Low FODMAP Diet for Functional Gastrointestinal Symptoms in Quiescent Inflammatory Bowel Disease: A Systematic Review of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. PICO and Research Question
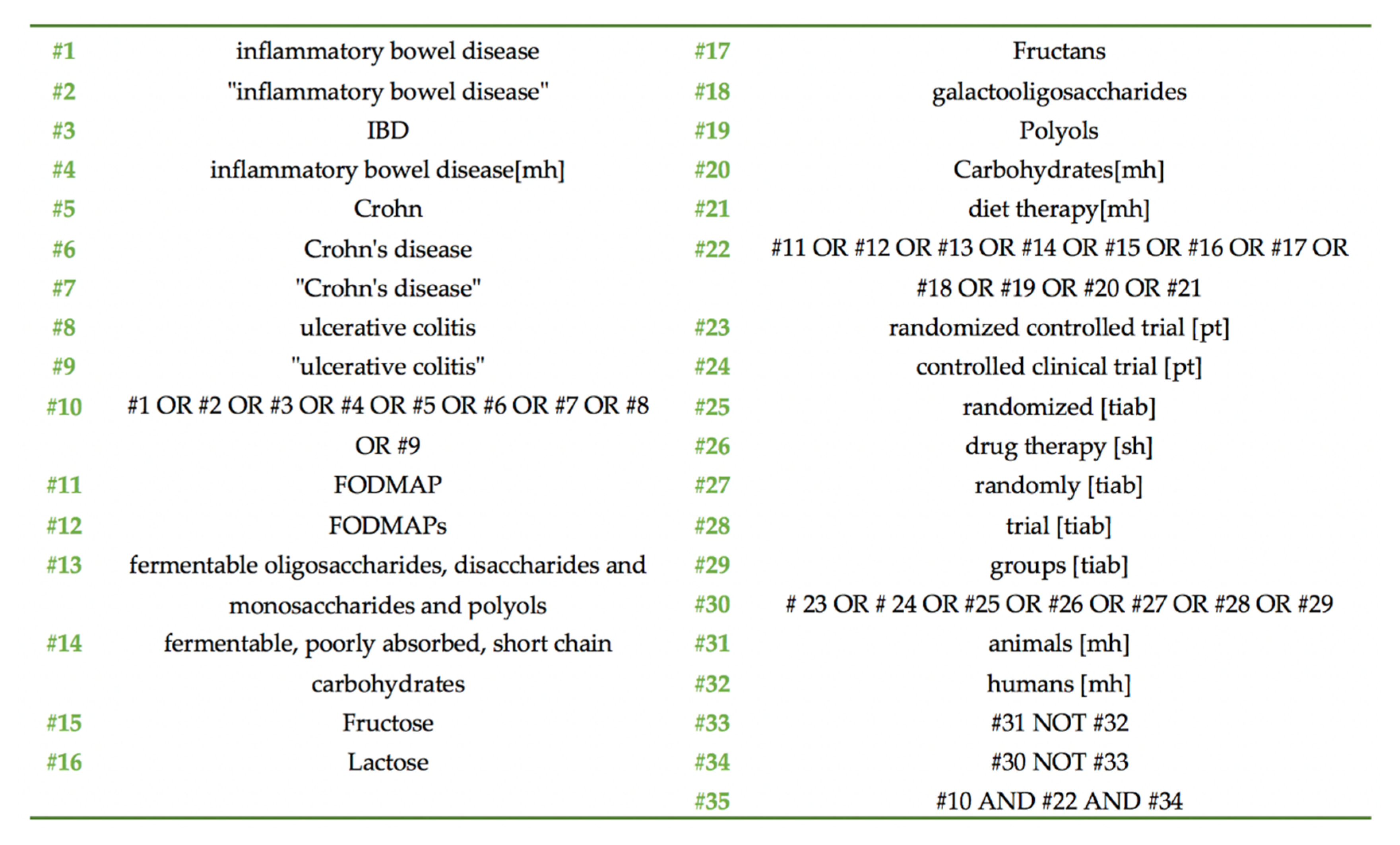
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Outcomes of Interest
2.5. Risk of Bias and Quality of the RCTs
2.6. Data Extraction
2.7. Data Synthesis
3. Results
3.1. Characteristics of the Trials
3.2. Intervention Characteristics
3.3. Outcomes of Interest
3.4. Risk of Bias of the Included RCTs
3.5. Effect of the LFD in QoL
3.6. Fecal Assays
3.7. Markers of Inflammation and Immunity
3.8. Relief of FGD Symptoms
3.9. Changes in the Dietary Intake Following an LFD
3.10. Effects on Gut Microbiota
3.11. Data Synthesis
3.12. Research in the Pipeline
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moayyedi, P.; Simrén, M.; Bercik, P. Evidence-based and mechanistic insights into exclusion diets for IBS. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 406–413. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Berean, K.J.; Burgell, R.E.; Muir, J.G.; Gibson, P.R. Intestinal gases: Influence on gut disorders and the role of dietary manipulations. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Frame, L.A.; Costa, E.; Jackson, S.A. Current explorations of nutrition and the gut microbiome: A comprehensive evaluation of the review literature. Nutr. Rev. 2020, 78, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.; Gkiouras, K.; Theodoridis, X.; Asteriou, E.; Forbes, A.; Bogdanos, D. Oral Adjuvant Curcumin Therapy for Attaining Clinical Remission in Ulcerative Colitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, J.L.; Hedin, C.R.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut 2011, 60, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.O. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut 2006, 55, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.; Gibson, P. The Complete Low FODMAP Diet: A Revolutionary Plan for Managing IBS and Other Digestive Disorders, 1st ed.; The Experiment, LLC: New York, NY, USA, 2013. [Google Scholar]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- Turco, R.; Salvatore, S.; Miele, E.; Romano, C.; Marseglia, G.L.; Staiano, A. Does a low FODMAPs diet reduce symptoms of functional abdominal pain disorders? A systematic review in adult and paediatric population, on behalf of Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 53. [Google Scholar] [CrossRef] [Green Version]
- Schumann, D.; Klose, P.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Nutrition 2018, 45, 24–31. [Google Scholar] [CrossRef]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef]
- Prince, A.C.; Myers, C.E.; Joyce, T.; Irving, P.; Lomer, M.; Whelan, K. Fermentable Carbohydrate Restriction (Low FODMAP Diet) in Clinical Practice Improves Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 1129–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halpin, S.J.; Ford, A.C. Prevalence of Symptoms Meeting Criteria for Irritable Bowel Syndrome in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2012, 107, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.I.; Sandler, R.S.; Kappelman, M.D.; Martin, C.F.; Chen, W.; Anton, K.; Long, M.D. Prevalence and Impact of Inflammatory Bowel Disease—Irritable Bowel Syndrome on Patient-reported Outcomes in CCFA Partners. Inflamm. Bowel Dis. 2017, 23, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Perera, L.P.; Radigan, M.; Guilday, C.; Banerjee, I.; Eastwood, D.; Babygirija, R.; Massey, B.T. Presence of Irritable Bowel Syndrome Symptoms in Quiescent Inflammatory Bowel Disease Is Associated with High Rate of Anxiety and Depression. Dig. Dis. Sci. 2019, 64, 1923–1928. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.; Ahuja, V.; Kedia, S.; Midha, V.; Mahajan, R.; Mehta, V.; Sudhakar, R.; Singh, A.; Kumar, A.; Puri, A.S.; et al. Diet and inflammatory bowel disease: The Asian Working Group guidelines. Indian J. Gastroenterol. 2019, 38, 220. [Google Scholar] [CrossRef] [Green Version]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.H.; Lomer, M.C.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Zhan, Y.; Dai, S. Is a low FODMAP diet beneficial for patients with inflammatory bowel disease? A meta-analysis and systematic review. Clin. Nutr. 2018, 37, 123–129. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Br. Med. J. 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Consistent Prebiotic Effect on Gut Microbiota With Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin. Transl. Gastroenterol. 2016, 7, e164. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients With Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, S.R.; Stagg, A.J.; Fromentin, S.; Ehrlich, S.D.; McCarthy, N.E.; Galleron, N.; Levenez, F.; Lomer, M.; Lindsay, J.O.; Irving, P.M.; et al. 902—Low Fodmap Diet Improves Functional-Like Gastrointestinal Symptoms but Reduces Bifidobacteria and Faecalibacterium Prausnitzii in Quiescent Inflammatory Bowel Disease: A Randomised Controlled Trial and Metagenomic Analysis. Gastroenterology 2018, 154, S-177. [Google Scholar] [CrossRef]
- Bodini, G.; Zanella, C.; Crespi, M.; Pumo, S.L.; Demarzo, M.G.; Savarino, E.; Savarino, V.; Giannini, E.G. A randomized, 6-wk trial of a low FODMAP diet in patients with inflammatory bowel disease. Nutrition 2019, 67–68, 110542. [Google Scholar] [CrossRef]
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Végh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 3356. [Google Scholar] [CrossRef]
- Pedersen, N.; Ankersen, D.; Felding, M.; Végh, Z.; Burisch, Z.; Munkholm, P. DOP067 Low FODMAP diet reduces irritable bowel symptoms and improves quality of life in patients with inflammatory bowel disease in a randomized controlled trial. J. Crohn’s Colitis 2014, 8, S47. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, N. EHealth: Self-management in inflammatory bowel disease and in irritable bowel syndrome using novel constant-care web applications. EHealth by constant-care in IBD and IBS. Dan. Med. J. 2015, 62, B5168. [Google Scholar]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology 2014, 146, 67–75. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [Green Version]
- Mangel, A.W.; Hahn, B.A.; Heath, A.T.; Northcutt, A.R.; Kong, S.; Dukes, G.E.; McSorley, D. Adequate Relief as an Endpoint in Clinical Trials in Irritable Bowel Syndrome. J. Int. Med. Res. 1998, 26, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet (Lond. Engl.) 1980, 1, 514. [Google Scholar] [CrossRef]
- Irvine, E.J.; Feagan, B.; Rochon, J.; Archambault, A.; Fedorak, R.N.; Groll, A.; Kinnear, D.; Saibil, F.; McDonald, J.W.; Canadian Crohn’s Relapse Prevention Trial Study Group. Quality of life: A valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Gastroenterology 1994, 106, 287–296. [Google Scholar] [CrossRef]
- Cheung, W.; Garratt, A.M.; Russell, I.T.; Williams, J.G. The UK IBDQ—A British version of the inflammatory bowel disease questionnaire. J. Clin. Epidemiol. 2000, 53, 297–306. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, S.; Parker, F.; Muir, J.; Gibson, P. Dietary Triggers of Abdominal Symptoms in Patients With Irritable Bowel Syndrome: Randomized Placebo-Controlled Evidence. Clin. Gastroenterol. Hepatol. 2008, 6, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Bodger, K.; Ormerod, C.; Shackcloth, D.; Harrison, M.; Collaborative on behalf of the IC. Development and validation of a rapid, generic measure of disease control from the patient’s perspective: The IBD-Control questionnaire. Gut 2014, 63, 1092–1102. [Google Scholar] [CrossRef] [Green Version]
- Jowett, S.L.; Seal, C.J.; Phillips, E.; Gregory, W.; Barton, J.R.; Welfare, M.R. Defining relapse of ulcerative colitis using a symptom-based activity index. Scand. J. Gastroenterol. 2003, 38, 164–171. [Google Scholar] [CrossRef]
- Halmos, E.P. A low FODMAP diet in patients with Crohn’s disease. J. Gastroenterol. Hepatol. 2016, 31, 14–15. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.; Morris, C.B.; Hu, Y.; Toner, B.B.; Diamant, N.; Whitehead, W.E.; Dalton, C.B.; Leserman, J.; Patrick, D.L.; Bangdiwala, S.I. Characterization of health related quality of life (HRQOL) for patients with functional bowel disorder (FBD) and its response to treatment. Am. J. Gastroenterol. 2007, 102, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Patrick, D.L.; Whitehead, W.E.; Toner, B.B.; Diamant, N.E.; Hu, Y.; Jia, H.; Bangdiwala, S.I. Further validation of the IBS-QOL: A disease-specific quality-of-life questionnaire. Am. J. Gastroenterol. 2000, 95, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.S.; Gibson, P.R. Development and validation of a comprehensive semi-quantitative food frequency questionnaire that includes FODMAP intake and glycemic index. J. Am. Diet. Assoc. 2010, 110, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Svedlund, J.; Sjödin, I.; Dotevall, G. GSRS—A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef]
- Camilleri, M.; Mayer, E.A.; Drossman, D.A.; Heath, A.; Dukes, G.E.; McSorley, D.; Kong, S.; Mangel, A.W.; Northcutt, A.R. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment. Pharmacol. Ther. 1999, 13, 1149–1159. [Google Scholar] [CrossRef]
- Kellow, J.; Lee, O.Y.; Chang, F.Y.; Thongsawat, S.; Mazlam, M.Z.; Yuen, H.; Gwee, K.A.; Bak, Y.T.; Jones, J.; Wagner, A. An Asia-Pacific, double blind, placebo controlled, randomised study to evaluate the efficacy, safety, and tolerability of tegaserod in patients with irritable bowel syndrome. Gut 2003, 52, 671–676. [Google Scholar] [CrossRef] [Green Version]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Juritsch, A.F.; Moreau, R. Role of soybean-derived bioactive compounds in inflammatory bowel disease. Nutr. Rev. 2018, 76, 618–638. [Google Scholar] [CrossRef]
- Casen, C.; Vebø, H.C.; Sekelja, M.; Hegge, F.T.; Karlsson, M.K.; Ciemniejewska, E.; Dzankovic, S.; Frøyland, C.; Nestestog, R.; Engstrand, L.; et al. Deviations in human gut microbiota: A novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment. Pharmacol. Ther. 2015, 42, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Quigley, E.M.M. Overlapping irritable bowel syndrome and inflammatory bowel disease: Less to this than meets the eye? Ther. Adv. Gastroenterol. 2016, 9, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, R.A.; Ali, R.A.R.; Lee, Y.Y. Irritable bowel syndrome and inflammatory bowel disease overlap syndrome: Pieces of the puzzle are falling into place. Intestig. Res. 2016, 14, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summers, M.A. A systematic review on the effect of fermentable oligosaccharide, disaccharide, monosaccharide and polyol manipulation on bifidobacteria abundance and gastrointestinal symptoms. Implications when following a low FODMAP diet. Clin. Nutr. ESPEN 2017, 22, 119. [Google Scholar] [CrossRef] [Green Version]
- Staudacher, H.M.; Whelan, K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: Probiotics, prebiotics and the low FODMAP diet. Proc. Nutr. Soc. 2016, 75, 306–318. [Google Scholar] [CrossRef]
- Gibson, P.R. Use of the low-FODMAP diet in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2017, 32, 40–42. [Google Scholar] [CrossRef] [Green Version]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; Jonkers, D.M.; Salonen, A.; Hanevik, K.; Raes, J.; Jalanka, J.; De Vos, W.M.; Manichanh, C.; Golic, N.; Enck, P.; et al. Intestinal Microbiota And Diet in IBS: Causes, Consequences, or Epiphenomena? Am. J. Gastroenterol. 2015, 110, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Catassi, G.; Lionetti, E.; Gatti, S.; Catassi, C. The Low FODMAP Diet: Many Question Marks for a Catchy Acronym. Nutrients 2017, 9, 292. [Google Scholar] [CrossRef]
- Simrén, M. Manipulating the Gut Microbiome as a Treatment Strategy for Functional Gastrointestinal Disorders. Gastroenterology 2018, 155, 960–962. [Google Scholar] [CrossRef] [Green Version]
- Larussa, T.; Suraci, E.; Marasco, R.; Imeneo, M.; Abenavoli, L.; Luzza, F. Self-Prescribed Dietary Restrictions are Common in Inflammatory Bowel Disease Patients and Are Associated with Low Bone Mineralization. Medicina (Kaunas) 2019, 55, 507. [Google Scholar] [CrossRef] [Green Version]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oii, S.; Correa, D.; Pak, S. Probiotics, prebiotics, and low FODMAP diet for irritable bowel syndrome—What is the current evidence? Complement. Ther. Med. 2019, 43, 73–80. [Google Scholar] [CrossRef]
- Krogsgaard, L.R.; Lyngesen, M.; Bytzer, P. Systematic review: Quality of trials on the symptomatic effects of the low FODMAP diet for irritable bowel syndrome. Aliment. Pharmacol. Ther. 2017, 45, 1506–1513. [Google Scholar] [CrossRef] [Green Version]
- Molina-Infante, J.; Serra, J.; Fernandez-Bañares, F.; Mearin, F. The low-FODMAP diet for irritable bowel syndrome: Lights and shadows. Gastroenterol. Hepatol. 2016, 39, 55–65. [Google Scholar] [CrossRef]
- Nazarenkov, N.; Beeken, L.; Seeger, K.; Ananthakrishnan, A.; Khalili, H.; Lewis, J.; Konijeti, G. P652 Nutritional adequacy of popular defined diets for inflammatory bowel disease. J. Crohn’s Colitis 2018, 12, S440–S441. [Google Scholar] [CrossRef]
- Sandhar, H.; Direkze, N.; Peake, S. P640 Evaluation of dietetic services and the impact of diet on disease activity for patients with inflammatory bowel disease at Imperial College Healthcare NHS Trust. J. Crohn’s Colitis 2019, 13, S438. [Google Scholar] [CrossRef]
- Maagaard, L.; Ankersen, D.V.; Végh, Z.; Burisch, J.; Jensen, L.; Pedersen, N.; Munkholm, P. Follow-up of patients with functional bowel symptoms treated with a low FODMAP diet. World J. Gastroenterol. 2016, 22, 4009. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Irving, P.M.; Lomer, M.C.E.; Whelan, K. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc. Nutr. Soc. 2017, 76, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No Effects of Gluten in Patients With Self-Reported Non-Celiac Gluten Sensitivity After Dietary Reduction of Fermentable, Poorly Absorbed, Short-Chain Carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef]
- Jairath, V.; Zou, G.Y.; Parker, C.E.; MacDonald, J.K.; AlAmeel, T.; Al Beshir, M.; Almadi, M.A.; Al-Taweel, T.; Atkinson, N.S.; Biswas, S.; et al. Placebo response and remission rates in randomised trials of induction and maintenance therapy for ulcerative colitis. Cochrane Database Syst. Rev. 2017, 9, CD011572. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh-Tabari, S.; Zamani, V. Systematic review with meta-analysis: The prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 132–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krogsgaard, L.R.; Lyngesen, M.; Bytzer, P. Letter: Bias in clinical trials of the symptomatic effects of the low FODMAP diet for irritable bowel syndrome-getting the facts right. Authors’ reply. Aliment. Pharmacol. Ther. 2017, 46, 386–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halmos, E.P.; Biesiekierski, J.R.; Newnham, E.D.; Burgell, R.E.; Muir, J.G.; Gibson, P.R. Inaccuracy of patient-reported descriptions of and satisfaction with bowel actions in irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13187. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Guidance for Industry Irritable Bowel Syndrome-Clinical Evaluation of Drugs for Treatment; U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2012.
- Whigham, L.; Joyce, T.; Harper, G.; Irving, P.M.; Staudacher, H.M.; Whelan, K.; Lomer, M.C.E. Clinical effectiveness and economic costs of group versus one-to-one education for short-chain fermentable carbohydrate restriction (low FODMAP diet) in the management of irritable bowel syndrome. J. Hum. Nutr. Diet. 2015, 28, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Am. J. Gastroenterol. 2016, 111, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome as Well as Traditional Dietary Advice: A Randomized Controlled Trial. Gastroenterology 2015, 149, 1399–1407. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.R.; Halmos, E.P.; Muir, J.G. Letter: Bias in clinical trials of the symptomatic effects of the low FODMAP diet for irritable bowel syndrome-getting the facts right. Aliment. Pharmacol. Ther. 2017, 46, 385–386. [Google Scholar] [CrossRef] [Green Version]
- Forbes, A. Nutrition and inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 350–354. [Google Scholar] [CrossRef]
- Halmos, E.P. When the low FODMAP diet does not work. J. Gastroenterol. Hepatol. 2017, 32, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Chapman, S.; Sibelli, A.; St-Clair Jones, A.; Forbes, A.; Chater, A.; Horne, R. Personalised Adherence Support for Maintenance Treatment of Inflammatory Bowel Disease: A Tailored Digital Intervention to Change Adherence-related Beliefs and Barriers. J. Crohn’s Colitis 2020, 14, 1394–1404. [Google Scholar] [CrossRef]


| First Author: | Bodini [26] | Cox [24,25] | Halmos [23] | Pedersen [27,28,29] |
|---|---|---|---|---|
| Publication: | Nutrition 2019 | Gastroenterology 2020; J. Crohns Colitis 2018 | Clin. Transl. Gastroenterol. 2017 | World J. Gastroenterol. 2017; Dan. Med. J. 2015; J. Crohns Colitis 2014 |
| Publication type: | Full-text (n = 1) | Full-text (n = 1) and poster abstract (n = 1) | Full-text (n = 1) | Full-text (n = 2) and poster abstract (n = 1) |
| Study duration: | NR | 2016–2017 | 2009–2011 | 2012–2013 |
| Origin: | Italy | UK | Australia | Denmark |
| Registry: | - | ISRCTN17061468 | ACTRN12612001185853 | - |
| Funding: | NR | Kenneth Rainin Fndn | National Health and Medical Research Council of Australia, Eva and Les Erdi Fndn, Monash University | NR |
| Ethical permission: | NR | London Dulwich | Eastern Health and Monash University Human Research and Ethics Committees | Ethics Committee of Science, Denmark |
| RCT Design: | Parallel | Parallel | Cross-over | Parallel |
| Randomization: | PC-generated sequence | Block, with a 1:1 ratio, stratified by diagnosis (CD/UC) and fCAL at screening | PC-generated order | A person not involved in the study generated the random sequence and numbered the envelopes |
| Masking: | Single-blind (clinician) | Single-blind (patients). The terms “fermentable carbohydrates”, “low FODMAP diet”, or the diet’s mechanisms were not mentioned to participants | Double-blind (?) § | Open-label |
| Multicenter: | - | √ | - | - |
| Recruitment site: | Ospedale Policlinico San Martino—IRCCS per l’Oncologia, Genoa | Two large gastroenterology clinics in London | Gastroenterology clinics and the internet | Tertiary hospital in Copenhagen |
| Participants: | N = 55 IBD-IBS patients on remission or with mild disease activity (PMS < 6 or HBi < 8) | N = 52 quiescent * IBD patients with FGD (IBS-D, IBS-M, IBS-U, FB, or FD), LFD naïve | N = 9 quiescent œ CD patients with FGS | N = 89 IBD patients with FGS in remission, or mild-to-moderate disease |
| Ethnicity: | NR | √ | NR | NR |
| CD/UC (n): | 35/20 | 26/26 | 9/0 | 28/61 |
| Criteria for IBS: | Rome IV [31] | Rome III [32] | Rome III [32] š | Rome III [32] |
| IBD Diagnostic criteria: | endoscopic, radiologic, and histologic evaluation | NR | NR | NR |
| Participant age: | 45 (20–75) † years | ≥18 years | 35 (29–41) ƒ years | 40 (20–70) † years |
| Intervention arm: | n = 26 | n = 27 | n = 9 | n = 44 |
| Control arm: | n = 29 | n = 25 | n = 9 | n = 45 |
| Inclusion criteria: | √ | √ | √ | √ |
| Exclusion criteria: | √ | √ | √ | √ |
| HLA-DQ2/DQ8: | NR | NR | All patients were negative | NR |
| First Author: | Bodini [26] | Cox [24,25] | Halmos [23] | Pedersen [27,28,29] |
|---|---|---|---|---|
| Relief assessment: | NR | GSQ | 100 mm VAS | NR |
| Improvement definition: | NR | Achieving a 50-point reduction in IBS-SSS | NR | Achieving a 50-point reduction in IBS-SSS |
| Intervention: | LFD No ONS was allowed | LFD | LFD ≤ 0.5 g per sitting [37] (three main meals and three snacks daily were delivered to patients) + small quantities of psyllium and resistant starch (daily average of 3 g psyllium and 5 g Hi-Maize 220 (National Starch and Chemical Company, Bridgewater, NJ, USA), to ensure similar fiber content | LFD |
| Comparator: | Standard diet | Sham exclusion diet of similar intensity, burden, and nutrient intake to the LFD | typical Australian diet | Normal diet |
| Assessment of dietary FODMAP intake: | Detailed meals with calculated FODMAP content (NOD) | Via FODMAP database (Monash University, Melbourne, Australia) | FODMAP content for all provided food underwent FODMAP analysis via high-performance liquid chromatography and enzymatic assays | NR |
| Intervention duration: | 6 weeks | 4 weeks | 21 days (each intervention) | 6 weeks |
| Wash-out duration: | N/A | N/A | >21 days (until the symptoms had returned to the same level as during their habitual diet) | N/A |
| Stool: | Sample | 7-day diary and fresh stool sample at baseline | 5-day samples | Sample |
| fCAL assay: | Quantum Blue fCAL (Buhlmann Lab) | ELISA | ELISA using a commercial kit (Buhlmann EK-Cal, Schönenbuch, Switzerland) | Home-administered collecting kit and ELISA |
| Compliance assessment: | Dietitian (weekly phone calls and food diaries) | With a question at the end of the trial: “During the 4-week trial I have followed the diet…”: never/rarely (<25% of the time), sometimes (25–50% of the time), frequently (51–75% of the time) or always (76–100% of the time) and with 7-day food diaries | Dietitian | FFQ [45] with the most commonly consumed high-FODMAP foods adapted to the Danish population |
| Dropouts (n): | - | n = 6 (2 withdrew consent, 1 became pregnant, 1 initiated steroids, and 1 antibiotics) n = 3 for low compliance | n = 1 for low LFD compliance | n = 11 (7 for difficulty in LFD compliance and 4 for lack of compliance with registering IBS symptoms) |
| Non-compliant (n): | NR | n = 3 | n = 1 from the LFD group | n = 7 from the LFD group |
| Adverse events (n): | NR | n = 2 IBD relapse (one in each group) n = 1 started antibiotics unrelated to IBD n = 1 abdominal pain (controls) n = 2 flu-like symptoms and sinusitis (one in each group) | NR | |
| Primary outcomes: | Δ in PMS, HBi, IBD-Q | Δ in IBS-SSS | Δ in fecal microbiota including total, butyrate-producing (C. leptum, F. prausnitzii, Roseburia spp.), traditionally prebiotic (Lactobacilli and Bifidobacteria spp.), and mucus-degrading bacteria (A. muciniphila, R. gnavus, R. torques) | Δ in HBi, SCCAI, patients reporting improvement |
| Secondary outcomes: | Δ in CRP levels, fCAL, anthropometry | Δ in GSRS, fecal SCFA (GLC), fecal pH (InLab, Mettler Toledo probe), CRP, BSFS, IBD-Q, HBi, PMS, IBD Control Q, fecal microbiome composition and function | fecal pH, total and specific fecal SCFA concentration, severity of GI symptoms (100 mm VAS), fecal frequency and weight, FWC, whole-gut transit time, comparison of data during interventional diets to habitual diet | Δ in IBS-SSS, QoL (HR-QoL, IBS-QoL), CRP, fCAL, SIBDQ, SF36, treatment satisfaction (VAS) |
| Microbiome composition: | - | Via quantitative metagenomic pipeline | PCR on DNA fecal samples | - |
| T-cell phenotype: | - | CD3, CD45RA+, CD45RA-, CD4, CD8, Vδ2 unconventional T-cells, integrin α4β7 | - | - |
| Timepoints: | Baseline and end (6 weeks) | Baseline and end of trial (4 weeks) | Start and end of each intervention | Baseline and 6 weeks |
| Analyses: | ITT n = 55 | ITT n = 52 PP n = 43 | PP n = 8 | ITT intervention n = 37 ITT controls n = 41 |
| Jadad score [22]: | 3 | 3 | 3 | 2 |
| Outcomes | Bodini [26] | Cox [24,25] | Halmos [23] | Pedersen [27,28,29] | ||
|---|---|---|---|---|---|---|
| Disease activity | CD | HBi | ↓ | NS | NS | |
| UC | PMS | NS | NS | |||
| SCCAI | ↓ | |||||
| IBD control score [38] | ↑ | |||||
| Stool analyses | Fecal Frequency | ↓ | NS | |||
| % with normal Consistency (BSFS) | NS | |||||
| Fecal weight | NS | |||||
| Fecal pH | NS | NS | ||||
| FWC | NS | |||||
| SCFA concentration | ↓ | NS | ||||
| Gut transit time | NS | |||||
| fCAL | ↓ | NS | NS | NS | ||
| Gene count | NS | |||||
| whole-gut transit time | NS | |||||
| Quality of life | IBD-Q | ↑ | ↑ | |||
| IBS-QoL | NS | |||||
| SIBDQ | ↑ | |||||
| SF36 | NS | |||||
| Inflammation markers | CRP | NS | NS | NS | ||
| Immunity | CD4 T-cells (n/%) | NS/NS | ||||
| CD8 T-cell s (n/%) | NS/NS | |||||
| α4β7 positive Vδ2 T-cells (n) | ↓ | |||||
| FGS | severity of GI symptoms (100 mm VAS) | ↓ | ||||
| Bloating (100 mm VAS) | ↓ | |||||
| Abdominal pain (100 mm VAS) | ↓ | |||||
| Wind (100 mm VAS) | ↓ | |||||
| Adequate relief (%) (GSQ) | ↑ | |||||
| GSRS | Flatulence | ↓ | ||||
| IBS-SSS | Total score | NS | ↓ | |||
| Pain duration | NS | ↓ | ||||
| Bloating | ↓ | NS | ||||
| Stool frequency & consistency | ↓ | ↓ | ||||
| Dietary intake | Energy (kcal) | ↓ | NS † | |||
| Starch (g) | NS | ↓ | ||||
| Protein (g) | ↓ | NS | ||||
| Fat (g) (total) | ↓ | NS | ||||
| Sugars (g) | ↓ | NS | ||||
| Calcium (mg) | ↓ | NR | ||||
| Iodine (µg) | ↓ | NR | ||||
| Phosphorous (mg) | ↓ | NR | ||||
| Fiber (g) | NS | NS * | ||||
| Bacteria | Cox [24,25] * | Halmos [23] † | |
|---|---|---|---|
| Relative (% Total) | Absolute (Copies of 16S rRNA Gene/g) | Relative (% Total) | |
| Total bacteria | NS | NS | NS |
| α-diversity | NS | ||
| β-diversity | NS | ||
| Phyla distribution | NS | ||
| Faecalibacterium prausnitzii | ↑ | NS | NS |
| Roseburia | NS | NS | |
| Lactobacilli sp. | NS | NS | |
| Bifidobacteria sp. | NS | NS | NS |
| Bifidobacterium adolescentis | ↓ | ||
| Bifidobacterium longum | ↓ | ||
| Bifidobacterium animalis | NS | ||
| Bifidobacterium bifidum | NS | ||
| Bifidobacterium breve | NS | ||
| Bifidobacterium dentium | ↓ | ||
| Bifidobacterium pseudocatenulatum | NS | ||
| Akkermansia muciniphila | ↓ | ↓ | |
| Ruminococcus gnavus | NS | NS | |
| Ruminococcus torques | NS | ↑ | |
| Clostridiumcluster IV | NS | NS | |
| Clostridium cluster XIVa | ↓ | ↓ | |
| Clinical Trial Identifier | Collaborators | Design | Intervention Duration | Sample | Intervention(s), Comparator(s) | Study Duration | Primary Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|
| NCT041 43633 * | (1) Hospital General de México | Parallel, single-blind (patients) RCT | 10 weeks | (1) Patients with IBS; (2) Patients with UC; (3) Healthy participants (N = 105 normal weight or overweight adults) | (1) LFD (55% CHO, 25% fat, 20% protein) in five meals daily (2) Standard diet (55% CHO, 25% fat, 20% protein) in five meals daily. Cruciferous vegetables, fruits, and condiments were eliminated and maintenance of a normal FODMAP content was sought | February 2018 to August 2020 | Nutritional status (serum TC, TG, Ca, Alb, Fe, Hb, Ht, vitamins B12 and D, and Cr levels) | (1) WHOQOL-BREF; (2) FFQ; (3) Body composition (BF, LBM) and anthropometry (arm, waist, and hips perimeters); (4) Gut microbiota (stool sample PCR); (5) Blood chemistry (Glu, Cr, HDL, LDL, TC); (6) IBS-SSS; (7) Symptoms severity |
| NCT024 69220 * | (1) North Denmark Hospital (2) Vendsyssel Hospital | Parallel RCT with quadruple masking | 8 weeks | Patients with UC and IBS (N = 45 adults) | (1) LFD and low-FODMAP ONS; (2) LFD and high-FODMAP ONS; | June 2015 to December 2020 | IBS-SSS | (1) SF36 (2) Pain (VAS) |
| NCT036 44602 † | (1) Shariati Hospital (2) Tehran University of Medical Sciences | Parallel, open-label RCT | 4 weeks | Patients with moderate UC (N = 32 adults) | (1) LFD (55% CHO, 25% fat, 20% protein) in six meals daily; (2) Standard UC care including PA advice, intake of low-fat dairy and meat, intake of vegetable oils and reduced refined sugars | July 2018 to April 2023 | (1) Gut microbiota (via PCR) | (1) Inflammation (NOD, via ELISA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grammatikopoulou, M.G.; Goulis, D.G.; Gkiouras, K.; Nigdelis, M.P.; Papageorgiou, S.T.; Papamitsou, T.; Forbes, A.; Bogdanos, D.P. Low FODMAP Diet for Functional Gastrointestinal Symptoms in Quiescent Inflammatory Bowel Disease: A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 3648. https://doi.org/10.3390/nu12123648
Grammatikopoulou MG, Goulis DG, Gkiouras K, Nigdelis MP, Papageorgiou ST, Papamitsou T, Forbes A, Bogdanos DP. Low FODMAP Diet for Functional Gastrointestinal Symptoms in Quiescent Inflammatory Bowel Disease: A Systematic Review of Randomized Controlled Trials. Nutrients. 2020; 12(12):3648. https://doi.org/10.3390/nu12123648
Chicago/Turabian StyleGrammatikopoulou, Maria G., Dimitrios G. Goulis, Konstantinos Gkiouras, Meletios P. Nigdelis, Stefanos T. Papageorgiou, Theodora Papamitsou, Alastair Forbes, and Dimitrios P. Bogdanos. 2020. "Low FODMAP Diet for Functional Gastrointestinal Symptoms in Quiescent Inflammatory Bowel Disease: A Systematic Review of Randomized Controlled Trials" Nutrients 12, no. 12: 3648. https://doi.org/10.3390/nu12123648
APA StyleGrammatikopoulou, M. G., Goulis, D. G., Gkiouras, K., Nigdelis, M. P., Papageorgiou, S. T., Papamitsou, T., Forbes, A., & Bogdanos, D. P. (2020). Low FODMAP Diet for Functional Gastrointestinal Symptoms in Quiescent Inflammatory Bowel Disease: A Systematic Review of Randomized Controlled Trials. Nutrients, 12(12), 3648. https://doi.org/10.3390/nu12123648










