Effect of Cocoa and Cocoa Products on Cognitive Performance in Young Adults
Abstract
:1. Introduction
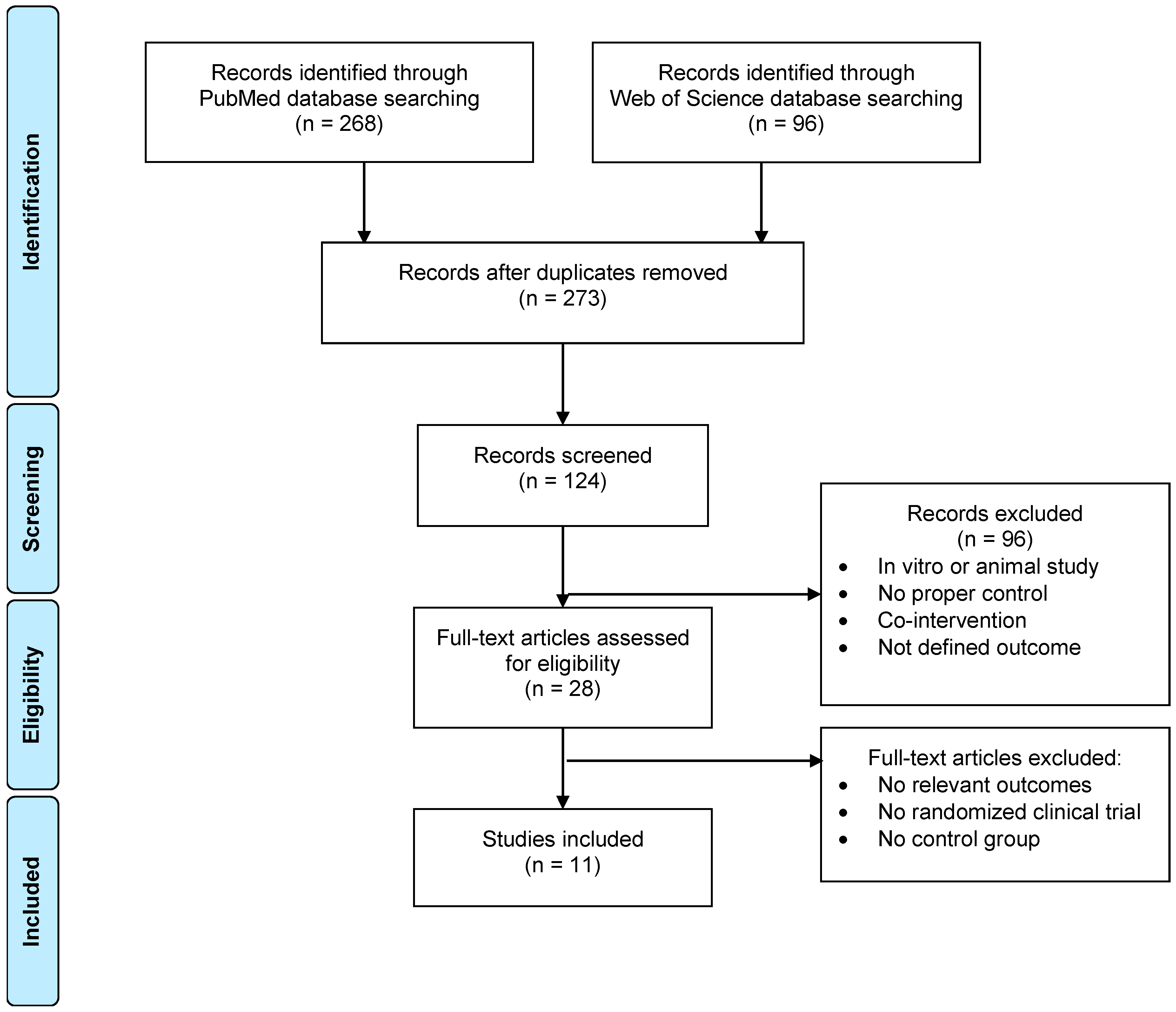
2. Materials and Methods
Literature Selection
3. Results and Discussion
3.1. Methodological Aspects
3.2. Results on Cardiovascular Endpoints
3.3. Results on Cognition Endpoints
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mintzer, J.; Donovan, K.A.; Kindy, A.Z.; Lock, S.L.; Chura, L.R.; Barracca, N. Lifestyle Choices and Brain Health. Front. Med. 2019, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pang, Y.; Liu, J.; Wang, J.; Xie, Z.; Huang, T. Association of healthy lifestyle with cognitive function among Chinese older adults. Eur. J. Clin. Nutr. 2020, 28. [Google Scholar] [CrossRef] [PubMed]
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [Green Version]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Bánáti, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res Rev 2017, 35, 222–240. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, L.J.; Barbagallo, M. Nutritional prevention of cognitive decline and dementia. Acta Biomed. 2018, 89, 276–290. [Google Scholar] [CrossRef]
- Moore, K.; Hughes, C.F.; Ward, M.; Hoey, L.; McNulty, H. Diet, nutrition and the ageing brain: Current evidence and new directions. Proc. Nutr. Soc. 2018, 77, 152–163. [Google Scholar] [CrossRef]
- Flanagan, E.; Lamport, D.; Brennan, L.; Burnet, P.; Calabrese, V.; Cunnane, S.C.; de Wilde, M.C.; Dye, L.; Farrimond, J.A.; Emerson Lombardo, N.; et al. Nutrition and the ageing brain: Moving towards clinical applications. Ageing Res Rev. 2020, 62, 101079. [Google Scholar] [CrossRef]
- Vinciguerra, F.; Graziano, M.; Hagnäs, M.; Frittitta, L.; Tumminia, A. Influence of the Mediterranean and Ketogenic Diets on Cognitive Status and Decline: A Narrative Review. Nutrients 2020, 12, 1019. [Google Scholar] [CrossRef] [Green Version]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-Based Dietary Patterns, Plant Foods, and Age-Related Cognitive Decline. Adv. Nutr. 2019, 10, S422–S436. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, J.Á.; Zafrilla, P.; Marhuenda, J. Cognitive Function and Consumption of Fruit and Vegetable Polyphenols in a Young Population: Is There a Relationship? Foods 2019, 8, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, A.; Trabelsi, K.; Müller, P.; Bouaziz, B.; Boukhris, O.; Glenn, J.M.; Bott, N.T.; Driss, T.; Chtourou, H.; Müller, N.; et al. The Effect of (Poly)phenol-Rich Interventions on Cognitive Functions and Neuroprotective Measures in Healthy Aging Adults: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A Review of the Cognitive Effects Observed in Humans Following Acute Supplementation with Flavonoids, and Their Associated Mechanisms of Action. Nutrients 2015, 7, 10290–10306. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 2564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, A.; Trabelsi, K.; Boukhris, O.; Bouaziz, B.; Müller, P.; Glenn, J.M.; Bott, N.T.; Müller, N.G.; Chtourou, H.; Driss, T.; et al. Effects of Polyphenol-Rich Interventions on Cognition and Brain Health in Healthy Young and Middle-Aged Adults: Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1598. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventos, R.-M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Socci, V.; Tempesta, D.; Desideri, G.; De Gennaro, L.; Ferrara, M. Enhancing Human Cognition with Cocoa Flavonoids. Front. Nutr. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Haskell-Ramsay, C.F.; Schmitt, J.; Actis-Goretta, L. The Impact of Epicatechin on Human Cognition: The Role of Cerebral Blood Flow. Nutrients 2018, 10, 986. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Reyes, P.K.; De Lara, J.C.-F.; González-Soto, M.; Tejero, M. Effects of Cocoa-Derived Polyphenols on Cognitive Function in Humans. Systematic Review and Analysis of Methodological Aspects. Plant Foods Hum. Nutr. 2020, 75, 1–11. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, A.N.; Pavlova, M.A.; Klosterhalfen, S.; Enck, P. Chocolate and the brain: Neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci. Biobehav. Rev. 2013, 7, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Ezra-Nevo, G.; Henriques, S.F.; Ribeiro, C. The diet-microbiome tango: How nutrients lead the gut brain axis. Curr. Opin. Neurobiol. 2020, 62, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Pestana, D.; Teixeira, D.; Couraud, P.-O.; Romero, I.; Weksler, B.; de Freitas, V.; Mateus, N.; Calhau, C. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2011, 2, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Gasperotti, M.; Passamonti, S.; Tramer, F.; Masuero, D.; Guella, G.; Mattivi, F.; Vrhovsek, U. Fate of microbial metabolites of dietary polyphenols in rats: Is the brain their target destination? ACS Chem. Neurosci. 2015, 39, 1341–1352. [Google Scholar] [CrossRef] [Green Version]
- Angelino, D.; Carregosa, D.; Domenech-Coca, C.; Savi, M.; Figueira, I.; Brindani, N.; Jang, S.; Lakshman, S.; Molokin, A.; Urban, J.F., Jr.; et al. 5-(Hydroxyphenyl)-γ-Valerolactone-Sulfate, a Key Microbial Metabolite of Flavan-3-ols, Is Able to Reach the Brain: Evidence from Different in Silico, In Vitro and In Vivo Experimental Models. Nutrients 2019, 11, 2678. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, M.; Sugimoto, N.; Katakura, M.; Matsuzaki, K.; Tanigami, H.; Yachie, A.; Ohno-Shosaku, T.; Shido, O. Theobromine up-regulates cerebral brain-derived neurotrophic factor and facilitates motor learning in mice. J. Nutr. Biochem. 2017, 39, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Lamport, D.J.; Dye, L.; Wightman, J.D.; Lawton, C.L. The effects of flavonoid and other polyphenol consumption on cognitive performance: A systematic research review of human experimental and epidemiological studies. Nutr. Aging 2012, 1, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Muralidhara, K.G. Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Academic Press: London, UK, 2015. [Google Scholar] [CrossRef]
- Nehlig, A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br. J. Clin. Pharmacol. 2013, 75, 716–727. [Google Scholar] [CrossRef]
- Schroeter, H.; Bahia, P.; Spencer, J.P.; Sheppard, O.; Rattray, M.; Cadenas, E.; Rice-Evans, C.; Williams, R.J. (−)Epicatechin stimulates ERK-dependent cyclic AMP response element activity and upregulates GluR2 in cortical neurons. J. Neurochem. 2007, 101, 1596–1606. [Google Scholar] [CrossRef]
- Abd El Mohsen, M.M.; Kuhnle, G.; Rechner, A.R.; Schroeter, H.; Rose, S.; Jenner, P.; Rice-Evans, C.A. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic. Biol. Med. 2002, 33, 1693–1702. [Google Scholar] [CrossRef]
- Mandel, S.A.; Avramovich-Tirosh, Y.; Reznichenko, L.; Zheng, H.; Weinreb, O.; Amit, T.; Youdim, M.B. Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress and PKC signaling pathway. Neurosignals 2005, 42, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl. S2), S215–S220. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Suga, T.; Ishibashi, A.; Takenaka, S.; Tanaka, D.; Hirano, Y. Flavanol-rich cocoa consumption enhances exercise-induced executive function improvements in humans. Nutrition 2018, 46, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Socci, V.; Tempesta, D.; Ferri, C.; De Gennaro, L.; Desideri, G.; Michele, F. Flavanol-rich chocolate acutely improves arterial function and working memory performance counteracting the effects of sleep deprivation in healthy individuals. J. Hypertens. 2016, 34, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, E.; Matsuzaki, K.; Sugimoto, N.; Tanabe, Y.; Hara, T.; Katakura, M.; Miyamoto, M.; Mishima, S.; Shido, O. Sub-Chronic Consumption of Dark Chocolate Enhances Cognitive Function and Releases Nerve Growth Factors: A Parallel-Group Randomized Trial. Nutrients 2019, 11, 2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon-Garciduenas, L.; Mora-Tiscareño, A.; Franco-Lira, M.; Cross, J.V.; Engle, R.; Aragón-Flores, M.; Gómez-Garza, G.; Jewells, V.; Medina-Cortina, H.; Solorio, E.; et al. Flavonol-rich dark cocoa significantly decreases plasma endothelin-1 and improves cognition in urban children. Front. Pharmacol. 2013, 4, 104. [Google Scholar] [CrossRef] [Green Version]
- ZMassee, L.A.; Ried, K.; Pase, M.; Travica, N.; Yoganathan, J.; Scholey, A.; Macpherson, H.; Kennedy, G.; Sali, A.; Pipingas, A. The acute and sub-chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: A randomized, controlled trial. Front. Pharmacol. 2015, 6, 93. [Google Scholar]
- Scholey, A.B.; French, S.J.; Morris, P.J.; Kennedy, D.O.; Milne, A.L.; Haskell, C.F. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J. Psychopharmacol. 2010, 24, 1505–1514. [Google Scholar] [CrossRef]
- Field, D.T.; Williams, C.M.; Butler, L.T. Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions. Physiol. Behav. 2011, 103, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Decroix, L.; van Schuerbeek, P.; Tonoli, C.; van Cutsem, J.; Soares, D.D.; Heyman, E.; Vanderhasselt, T.; Verrelst, R.; Raeymaekers, H.; de Mey, J.; et al. The effect of acute cocoa flavanol intake on the BOLD response and cognitive function in type 1 diabetes: A randomized, placebo-controlled, double-blinded cross-over pilot study. Psychopharmacology 2019, 236, 3421–3428. [Google Scholar] [CrossRef]
- Karabay, A.; Saija, J.D.; Field, D.T.; Akyürek, E.G. The acute effects of cocoa flavanols on temporal and spatial attention. Psychopharmacology 2018, 235, 1497–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamport, D.J.; Christodoulou, E.; Achilleos, C. Beneficial Effects of Dark Chocolate for Episodic Memory in Healthy Young Adults: A Parallel-Groups Acute Intervention with a White Chocolate Control. Nutrients 2020, 12, 483. [Google Scholar] [CrossRef] [Green Version]
- DeCarli, C. Cerebrovascular disease: Assessing the brain as an end-organ of vascular disease. Nat. Rev. Cardiol. 2012, 9, 435–436. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.P.E. The interactions of flavonoids within neuronal signaling pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, C.; Dejam, A.; Kleinbongard, P.; Schewe, T.; Sies, H.; Kelm, M. Vascular effects of cocoa rich in flavan-3-ols. J. Am. Med. Assoc. 2003, 290, 1030–1031. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 03, 1024–1029. [Google Scholar] [CrossRef] [Green Version]
- Dugo, L.; Tripodo, G.; Santi, L.; Fanali, C. Cocoa Polyphenols: Chemistry, Bioavailability and Effects on Cardiovascular Performance. Curr. Med. Chem. 2018, 25, 4903–4917. [Google Scholar] [CrossRef]
- Moreno-Ulloa, A.; Mendez-Luna, D.; Beltrán-Partida, E.; Castillo, C.; Guevara, G.; Ramírez-Sánchez, I.; Correa-Basurto, J.; Ceballos, G.; Villarreal, F. The effects of (-)-epicatechin on endothelial cells involve the G protein-coupled estrogen receptor (GPER). Pharmacol. Res. 2015, 100, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D.L. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr. Dis. Treat. 2008, 2, 433–440. [Google Scholar]
- Fisher, N.D.L.; Sorond, F.A.; Hollenberg, N.K. Cocoa Flavanols and Brain Perfusion. J. Cardiovasc. Pharmacol. 2006, 47, S210–S214. [Google Scholar] [CrossRef]
- Patel, A.K.; Rogers, J.T.; Huang, X. Flavanols, Mild Cognitive Impairment, and Alzheimer’s Dementia. Int. J. Clin. Exp. Med. 2008, 1, 181–191. [Google Scholar] [PubMed]
- Gormaz, J.G.; Valls, N.; Sotomayor, C.; Turner, T.; Rodrigo, R. Potential Role of Polyphenols in the Prevention of Cardiovascular Diseases: Molecular Bases. Curr. Med. Chem. 2016, 23, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cilleros, D.; Ramos, S.; Goya, L.; Martín, M.A. Colonic metabolites from flavanols stimulate nitric oxide production in human endothelial cells and protect against oxidative stress-induced toxicity and endothelial dysfunction. Food Chem. Toxicol. 2018, 115, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schömig, E. Effects of Low Habitual Cocoa Intake on Blood Pressure and Bioactive Nitric Oxide. JAMA 2007, 298, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, L.; A Kroon, P.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; A Le Cornu, K.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Sullivan, T.R.; Fakler, P.; Frank, O.; Stocks, N. Does chocolate reduce blood pressure? A meta-analysis. BMC Med. 2010, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ried, K.; Sullivan, T.R.; Fakler, P.; Frank, O.; Stocks, N.P. Effect of cocoa on blood pressure. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Faridi, Z.; Njike, V.Y.; Dutta, S.; Ali, A.; Katz, D.L. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2008, 8, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high polyphenol dark chocolate. J. Nutr. 2008, 38, 1671–1676. [Google Scholar] [CrossRef]
- Monahan, K.D.; Feehan, R.P.; Kunselman, A.R.; Preston, A.G.; Miller, D.L.; Lott, M.E. Dose-dependent increases in flow-mediated dilation following acute cocoa ingestion in healthy older adults. J. Appl. Physiol. 2011, 111, 1568–1574. [Google Scholar] [CrossRef] [Green Version]
- Njike, V.Y.; Faridi, Z.; Shuval, K.; Dutta, S.; Kay, C.D.; West, S.G.; Kris-Etherton, P.M.; Katz, D.L. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int. J. Cardiol. 2011, 149, 83–88. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Aguilar, H.; Ceballos, G.; Villarreal, F. (−)- Epicatechin-induced calcium independent eNOS activation: Roles of HSP90 and AKT. Mol. Cell Biochem. 2012, 70, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.J.; Spencer, J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Rad. Biol. Med. 2012, 2, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Fisher, N.D.; Hughes, M.; Gerhard-Herman, M.; Hollenberg, N.K. Flavanol rich cocoa induces nitric oxide-dependent vasodilation in healthy humans. J. Hypertens. 2003, 1, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Garcia, G.; Pimpao, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 1145. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, N.K.; Fisher, N.D.; McCullough, M.L. Flavanols, the Kuna, cocoa consumption, and nitric oxide. J. Am. Soc. Hypertens. 2009, 3, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Kalt, W.; Hanneken, A.; Milbury, P.; Tremblay, F. Recent research on polyphenolics in vision and eye health. J. Agric. Food Chem. 2010, 58, 4001–4007. [Google Scholar] [CrossRef]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and chocolate in human health and disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef] [Green Version]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Tagougui, S.; Heyman, E.; Meeusen, R. Acute cocoa flavanol improves cerebral oxygenation without enhancing executive function at rest or after exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 1225–1232. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Necozione, S.; Di Giosia, P.; Barnabei, R.; Allegaert, L.; Bernaert, H.; Ferri, C. Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. J. Hypertens. 2015, 33, 294–303. [Google Scholar] [CrossRef]
- Brickman, A.M.; A Khan, U.; A Provenzano, F.; Yeung, L.-K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; A Small, S. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: The cocoa, cognition, and aging (cocoa) study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Pal, D.; Moutsiana, C.; Field, D.T.; Williams, C.M.; Spencer, J.P.E.; Butler, L.T. The effect of flavanol-rich cocoa on cerebral perfusion in healthy older adults during conscious resting state: A placebo controlled, crossover, acute trial. Psychopharmacol. 2015, 232, 3227–3234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Country, M.W. Retinal metabolism: A comparative look at energetics in the retina. Brain Res. 2017, 1672, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Krępa, E.; Domaszewski, P.; Pokora, I.; Żebrowska, A.; Gdańska, A.; Podgórski, T. Effects of medium-term green tea extract supplementation combined with CrossFit workout on blood antioxidant status and serum brain-derived neurotrophic factor in young men: A pilot study. J. Int. Soc. Sports Nutr. 2019, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dinges, D.F. Cocoa Flavanols, Cerebral Blood Flow, Cognition, and Health: Going Forward. J. Cardiovasc. Pharmacol. 2006, 47, S223–S225. [Google Scholar] [CrossRef]
- Van Praag, H.; Lucero, M.J.; Yeo, G.W.; Stecker, K.; Heivand, N.; Zhao, C.; Yip, E.; Afanador, M.; Schroeter, H.; Hammerstone, J.; et al. Plant-Derived Flavanol (-)Epicatechin Enhances Angiogenesis and Retention of Spatial Memory in Mice. J. Neurosci. 2007, 27, 5869–5878. [Google Scholar] [CrossRef]
- Katusic, Z.S.; Austin, S.A. Endothelial nitric oxide: Protector of a healthy mind. Eur. Hear. J. 2014, 35, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Kelleher, R.J., III; Govindarajan, A.; Jung, H.Y.; Kang, H.; Tonegawa, S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 2004, 116, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Cordero-Herrera, I.; Martín, M.A.; Goya, L. and Ramos, S. Cocoa flavonoids protect hepatic cells against high glucose-induced oxidative stress: Relevance of MAPKs. Mol. Nutr. Food Res. 2015, 59, 597–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyarzu, P.; Malin, D.H.; Lau, F.C.; Taglialatela, G.; Moon, W.D.; Jennings, R.; Moy, E.; Moy, D.; Lippold, S.; Shukitt-Hale, B.; et al. Blueberry supplemented diet: Effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutr. Neurosci. 2004, 7, 75–83. [Google Scholar] [CrossRef]
- Valente, T.; Hidalgo, J.; Bolea, I.; Ramirez, B.; Anglés, N.; Reguant, J.; Morelló, J.R.; Gutiérrez, C.; Boada, M.; Unzeta, M. A diet enriched in polyphenols and polyunsaturated fatty acids, LMN diet, induces neurogenesis in the subventricular zone and hippocampus of adult mouse brain. J. Alzheimer Dis. 2009, 18, 849–865. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F.; Nguyen, T.T. Natural mood foods: The actions of polyphenols against psychiatric and cognitive disorders. Nutr. Neurosci. 2012, 15, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Neshatdoust, S.; Saunders, C.; Castle, S.M.; Vauzour, D.; Williams, C.; Butler, L.; Lovegrove, J.A.; Spencer, J.P. High-flavonoid intake induces cognitive improvements linked to changes in serum brain-derived neurotrophic factor: Two randomised, controlled trials. Nutr. Healthy Aging 2016, 4, 81–93. [Google Scholar] [CrossRef] [Green Version]
| Reference | Participants (Years Old) | Type of Study | Flavanol Amount | Control Group | Time * | Outcomes | Results |
|---|---|---|---|---|---|---|---|
| [33] | 16 (female only) 18–30 | acute/chronic (5 days) | 172 mg CF for 5 days | 13 mg CF | 90 min | blood oxygenation level-dependent by fMRI | blood oxygenation in active brain regions fMRI |
| 4 24–31 | acute | 516 mg CF | 39 mg | before, 2, 4& 6 h | cerebral blood flow increased 60% at 2 h after ingestion | ||
| [39] | 30 (17 female) 21.9 ± 0.61 | acute | 520 mg & 994 mg of CF | 46 mg | 90 min | cognitive demand battery | improvement in serial 3 subtraction for all time point at 520 mg but not 994 mg and the same for fatigue improvement. |
| [40] | 30 (22 female) 18–25 | acute | 773 mg CF (222 mg theobromine y 35 mg caffeine) | Trace amounts | 2 h | visual contrast sensitivity and memory tasks | visual contrast sensitivity and integration time threshold, no effect on memory tasks |
| [37] | 18 (11 female) 10.55 ± 1.45 | chronic (10.11 ± 3.4 days) | 680 mg CF (30 g cocoa) | no flavanol for 15 days. | 4 h | Inflammation markers and short memory tests | decrease plasma endothelin1 and inflammatory mediators. In total, 83% of children showed a marginally significant improvement in one or both short memory tasks |
| [38] | 40 (27 female) 24.13 ± 4.47 | acute/chronic (28 days) | 250 mg CF (Tablet with 3 g cocoa) | placebo tablet | 2 h | subtraction, rapid visual processing, and a mental fatigue scale | improved self-reported mental fatigue and performance on the Serial Sevens task |
| [35] | 32 (16 female) 25.31 ± 3.60 | acute | 520 mg CF | 88.5 mg CF | 90 min | cognitive functions in sleep deprivation conditions | working memory accuracy is higher only in female after high flavanol chocolate consumption in sleep deprivation conditions than after low flavanol in the same conditions |
| [34] | 10 (0 female) 22.6 ± 0.3 | acute | 563 mg CF | 38 mg CF (Energy matched beverage) | 70 min | executive function and memory function (Stroop task, face-name matching) | CF could enhance exercise-induced improvement in executive function, but not in memory function |
| [41] | 20 (ND females) 23.2 ± 4.3 | acute | 530 mg CF | Capsules matched for theobromine and caffeine | 2 h | cognition battery and hemodynamic changes and neuronal activity | CF intake improved neurovascular coupling, but did not affect neuronal activity and cognitive performance in both normoxia and hypoxia |
| [42] | 48 (24 female) 22.15 ± 0.01 | acute | 747 mg and 37 4 mg CF | alkalinized cocoa | 2 h | cognition and visual functions | CF does not influence temporal attention, but does decrease reaction time in visual search with medium effect size and without losing accuracy |
| [36] | 20 (6 female) 20–31 | chronic (30 days) | 24 g/day dark chocolate | White chocolate | 20 h | cognitive function (Stroop and digital cancellation test). Prefrontal CBF | Increased Plasma Nerve Growth Factor, enhancing cognitive function performance |
| [43] | 98 (57 female) 20.7 ± 0.18 | acute | 35 g dark chocolate | White chocolate | 2 h | verbal episodic memory | dark chocolate consumption can benefit verbal episodic memory |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, M.A.; Goya, L.; de Pascual-Teresa, S. Effect of Cocoa and Cocoa Products on Cognitive Performance in Young Adults. Nutrients 2020, 12, 3691. https://doi.org/10.3390/nu12123691
Martín MA, Goya L, de Pascual-Teresa S. Effect of Cocoa and Cocoa Products on Cognitive Performance in Young Adults. Nutrients. 2020; 12(12):3691. https://doi.org/10.3390/nu12123691
Chicago/Turabian StyleMartín, María Angeles, Luis Goya, and Sonia de Pascual-Teresa. 2020. "Effect of Cocoa and Cocoa Products on Cognitive Performance in Young Adults" Nutrients 12, no. 12: 3691. https://doi.org/10.3390/nu12123691
APA StyleMartín, M. A., Goya, L., & de Pascual-Teresa, S. (2020). Effect of Cocoa and Cocoa Products on Cognitive Performance in Young Adults. Nutrients, 12(12), 3691. https://doi.org/10.3390/nu12123691







