Sarcopenia Risk Evaluation in a Sample of Hospitalized Elderly Men and Women: Combined Use of the Mini Sarcopenia Risk Assessment (MSRA) and the SARC-F
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Anthropometry
2.3. Body Composition
2.4. Muscle Strength
2.5. Definition of Sarcopenic Subjects Based on EWGSOP2 Criteria
2.6. SARC-F and Mini Sarcopenia Risk Assessment Questionnaire
2.7. Covariates
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Cerri, A.P.; Bellelli, G.; Mazzone, A.; Pittella, F.; Landi, F.; Zambon, A.; Annoni, G. Sarcopenia and malnutrition in acutely ill hospitalized elderly: Prevalence and outcomes. Clin. Nutr. 2015, 34, 745–751. [Google Scholar] [CrossRef]
- Batsis, J.A.; Mackenzie, T.A.; Barre, L.K.; LopezJimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity and mortality in older adults: Results from the National Health and Nutrition Examination Survey III. Eur. J. Clin. Nutr. 2014, 68, 1001–1007. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B.; et al. The Loss of Skeletal Muscle Strength, Mass, and Quality in Older Adults: The Health, Aging and Body Composition Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Rossi, A.P.; Harris, T.B.; Fantin, F.; Armellini, F.; Zamboni, M. The multidomain mobility lab in older persons: From bench to bedside. The assessment of body composition in older persons at risk of mobility limitations. Curr. Pharm. Des. 2014, 20, 3245–3255. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.P.; Micciolo, R.; Rubele, S.; Fantin, F.; Caliari, C.; Zoico, E.; Mazzali, G.; Ferrari, E.; Volpato, S.; Zamboni, M. Assessing the risk of sarcopenia in the elderly: The Mini Sarcopenia Risk Assessment (MSRA) questionnaire. J. Nutr. Health Aging 2017, 21, 743–749. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A Simple Questionnaire to Rapidly Diagnose Sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lu, J.; Jiang, J.; Zeng, Y.; Tang, H. Comparison of four sarcopenia screening tools in nursing home residents. Aging Clin. Exp. Res. 2018, 31, 1481–1489. [Google Scholar] [CrossRef]
- Visser, M.; Fuerst, T.; Lang, T.; Salamone, L.; Harris, T.B. For The Health; Body Composition Working Group Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. J. Appl. Physiol. 1999, 87, 1513–1520. [Google Scholar] [CrossRef]
- Rossi, A.P.; Zanandrea, V.; Zoico, E.; Zanardo, M.; Caliari, C.; Confente, S.; Gabriele, S.; Mazzali, G.; Fantin, F.; Zamboni, M. Inflammation and nutritional status as predictors of physical performance and strength loss during hospitalization. Eur. J. Clin. Nutr. 2016, 70, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, T.; Guralnik, J.M.; Foley, D.; Masaki, K.; Leveille, S.; Curb, J.D.; White, L. Midlife Hand Grip Strength as a Predictor of Old Age Disability. JAMA 1999, 281, 558–560. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of Sarcopenia among the Elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Katz, S. Assessing Self-maintenance: Activities of Daily Living, Mobility, and Instrumental Activities of Daily Living. J. Am. Geriatr. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837. [Google Scholar] [CrossRef]
- Zhou, X.-H.; Obuchowski, N.A.; McClish, D.K. Statistical Methods in Diagnostic Medicine, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2011; pp. 23–28. ISBN 978-0-470-18314-4. [Google Scholar]
- SPSS Inc. SPSS-X User’s Guide, 2nd ed.; Mc Graw-Hill: New York, NY, USA, 1986; ISBN 10: 0070465533. [Google Scholar]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachex Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Yang, M.; Hu, X.; Xie, L.; Zhang, L.; Zhou, J.; Lin, J.; Wang, Y.; Li, Y.; Han, Z.; Zhang, D.; et al. Comparing Mini Sarcopenia Risk Assessment With SARC-F for Screening Sarcopenia in Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2019, 20, 53–57. [Google Scholar] [CrossRef]
- Li, M.; Kong, Y.; Chen, H.; Chu, A.; Song, G.; Cui, Y. Accuracy and prognostic ability of the SARC-F questionnaire and Ishii’s score in the screening of sarcopenia in geriatric inpatients. Braz. J. Med. Biol. Res. 2019, 52, e8204. [Google Scholar] [CrossRef]
- Tanaka, S.; Kamiya, K.; Hamazaki, N.; Matsuzawa, R.; Nozaki, K.; Maekawa, E.; Noda, C.; Yamaoka-Tojo, M.; Matsunaga, A.; Masuda, T.; et al. Utility of SARC-F for Assessing Physical Function in Elderly Patients With Cardiovascular Disease. J. Am. Med. Dir. Assoc. 2017, 18, 176–181. [Google Scholar] [CrossRef]
- Krzymińska-Siemaszko, R.; Tobis, S.; Lewandowicz, M.; Wieczorowska-Tobis, K. Comparison of four sarcopenia screening questionnaires in community-dwelling older adults from Poland using six sets of international diagnostic criteria of sarcopenia. PLoS ONE 2020, 15, e0231847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lu, M.; Wang, X.; Guo, Y. The role of sarcopenia questionnaires in hospitalized patients with chronic heart failure. Aging Clin. Exp. Res. 2020, 1–6. [Google Scholar] [CrossRef]
- Rossi, A.P.; Fantin, F.; Micciolo, R.; Bertocchi, M.; Bertassello, P.; Zanandrea, V.; Zivelonghi, A.; Bissoli, L.; Zamboni, M. Identifying Sarcopenia in Acute Care Setting Patients. J. Am. Med. Dir. Assoc. 2014, 15, 303.e7–303.e12. [Google Scholar] [CrossRef]
- Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Manca, G.M.; Rizzo, M.R.; et al. Prevalence and Clinical Correlates of Sarcopenia, Identified According to the EWGSOP Definition and Diagnostic Algorithm, in Hospitalized Older People: The GLISTEN Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1575–1581. [Google Scholar] [CrossRef]
- Buckinx, F.; Reginster, J.-Y.; Dardenne, N.; Croisiser, J.-L.; Kaux, J.-F.; Beaudart, C.; Slomian, J.; Bruyère, O. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: A cross-sectional study. BMC Musculoskelet. Disord. 2015, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, H. Accuracy of segmental multi-frequency bioelectrical impedance analysis for assessing whole-body and appendicular fat mass and lean soft tissue mass in frail women aged 75 years and older. Eur. J. Clin. Nutr. 2013, 67, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Sobestiansky, S.; Michaelsson, K.; Cederholm, T. Sarcopenia prevalence and associations with mortality and hospitalisation by various sarcopenia definitions in 85–89 year old community-dwelling men: A report from the ULSAM study. BMC Geriatr. 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
| Non Sarcopenic (n = 86) | Sarcopenic (n = 66) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | p | |
| Age (years) | 79.79 ± 5.47 | 66–93 | 82.88 ± 5.18 | 70–94 | = 0.015 |
| Gender (male) | 60 (69.8%) | 34 (51.5%) | N.S. | ||
| Weight (kg) | 76.36 ± 14.03 | 51–111 | 64.39 ± 12.06 | 39–91 | <0.001 |
| Height (m) | 1.66 ± 0.08 | 1.52–1.80 | 1.62 ± 0.08 | 1.49–1.80 | = 0.022 |
| BMI (kg/m2) | 27.40 ± 4.15 | 26.95–29.85 | 24.33 ± 3.74 | 23.15–25.21 | = 0.001 |
| ADL score | 5.4 ± 0.4 | 1–6 | 5.0 ± 0.3 | 1–6 | <0.05 |
| MMSE | 26.55 ± 3.3 | 21.70–30 | 26.69 ± 2.80 | 21.30–30 | N.S. |
| Charlson Comorbidity Index | 2.06 ± 1.56 | 0–8 | 2.75 ± 2.07 | 0–9 | <0.05 |
| Charlson Age Comorbidity Index | 5.80 ± 1.83 | 2–12 | 6.53 ± 2.32 | 2–13 | <0.05 |
| 7-item MSRA score | 23.25 ± 7.06 | 0–35 | 18.93 ± 6.58 | 10–35 | <0.01 |
| 5-item MSRA score | 35.81 ± 12.43 | 0–55 | 27.87 ± 13.17 | 0–55 | <0.01 |
| SARC-F score | 1.60 ± 1.52 | 0–6 | 3.39 ± 2.22 | 0–6 | <0.05 |
| 7-item MSRA score ≤25 (%) | 16 (18.6%) | 50 (75.7%) | <0.001 | ||
| 5-item MSRA score ≤40 (%) | 44 (51.2%) | 52 (78.8%) | <0.05 | ||
| SARC-F score ≥3 (%) | 18 (20.1%) | 42 (63.6%) | <0.001 | ||
| Combined SARC-F/7-item MSRA (%) | 0 (0.0%) | 42 (63.6%) | <0.001 | ||
| Combined SARC-F/5-item MSRA (%) | 2 (2.3%) | 28 (42.4%) | <0.001 | ||
| Handgrip (kg) | 29.40 ± 8.16 | 10.50−42.50 | 18.88 ± 5.50 | 6.50−26.80 | <0.001 |
| Gait-speed (<0.8 m/s) | 14 (41.2%) | 39 (92.9%) | <0.001 | ||
| SMI (kg/m2) | 6.85 ± 1.09 | 6.86–7.52 | 5.63 ± 0.74 | 4.23–5.83 | <0.001 |
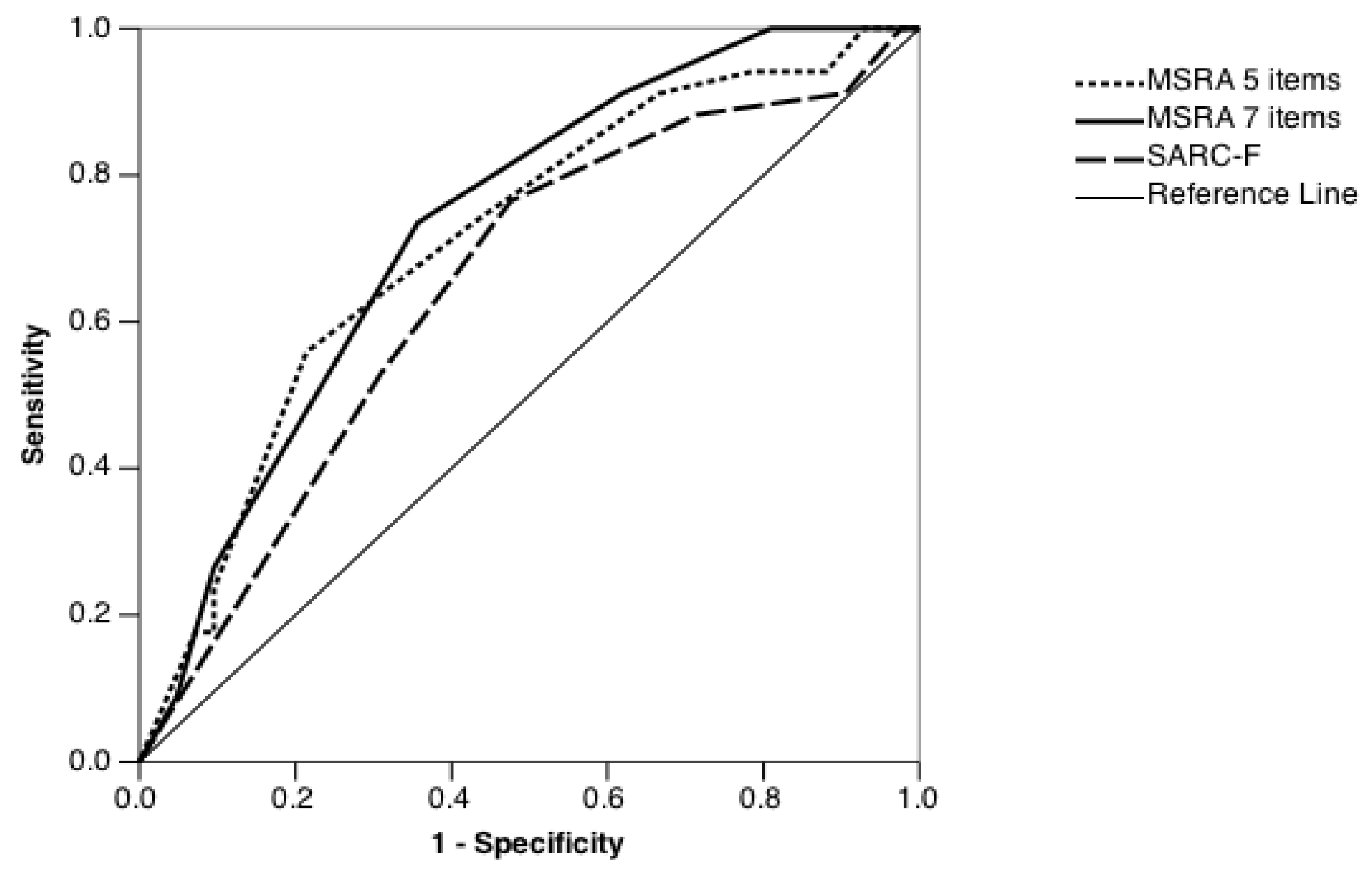
| 7-item MSRA | |||
| Score | Sensitivity | 1–Specificity | Youden Index |
| 2.5 | 0.024 | 0 | 0.024 |
| 7.5 | 0.048 | 0 | 0.048 |
| 12.5 | 0.190 | 0 | 0.190 |
| 17.5 | 0.381 | 0.088 | 0.293 |
| 22.5 | 0.643 | 0.265 | 0.378 |
| 27.5 | 0.905 | 0.735 | 0.170 |
| 32.5 | 0.952 | 0.912 | 0.040 |
| 36 | 1 | 1 | 0 |
| 5-item MSRA | |||
| Score | Sensitivity | 1–Specificity | Youden Index |
| 5 | 0.071 | 0 | 0.071 |
| 12.5 | 0.119 | 0.059 | 0.06 |
| 17.5 | 0.214 | 0.059 | 0.155 |
| 22.5 | 0.333 | 0.088 | 0.245 |
| 27.5 | 0.571 | 0.265 | 0.306 |
| 32.5 | 0.714 | 0.382 | 0.332 |
| 37.5 | 0.786 | 0.441 | 0.345 |
| 42.5 | 0.905 | 0.765 | 0.140 |
| 47.5 | 0.905 | 0.824 | 0.081 |
| 52.5 | 0.929 | 0.824 | 0.105 |
| 56 | 1 | 1 | 0 |
| SARC-F | |||
| Score | Sensitivity | 1–Specificity | Youden Index |
| 0.5 | 0.881 | 0.706 | 0.175 |
| 1.5 | 0.69 | 0.471 | 0.219 |
| 2.5 | 0.524 | 0.235 | 0.289 |
| 3.5 | 0.333 | 0.118 | 0.215 |
| 4.5 | 0.286 | 0.118 | 0.168 |
| 5.5 | 0.095 | 0.088 | 0.007 |
| 6.5 | 0.048 | 0.029 | 0.019 |
| 7.5 | 0.024 | 0 | 0.024 |
| Specificity | Sensitivity | Negative Predictive Value | Positive Predictive Value | |
|---|---|---|---|---|
| 7-item MSRA score ≤30 | 0.186 | 0.879 | 0.671 | 0.454 |
| 7-item MSRA score ≤25 (new cut-off) | 0.651 | 0.757 | 0.777 | 0.624 |
| 5-item MSRA score ≤45 | 0.163 | 0.909 | 0.700 | 0.454 |
| 5-item MSRA score ≤40 (new cut-off) | 0.679 | 0.688 | 0.739 | 0.622 |
| SARC-F score ≥4 | 0.651 | 0.576 | 0.667 | 0.559 |
| SARC-F score ≥3 (new cut-off) | 0.765 | 0.524 | 0.677 | 0.631 |
| Combined SARC-F/7-item MSRA | 1.00 | 0.636 | 0.782 | 1.00 |
| Combined SARC-F/5-item MSRA | 0.977 | 0.424 | 0.689 | 0.934 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, A.P.; Caliari, C.; Urbani, S.; Fantin, F.; Brandimarte, P.; Martini, A.; Zoico, E.; Zoso, G.; Babbanini, A.; Zanotelli, A.; et al. Sarcopenia Risk Evaluation in a Sample of Hospitalized Elderly Men and Women: Combined Use of the Mini Sarcopenia Risk Assessment (MSRA) and the SARC-F. Nutrients 2021, 13, 635. https://doi.org/10.3390/nu13020635
Rossi AP, Caliari C, Urbani S, Fantin F, Brandimarte P, Martini A, Zoico E, Zoso G, Babbanini A, Zanotelli A, et al. Sarcopenia Risk Evaluation in a Sample of Hospitalized Elderly Men and Women: Combined Use of the Mini Sarcopenia Risk Assessment (MSRA) and the SARC-F. Nutrients. 2021; 13(2):635. https://doi.org/10.3390/nu13020635
Chicago/Turabian StyleRossi, Andrea P., Cesare Caliari, Silvia Urbani, Francesco Fantin, Piero Brandimarte, Angela Martini, Elena Zoico, Giulia Zoso, Alessio Babbanini, Alfredo Zanotelli, and et al. 2021. "Sarcopenia Risk Evaluation in a Sample of Hospitalized Elderly Men and Women: Combined Use of the Mini Sarcopenia Risk Assessment (MSRA) and the SARC-F" Nutrients 13, no. 2: 635. https://doi.org/10.3390/nu13020635
APA StyleRossi, A. P., Caliari, C., Urbani, S., Fantin, F., Brandimarte, P., Martini, A., Zoico, E., Zoso, G., Babbanini, A., Zanotelli, A., & Zamboni, M. (2021). Sarcopenia Risk Evaluation in a Sample of Hospitalized Elderly Men and Women: Combined Use of the Mini Sarcopenia Risk Assessment (MSRA) and the SARC-F. Nutrients, 13(2), 635. https://doi.org/10.3390/nu13020635






