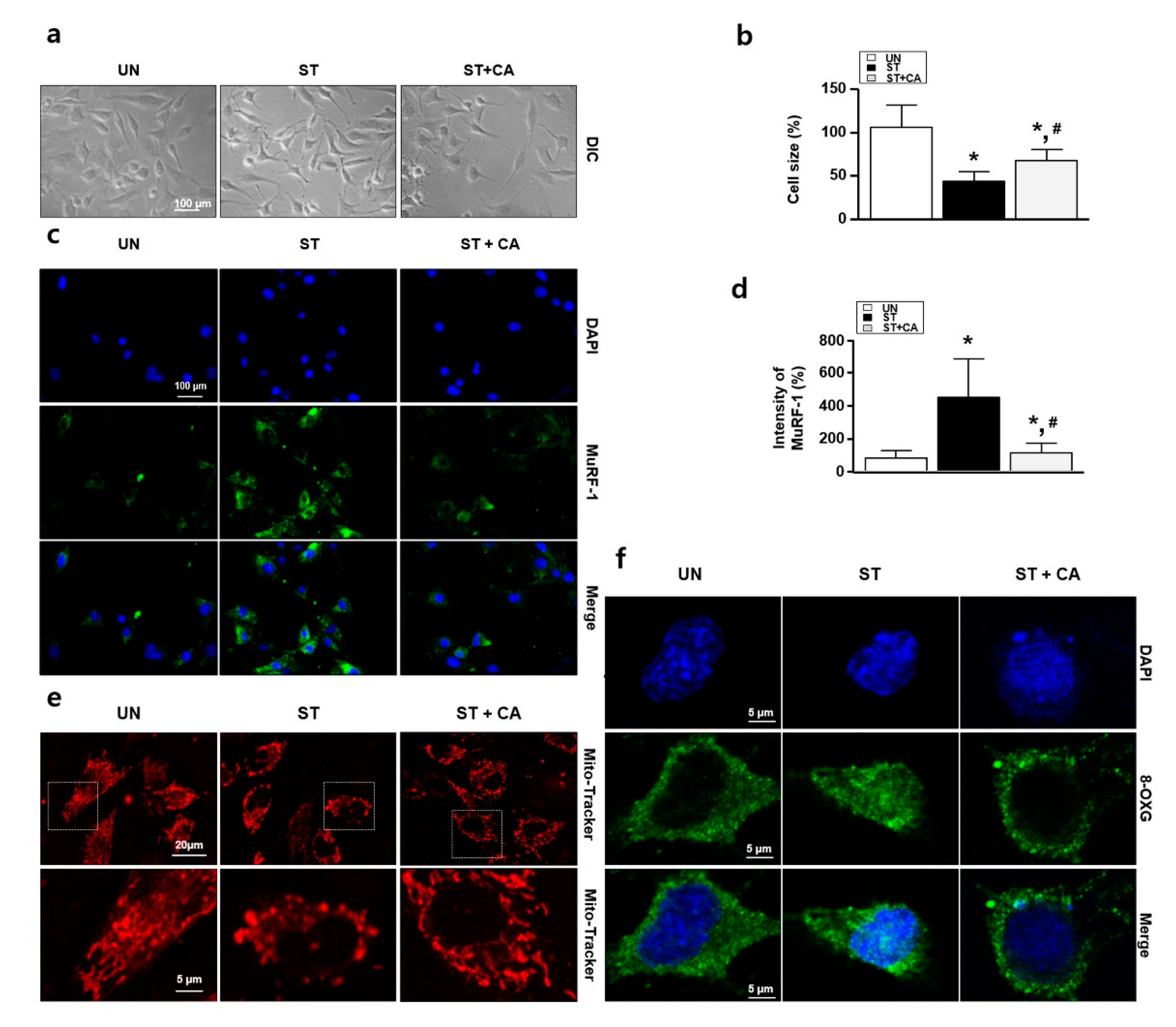
3.2. Camphene Reduces the Starvation-Induced Oxidative Stress and Atrophy in L6 Skeletal Muscle Cells
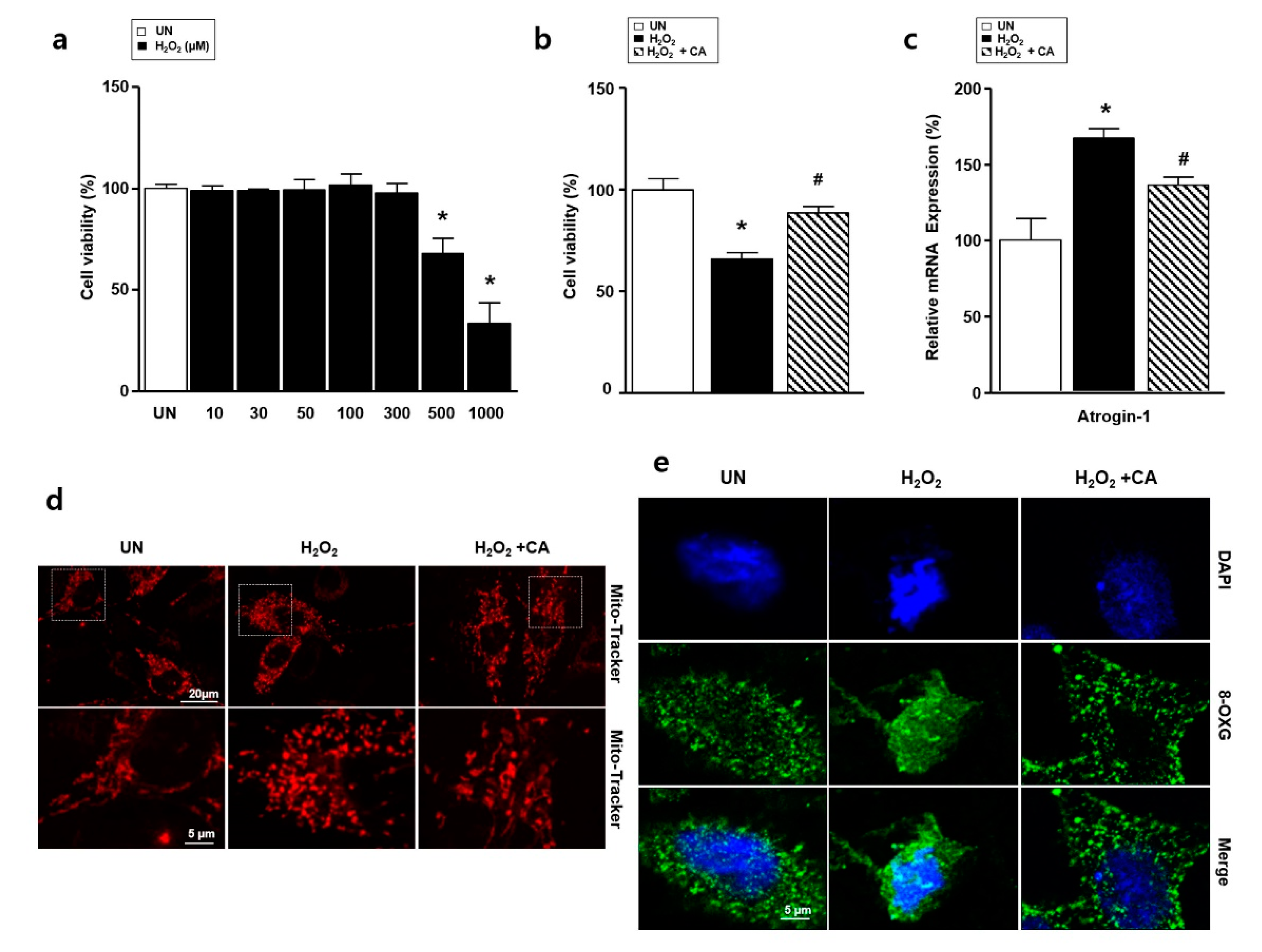
3.3. Camphene Reduces Hydrogen Peroxide (H2O2)-Induced Oxidative Stress in L6 Skeletal Muscle Cells
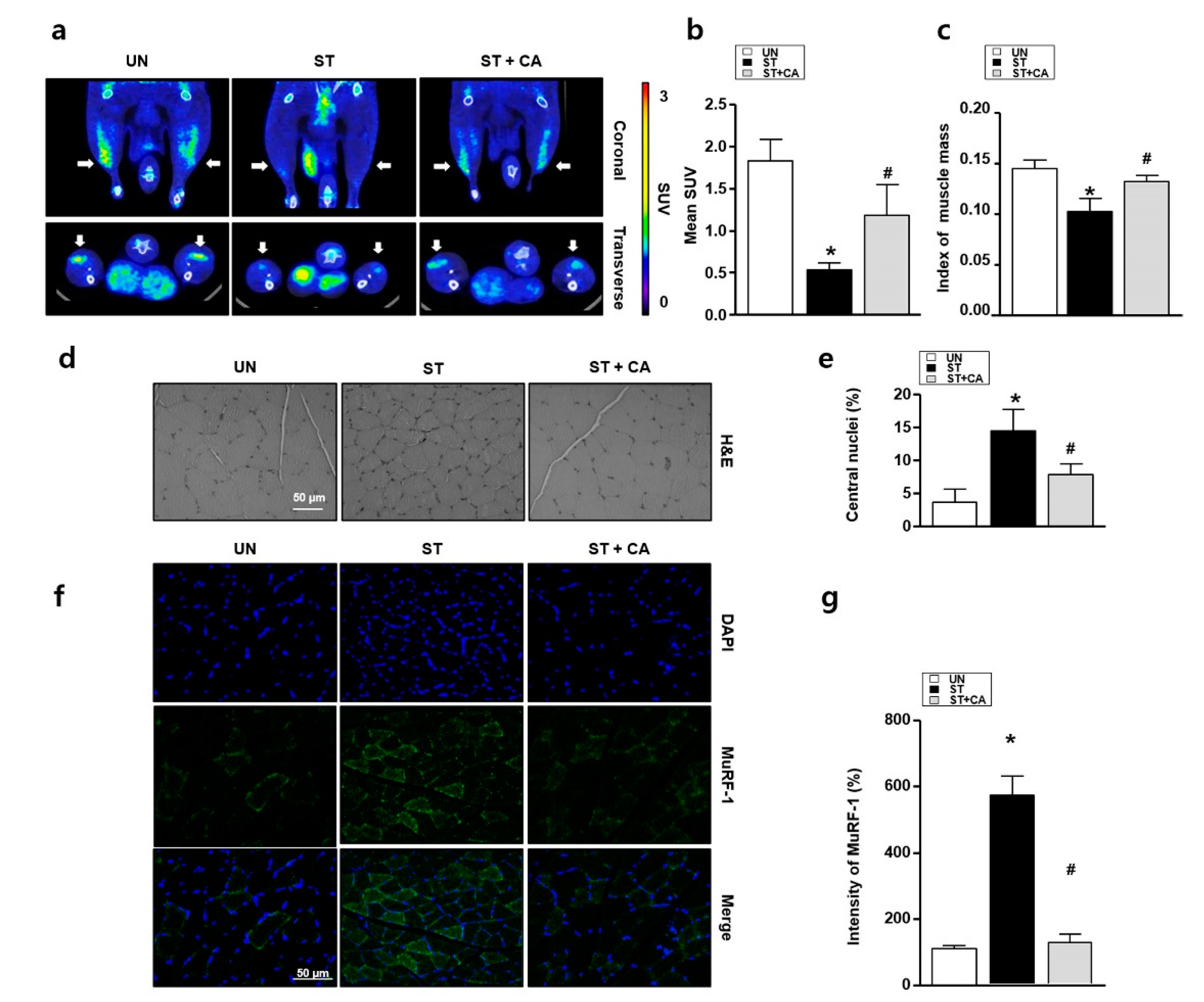
3.4. Camphene Inhibits Starvation-Induced Skeletal Muscle Atrophy in Rats
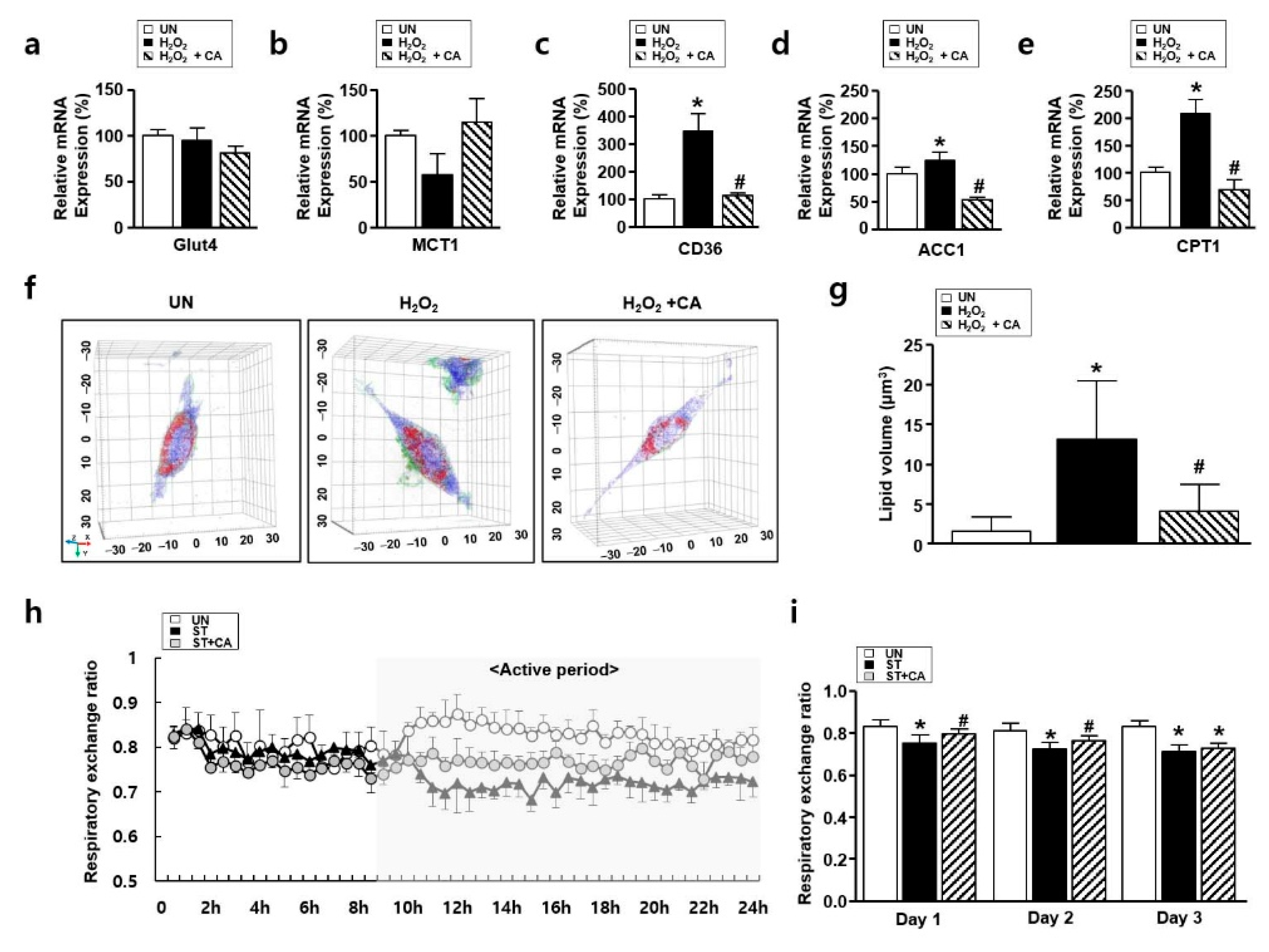
3.5. The Effect of Camphene on Lipid Metabolism In Vitro and In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Powers, S.K.; Lynch, G.S.; Murphy, K.T.; Reid, M.B.; Zijdewind, I. Disease-induced skeletal muscle atrophy and fatigue. Med. Sci. Sports Exerc. 2016, 48, 2307–2319. [Google Scholar] [CrossRef]
- Mckinnell, I.W.; Rudnicki, M.A. Molecular mechanisms of muscle atrophy. Cell 2004, 119, 907–910. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Cholewa, J.; Zhao, Y.; Shang, H.-Y.; Yang, Y.-Q.; Araújo Pessôa, K.; Su, Q.-S.; Lima-Soares, F.; Zanchi, N.E. Targeting inflammation and downstream protein metabolism in sarcopenia: A brief updated description of concurrent exercise and leucine-based multimodal intervention. Front. Physiol. 2017, 8, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukushima, H.; Fujii, Y.; Koga, F. Metabolic and molecular basis of sarcopenia: Implications in the management of urothelial carcinoma. Int. J. Mol. Sci. 2019, 20, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, S.K.; Smuder, A.J.; Judge, A.R. Oxidative stress and disuse muscle atrophy: Cause or consequence? Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 240–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakellariou, G.K.; Pearson, T.; Lightfoot, A.P.; Nye, G.A.; Wells, N.; Giakoumaki, I.I.; Vasilaki, A.; Griffiths, R.D.; Jackson, M.J.; McArdle, A. Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci. Rep. 2016, 6, 33944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Morton, A.B.; Ahn, B.; Smuder, A.J. Redox control of skeletal muscle atrophy. Free Radic. Biol. Med. 2016, 98, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [Green Version]
- Vial, G.; Coudy-Gandilhon, C.; Pinel, A.; Wauquier, F.; Chevenet, C.; Béchet, D.; Wittrant, Y.; Coxam, V.; Soubrier, M.; Tournadre, A.; et al. Lipid accumulation and mitochondrial abnormalities are associated with fiber atrophy in the skeletal muscle of rats with collagen-induced arthritis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158574. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juel, C.; Halestrap, A.P. Lactate transport in skeletal muscle—Role and regulation of the monocarboxylate transporter. J. Physiol. 1999, 517, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.Y.; Lee, T.; Jeong, Y. High-fat diet increases fat oxidation and promotes skeletal muscle fatty acid transporter expression in exercise-trained mice. J. Med. Food. 2020, 23, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glund, S.; Schoelch, C.; Thomas, L.; Niessen, H.G.; Stiller, D.; Roth, G.J.; Neubauer, H. Inhibition of acetyl-CoA carboxylase 2 enhances skeletal muscle fatty acid oxidation and improves whole-body glucose homeostasis in db/db mice. Diabetologia. 2012, 55, 2044–2053. [Google Scholar] [CrossRef]
- De Lavor, É.M.; Fernandes, A.W.C.; de Andrade Teles, R.B.; Leal, A.E.B.P.; de Oliveira Júnior, R.G.; Gama e Silva, M.; de Oliveira, A.P.; Silva, J.C.; de Moura Fontes Araújo, M.T.; Coutinho, H.D.M.; et al. Essential oils and their major compounds in the treatment of chronic inflammation: A review of antioxidant potential in preclinical studies and molecular mechanisms. Oxid. Med. Cell Longev. 2018, 2018, 6468593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hadzopoulou-Cladaras, M. Camphene, a plant-derived monoterpene, reduces plasma cholesterol and triglycerides in hyperlipidemic rats independently of HMG-CoA reductase activity. PLoS ONE 2011, 6, e20516. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.; Lee, D.; Jung, S.H.; Lee, K.J.; Jin, H.; Kim, S.J.; Lee, H.M.; Kim, B.; Won, K.J. Sabinene prevents skeletal muscle atrophy by inhibiting the MAPK-MuRF-1 pathway in rats. Int. J. Mol. Sci. 2019, 20, 4955. [Google Scholar] [CrossRef] [Green Version]
- Pasetto, L.; Olivari, D.; Nardo, G.; Trolese, M.C.; Bendotti, C.; Piccirillo, R.; Bonetto, V. Micro-computed tomography for non-invasive evaluation of muscle atrophy in mouse models of disease. PLoS ONE 2018, 13, e0198089. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, H.; Yun, H.Y.; Kim, B.; Lee, C.H.; Suh, H.; Lim, K. Silk peptide intake increases fat oxidation at rest in exercised mice. J. Nutr. Sci. Vitaminol. 2013, 59, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, J.; Yum, H.Y.; Suh, H.; Lim, K. Silk peptide treatment can improve the exercise performance of mice. J. Int. Soc. Sports Nutr. 2014, 11, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, K.P.; Lee, D.W.; Lim, K. Piperine enhances carbohydrate/fat metabolism in skeletal muscle during acute exercise in mice. Nutr. Metab. 2017, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Ahn, S.H.; Lee, K.P.; Park, S.-Y.; Cheon, J.H.; Choi, J.-Y.; Kim, K. The natural compound dansameum reduces foam cell formation by downregulating CD36 and peroxisome proliferator-activated receptor-gamma; expression. Pharmacogn. Mag. 2018, 13, S868–S874. [Google Scholar] [PubMed]
- Cadena, S.M.; Zhang, Y.; Fang, J.; Brachat, S.; Kuss, P.; Giorgetti, E.; Stodieck, L.S.; Kneissel, M.; Glass, D.J. Skeletal muscle in MuRF1 null mice is not spared in low-gravity conditions, indicating atrophy proceeds by unique mechanisms in space. Sci. Rep. 2019, 9, 9397. [Google Scholar] [CrossRef]
- Seo, E.; Kang, H.; Choi, H.; Choi, W.; Jun, H.S. Reactive oxygen species-induced changes in glucose and lipid metabolism contribute to the accumulation of cholesterol in the liver during aging. Aging Cell 2019, 18, e12895. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Ahn, J.W.; Jo, Y.; Kang, H.Y.; Kim, H.J.; Cheon, Y.; Kim, J.W.; Park, Y.; Lee, S.; Park, K. Label-free tomographic imaging of lipid droplets in foam cells for machine-learning-assisted therapeutic evaluation of targeted nanodrugs. ACS Nano 2020, 14, 1856–1865. [Google Scholar] [CrossRef]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef] [Green Version]
- Ábrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of oxidative stress as key regulator of muscle wasting during cachexia. Oxid. Med. Cell Longev. 2018, 2018, 2063179. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants—Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. In Vitro 2009, 23, 295–301. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Lipid modulation of skeletal muscle mass and function. J. Cachexia Sarcopeni. 2016, 8, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [PubMed] [Green Version]
- Sánchez-Sánchez, J.L.; Mañas, A.; García-García, F.J.; Ara, I.; Carnicero, J.A.; Walter, S.; Rodríguez-Mañas, L. Sedentary behaviour, physical activity, and sarcopenia among older adults in the TSHA: Isotemporal substitution model. J. Cachexia Sarcopeni 2019, 10, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Fukawa, T.; Yan-Jiang, B.C.; Min-Wen, J.C.; Jun-Hao, E.T.; Huang, D.; Qian, C.N.; Ong, P.; Li, Z.; Chen, S.; Mak, S.Y.; et al. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat. Med. 2016, 22, 666–671. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty infiltration of skeletal muscle: Mechanisms and comparisons with bone marrow adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, J.; Lim, K. Nutrition supplements to stimulate lipolysis: A review in relation to endurance exercise capacity. J. Nutr. Sci. Vitaminol. 2016, 62, 141–161. [Google Scholar] [CrossRef] [Green Version]
| Gene Product | Primer Sequence |
|---|---|
| Glut4 | Forward: 5′-AGAGTCTAAAGCGCCT-3′ |
| Reverse: 5′- CCGAGACCAACGTGAA-3′ | |
| ACC1 | Forward: 5′-AGGAAGATGGTGTCCCGCTCTG-3′ |
| Reverse: 5′-GGGGAGATGTGCTGGGTCAT-3′ | |
| MCT1 | Forward: 5′-AGAAGTCAGCCTTCCTCCTTT-3′ |
| Reverse: 5′-CCACAAGCCCAGTATGTGTAT-3′ | |
| CD36 | Forward: 5′-CGGCGATGAGAAAGCAGA-3′ |
| Reverse: 5′-ACTCCAACACCAAGTAAGACCA-3′ | |
| CPT1 | Forward: 5′-GTGCTGGAGGTGGCTTTGGT-3′ |
| Reverse: 5′-TGCTTGACGGATGTGGTTCC-3′ | |
| Atrogin-1 | Forward: 5′-GAACATCATGCAGAGGCTGA-3′ |
| Reverse: 5′-GTAGCCGGTCTTCACTGAGC-3′ | |
| β-actin | Forward: 5′-GGCCAACCGTGAAAAGATG-3′ |
| Reverse: 5′-GGATCTTCATGAGGTAGTCTGTC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.; Kim, J.; Moon, B.S.; Park, S.M.; Jung, D.E.; Kang, S.Y.; Lee, S.J.; Oh, S.J.; Kwon, S.H.; Nam, M.H.; et al. Camphene Attenuates Skeletal Muscle Atrophy by Regulating Oxidative Stress and Lipid Metabolism in Rats. Nutrients 2020, 12, 3731. https://doi.org/10.3390/nu12123731
Baek S, Kim J, Moon BS, Park SM, Jung DE, Kang SY, Lee SJ, Oh SJ, Kwon SH, Nam MH, et al. Camphene Attenuates Skeletal Muscle Atrophy by Regulating Oxidative Stress and Lipid Metabolism in Rats. Nutrients. 2020; 12(12):3731. https://doi.org/10.3390/nu12123731
Chicago/Turabian StyleBaek, Suji, Jisu Kim, Byung Seok Moon, Sun Mi Park, Da Eun Jung, Seo Young Kang, Sang Ju Lee, Seung Jun Oh, Seung Hae Kwon, Myung Hee Nam, and et al. 2020. "Camphene Attenuates Skeletal Muscle Atrophy by Regulating Oxidative Stress and Lipid Metabolism in Rats" Nutrients 12, no. 12: 3731. https://doi.org/10.3390/nu12123731
APA StyleBaek, S., Kim, J., Moon, B. S., Park, S. M., Jung, D. E., Kang, S. Y., Lee, S. J., Oh, S. J., Kwon, S. H., Nam, M. H., Kim, H. O., Yoon, H. J., Kim, B. S., & Lee, K. P. (2020). Camphene Attenuates Skeletal Muscle Atrophy by Regulating Oxidative Stress and Lipid Metabolism in Rats. Nutrients, 12(12), 3731. https://doi.org/10.3390/nu12123731






