Verbal Memory Performance in Depressed Children and Adolescents: Associations with EPA but Not DHA and Depression Severity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.2.1. Sociodemographic Variables
2.2.2. Independent Variables
Severity of Depression (CDRS-R)
EPA and DHA Statuses
2.2.3. Outcome Variables
Cognitive Tests—Memory
- Verbal Memory: VLMTWe used a validated German version (Verbaler Lern- und Merkfähigkeitstest (VLMT) [88]) of the Auditory Verbal Learning Test (AVLT) [89], in which a list of 15 semantically independent words is presented auditorily to an individual and he or she is asked to remember and reproduce as many words as possible. This process is repeated five times. Then, a second list of 15 words (= interference list (I)) is presented and the individual is asked to remember and reproduce as many words as possible from the second list. In the next step, the individual is asked to reproduce the words from the first list. After 20–30 min, he or she is once again asked to reproduce the first list of words. In the last step, the individual is asked to recognize the words from a list of 50 semantically or phonetically related and unrelated words. The test measures declarative verbal memory capacity. Short-term verbal memory is characterized by the number of words correctly reproduced by the individual in each of the five rounds (T1, T2, T3, T4, and T5). The long-term memory parameters are T7, which is the number of words from the first list recalled after 20–30 min, and T5–T7, which is the difference between the number of words recalled at T5 and T7. The interference score is I, which represents the number of correctly reproduced words from the interference list, T6, which is the number of words from the first list recalled after interference, and T5–6, which is the difference between the number of words reproduced from the first list before and after interference. Lastly, recognition (W) is the number of words correctly identified as belonging to the first list, and W–F is the correctly identified words minus the words wrongly attributed to the first list.
- Numeric Memory: WISC-IV Digit SpanThe digit span subtest from the Wechsler Intelligence Scale for Children—Fourth Edition (WISC-IV) [90] consists of two parts, namely, forward and backward. In the first part, the individual has to reproduce sequences of digits of increasing length. In the second part, the individual is instructed to repeat a sequence of digits in reverse order. The test measures numeric short-term memory and working memory.
2.2.4. Control Variables
IQ: Reynolds Intellectual Assessment Scales and Screening (RIAS)
C-Reactive Protein (CRP)
Body Mass Index (BMI)
2.3. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Main Analysis—EPA Status and Depression Severity in Relation to Memory
3.3. Main Analysis—DHA Status and Depression Severity in Relation to Memory
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Kessler, R.C.; Bromet, E.J. The Epidemiology of Depression Across Cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Sampson, N.A.; Berglund, P.; Gruber, M.J.; Al-Hamzawi, A.; Andrade, L.; Bunting, B.; Demyttenaere, K.; Florescu, S.; De Girolamo, G.; et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol. Psychiatr. Sci. 2015, 24, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Amminger, G.P.; Aguilar-Gaxiola, S.; Alonso, J.; Lee, S.; Ustun, T.B. Age of onset of mental disorders: A review of recent literature. Curr. Opin. Psychiatry 2007, 20, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Zisook, S.; Lesser, I.; Stewart, J.W.; Wisniewski, S.R.; Balasubramani, G.; Fava, M.; Gilmer, W.S.; Dresselhaus, T.R.; Thase, M.E.; Nierenberg, A.A.; et al. Effect of Age at Onset on the Course of Major Depressive Disorder. Am. J. Psychiatry 2007, 164, 1539–1546. [Google Scholar] [CrossRef]
- Costello, E.J.; Mustillo, S.; Keeler, G.; Angold, A. Prevalence of Psychiatric Disorders in Childhood and Adolescence. In Mental Health Services: A Public Health Perspective; Oxford University Press: Oxford, UK, 2004; pp. 111–128. [Google Scholar]
- Costello, E.J.; Egger, H.; Angold, A. 10-Year Research Update Review: The Epidemiology of Child and Adolescent Psychiatric Disorders: I. Methods and Public Health Burden. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 972–986. [Google Scholar] [CrossRef]
- Costello, E.J.; Erkanli, A.; Angold, A. Is there an epidemic of child or adolescent depression? J. Child Psychol. Psychiatry 2006, 47, 1263–1271. [Google Scholar] [CrossRef]
- Avenevoli, S.; Swendsen, J.; Shelli, A.; Burstein, M.; Merikangas, K.R. Major Depression in the National Comorbidity Survey–Adolescent Supplement: Prevalence, Correlates, and Treatment. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 37–44.e2. [Google Scholar] [CrossRef]
- World Health Organization. Depression and Other Common Mental Disorders—Global Health Estimates; Licence: CC BY-NC-SA 3.0; IGO: Geneva, Switzerland, 2017; pp. 1–24. [Google Scholar]
- Erskine, H.E.; Moffitt, T.E.; Copeland, W.E.; Costello, E.J.; Ferrari, A.J.; Patton, G.; Degenhardt, L.; Vos, T.; Whiteford, H.A.; Scott, J.G. A heavy burden on young minds: The global burden of mental and substance use disorders in children and youth. Psychol. Med. 2015, 45, 1551–1563. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; ISBN 97-8144-731-2-628. [Google Scholar]
- Knight, M.J.; Lyrtzis, E.; Baune, B.T. The association of cognitive deficits with mental and physical Quality of Life in Major Depressive Disorder. Compr. Psychiatry 2020, 97, 152147. [Google Scholar] [CrossRef]
- Cambridge, O.R.; Knight, M.J.; Mills, N.; Baune, B.T. The clinical relationship between cognitive impairment and psychosocial functioning in major depressive disorder: A systematic review. Psychiatry Res. 2018, 269, 157–171. [Google Scholar] [CrossRef]
- Hammar, Å. Cognitive functioning in major depression—A summary. Front. Hum. Neurosci. 2009, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Bruder, G.E.; Alvarenga, J.E.; Alschuler, D.M.; Abraham, K.; Keilp, J.G.; Hellerstein, D.J.; Stewart, J.W.; McGrath, P.J. Neurocognitive predictors of antidepressant clinical response. J. Affect. Disord. 2014, 166, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Etkin, A.; Patenaude, B.; Song, Y.J.C.; Usherwood, T.; Rekshan, W.; Schatzberg, A.F.; Rush, A.J.; Williams, L.M. A Cognitive–Emotional Biomarker for Predicting Remission with Antidepressant Medications: A Report from the iSPOT-D Trial. Neuropsychopharmacology 2014, 40, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.M. Adolescent depression: Diagnosis, treatment, and educational attainment. Health Econ. 2008, 17, 1215–1235. [Google Scholar] [CrossRef]
- Morey-Nase, C.; Phillips, L.J.; Bryce, S.; Hetrick, S.; Wright, A.L.; Caruana, E.; Allott, K. Subjective experiences of neurocognitive functioning in young people with major depression. BMC Psychiatry 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Ahern, E.; Semkovska, M. Cognitive functioning in the first-episode of major depressive disorder: A systematic review and meta-analysis. Neuropsychology 2017, 31, 52–72. [Google Scholar] [CrossRef]
- Porter, R.; Robinson, L.J.; Malhi, G.S.; Gallagher, P. The neurocognitive profile of mood disorders—A review of the evidence and methodological issues. Bipolar Disord. 2015, 17, 21–40. [Google Scholar] [CrossRef]
- Wagner, S.; Doering, B.; Helmreich, I.; Lieb, K.; Tadić, A. A meta-analysis of executive dysfunctions in unipolar major depressive disorder without psychotic symptoms and their changes during antidepressant treatment. Acta Psychiatr. Scand. 2012, 125, 281–292. [Google Scholar] [CrossRef]
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol. Med. 2014, 44, 2029–2040. [Google Scholar] [CrossRef]
- Lim, J.; Oh, I.K.; Han, C.; Huh, Y.J.; Jung, I.-K.; Patkar, A.A.; Steffens, D.C.; Jang, B.-H. Sensitivity of cognitive tests in four cognitive domains in discriminating MDD patients from healthy controls: A meta-analysis. Int. Psychogeriatr. 2013, 25, 1543–1557. [Google Scholar] [CrossRef]
- Snyder, H.R. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychol. Bull. 2013, 139, 81–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.; Hermens, D.F.; Porter, M.A.; Redoblado-Hodge, M.A. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J. Affect. Disord. 2012, 140, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, S.G.; Knorr, U.; Kessing, L.V. Cognitive impairment in the remitted state of unipolar depressive disorder: A systematic review. J. Affect. Disord. 2011, 134, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Biringer, E.; Mykletun, A.; Sundet, K.; Kroken, R.; Stordal, K.I.; Lund, A. A longitudinal analysis of neurocognitive function in unipolar depression. J. Clin. Exp. Neuropsychol. 2007, 29, 879–891. [Google Scholar] [CrossRef]
- Semkovska, M.; Quinlivan, L.; O’Grady, T.; Johnson, R.; Collins, A.; O’Connor, J.; Knittle, H.; Ahern, E.; Gload, T. Cognitive function following a major depressive episode: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 851–861. [Google Scholar] [CrossRef]
- Wagner, S.; Müller, C.; Helmreich, I.; Huss, M.; Tadić, A. A meta-analysis of cognitive functions in children and adolescents with major depressive disorder. Eur. Child Adolesc. Psychiatry 2015, 24, 5–19. [Google Scholar] [CrossRef]
- Brooks, B.L.; Iverson, G.L.; Sherman, E.M.S.; Roberge, M.-C. Identifying Cognitive Problems in Children and Adolescents with Depression Using Computerized Neuropsychological Testing. Appl. Neuropsychol. 2010, 17, 37–43. [Google Scholar] [CrossRef]
- Goodall, J.; Fisher, C.; Hetrick, S.; Phillips, L.; Parrish, E.M.; Allott, K. Neurocognitive Functioning in Depressed Young People: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2018, 28, 216–231. [Google Scholar] [CrossRef]
- Vilgis, V.; Silk, T.; Vance, A. Executive function and attention in children and adolescents with depressive disorders: A systematic review. Eur. Child Adolesc. Psychiatry 2015, 24, 365–384. [Google Scholar] [CrossRef]
- Günther, T.; Holtkamp, K.; Jolles, J.; Herpertz-Dahlmann, B.; Konrad, K. Verbal memory and aspects of attentional control in children and adolescents with anxiety disorders or depressive disorders. J. Affect. Disord. 2004, 82, 265–269. [Google Scholar] [CrossRef]
- McDermott, L.M.; Ebmeier, K. A meta-analysis of depression severity and cognitive function. J. Affect. Disord. 2009, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, F.T.; Brent, D.; Clark, L.; Tavitian, L.; McHugh, R.M.; Sahakian, B.J.; Phillips, M.L. Neurocognitive impairment in adolescent major depressive disorder: State vs. trait illness markers. J. Affect. Disord. 2011, 133, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, J.D.; Kakar, R.; McIntyre, R.S. The Cognitive Effects of Antidepressants in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int. J. Neuropsychopharmacol. 2016, 19, pyv082. [Google Scholar] [CrossRef] [PubMed]
- Bennabi, D.; Haffen, E.; Van Waes, V. Vortioxetine for Cognitive Enhancement in Major Depression: From Animal Models to Clinical Research. Front. Psychiatry 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Biringer, E.; Rongve, A.; Lund, A. A Review of Modern Antidepressants Effects on Neurocognitive Function. Curr. Psychiatry Rev. 2009, 5, 164–174. [Google Scholar] [CrossRef]
- Prado, C.E.; Watt, S.; Crowe, S.F. A meta-analysis of the effects of antidepressants on cognitive functioning in depressed and non-depressed samples. Neuropsychol. Rev. 2018, 28, 32–72. [Google Scholar] [CrossRef]
- Skandali, N.; Rowe, J.B.; Voon, V.; Deakin, J.B.; Cardinal, R.N.; Cormack, F.; Passamonti, L.; Bevan-Jones, W.R.; Regenthal, R.; Chamberlain, S.R.; et al. Dissociable effects of acute SSRI (escitalopram) on executive, learning and emotional functions in healthy humans. Neuropsychopharmacology 2018, 43, 2645–2651. [Google Scholar] [CrossRef]
- Bortolato, B.; Miskowiak, K.W.; Köhler, C.A.; Maes, M.; Fernandes, B.S.; Berk, M.; Carvalho, A.F. Cognitive remission: A novel objective for the treatment of major depression? BMC Med. 2016, 14, 1–18. [Google Scholar] [CrossRef]
- Zuckerman, H.; Pan, Z.; Park, C.; Brietzke, E.; Musial, N.; Shariq, A.S.; Iacobucci, M.; Yim, S.J.; Lui, L.M.W.; Rong, C.; et al. Recognition and Treatment of Cognitive Dysfunction in Major Depressive Disorder. Front. Psychiatry 2018, 9, 655. [Google Scholar] [CrossRef]
- Shilyansky, C.; Williams, L.M.; Gyurak, A.; Harris, A.; Usherwood, T.; Etkin, A. Effect of antidepressant treatment on cognitive impairments associated with depression: A randomised longitudinal study. Lancet Psychiatry 2016, 3, 425–435. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary Aspects of Diet: The Omega-6/Omega-3 Ratio and the Brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, X.; Zhang, D. Fish consumption and risk of depression: A meta-analysis. J. Epidemiol. Community Health 2015, 70, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-Y.; Huang, S.-Y.; Su, K.-P. A Meta-Analytic Review of Polyunsaturated Fatty Acid Compositions in Patients with Depression. Biol. Psychiatry 2010, 68, 140–147. [Google Scholar] [CrossRef]
- Edwards, R.; Peet, M.; Shay, J.; Horrobin, D. Depletion of docosahexaenoic acid in red blood cell membranes of depressive patients. Biochem. Soc. Trans. 1998, 26, S142. [Google Scholar] [CrossRef]
- Adams, P.B.; Lawson, S.; Sanigorski, A.; Sinclair, A.J. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids 1996, 31, S157–S161. [Google Scholar] [CrossRef]
- Sublette, M.E.; Ellis, S.P.; Geant, A.L.; Mann, J.J. Meta-Analysis of the Effects of Eicosapentaenoic Acid (EPA) in Clinical Trials in Depression. J. Clin. Psychiatry 2011, 72, 1577–1584. [Google Scholar] [CrossRef]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of Omega-3 Fatty Acids in the Treatment of Depressive Disorders: A Comprehensive Meta-Analysis of Randomized Clinical Trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef]
- Hallahan, B.; Ryan, T.; Hibbeln, J.R.; Murray, I.T.; Glynn, S.; Ramsden, C.E.; SanGiovanni, J.P.; Davis, J.M. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br. J. Psychiatry 2016, 209, 192–201. [Google Scholar] [CrossRef]
- Mocking, R.J.T.; Harmsen, I.; Assies, J.; Koeter, M.W.J.; Ruhé, H.G.; Schene, A.H. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramaniapillai, M.; Fan, B.; Lu, C.; Mclntyer, R.S. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Appleton, K.M.; Rogers, P.J.; Ness, A.R. Updated systematic review and meta-analysis of the effects of n−3 long-chain polyunsaturated fatty acids on depressed mood. Am. J. Clin. Nutr. 2010, 91, 757–770. [Google Scholar] [CrossRef]
- Bloch, M.H.; Hannestad, J. Omega-3 fatty acids for the treatment of depression: Systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 1272–1282. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Kuang, L.; Meng, H.; Zhou, X. Omega-3 fatty acids for the treatment of depressive disorders in children and adolescents: A meta-analysis of randomized placebo-controlled trials. Child Adolesc. Psychiatry Ment. Health 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Rapaport, M.H.; Nierenberg, A.A.; Schettler, P.J.; Kinkead, B.L.; Cardoos, A.; Walker, R.S.W.; Mischoulon, D. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: A proof-of-concept study. Mol. Psychiatry 2016, 21, 71–79. [Google Scholar] [CrossRef]
- Young, J.J.; Bruno, D.; Pomara, N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 2014, 169, 15–20. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Koletzko, B.; Lien, E.; Agostoni, C.; Böhles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J. Perinat. Med. 2008, 36, 5–14. [Google Scholar] [CrossRef]
- Baumgartner, J. Effects of Omega-3 Fatty Acid Supplementation on Cognition in Children. In Handbook of Lipids in Human Function; Elsevier: Amsterdam, The Netherlands, 2016; pp. 331–375. [Google Scholar]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef]
- Lassek, W.D.; Gaulin, S.J. Sex Differences in the Relationship of Dietary Fatty Acids to Cognitive Measures in American Children. Front. Evol. Neurosci. 2011, 3, 3. [Google Scholar] [CrossRef]
- Darcey, V.L.; McQuaid, G.A.; Fishbein, D.H.; VanMeter, J.W. Dietary Long-Chain Omega-3 Fatty Acids Are Related to Impulse Control and Anterior Cingulate Function in Adolescents. Front. Neurosci. 2019, 12, 1012. [Google Scholar] [CrossRef]
- Montgomery, P.; Burton, J.R.; Sewell, R.P.; Spreckelsen, T.F.; Richardson, A.J. Low Blood Long Chain Omega-3 Fatty Acids in UK Children Are Associated with Poor Cognitive Performance and Behavior: A Cross-Sectional Analysis from the DOLAB Study. PLoS ONE 2013, 8, e66697. [Google Scholar] [CrossRef]
- Van Der Wurff, I.S.M.; Von Schacky, C.; Berge, K.; Zeegers, M.P.A.; Kirschner, P.A.; De Groot, R.H.M. Association between Blood Omega-3 Index and Cognition in Typically Developing Dutch Adolescents. Nutrients 2016, 8, 13. [Google Scholar] [CrossRef]
- Emery, S.; Häberling, I.; Berger, G.; Walitza, S.; Schmeck, K.; Albert, T.; Baumgartner, N.; Strumberger, M.; Albermann, M.; Drechsler, R. Omega-3 and its domain-specific effects on cognitive test performance in youths: A meta-analysis. Neurosci. Biobehav. Rev. 2020, 112, 420–436. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Q.; Chu, J.; Zeng, W.; Yang, M.; Zhu, S. Effect of n−3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1422–1436. [Google Scholar] [CrossRef]
- Chang, J.P.-C.; Su, K.-P.; Mondelli, V.; Pariante, C.M. Omega-3 Polyunsaturated Fatty Acids in Youths with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Clinical Trials and Biological Studies. Neuropsychopharmacology 2018, 43, 534–545. [Google Scholar] [CrossRef]
- Bloch, M.H.; Qawasmi, A. Omega-3 Fatty Acid Supplementation for the Treatment of Children with Attention-Deficit/Hyperactivity Disorder Symptomatology: Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 991–1000. [Google Scholar] [CrossRef]
- Cooper, R.E.; Tye, C.; Kuntsi, J.; Vassos, E.; Asherson, P. Omega-3 polyunsaturated fatty acid supplementation and cognition: A systematic review and meta-analysis. J. Psychopharmacol. 2015, 29, 753–763. [Google Scholar] [CrossRef]
- Naderali, E.K.; Naderali, M.-M.; Abubakari, A.-R. Omega-3 fatty acid supplementation and cognitive function: Are smaller dosages more beneficial? Int. J. Gen. Med. 2014, 7, 463–473. [Google Scholar] [CrossRef]
- Mazereeuw, G.; Lanctôt, K.L.; Chau, S.A.; Swardfager, W.; Herrmann, N. Effects of omega-3 fatty acids on cognitive performance: A meta-analysis. Neurobiol. Aging 2012, 33, 1482.e17–1482.e29. [Google Scholar] [CrossRef]
- Karr, J.E.; Alexander, J.E.; Winningham, R.G. Omega-3 polyunsaturated fatty acids and cognition throughout the lifespan: A review. Nutr. Neurosci. 2011, 14, 216–225. [Google Scholar] [CrossRef]
- Chang, J.P.-C.; Su, K.-P.; Mondelli, V.; Satyanarayanan, S.K.; Yang, H.-T.; Chiang, Y.-J.; Chen, H.-T.; Pariante, C.M. High-dose eicosapentaenoic acid (EPA) improves attention and vigilance in children and adolescents with attention deficit hyperactivity disorder (ADHD) and low endogenous EPA levels. Transl. Psychiatry 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Knochel, C.; Voss, M.; Gruter, F.; Alves, G.S.; Matura, S.; Sepanski, B.; Stablein, M.; Wenzler, S.; Prvulovic, D.; Carvalho, A.F.; et al. Omega 3 Fatty Acids: Novel Neurotherapeutic Targets for Cognitive Dysfunction in Mood Disorders and Schizophrenia? Curr. Neuropharmacol. 2015, 13, 663–680. [Google Scholar] [CrossRef]
- Rogers, P.J.; Appleton, K.M.; Kessler, D.; Peters, T.J.; Gunnell, D.; Hayward, R.C.; Heatherley, S.V.; Christian, L.M.; McNaughton, S.A.; Ness, A.R. No effect ofn-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: A randomised controlled trial. Br. J. Nutr. 2008, 99, 421–431. [Google Scholar] [CrossRef]
- Vesco, A.T.; Young, A.S.; Arnold, L.E.; Fristad, M.A. Omega-3 supplementation associated with improved parent-rated executive function in youth with mood disorders: Secondary analyses of the omega 3 and therapy (OATS) trials. J. Child Psychol. Psychiatry 2017, 59, 628–636. [Google Scholar] [CrossRef]
- Häberling, I.; Berger, G.; Schmeck, K.; Held, U.; Walitza, S. Omega-3 Fatty Acids as a Treatment for Pediatric Depression. A Phase III, 36 Weeks, Multi-Center, Double-Blind, Placebo-Controlled Randomized Superiority Study. Front. Psychiatry 2019, 10. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision (DSM-IV-TR); American Psychiatric Association: Arlington, VA, USA, 2000; ISBN 0-89042-334-2. [Google Scholar]
- Poznanski, E.; Mokros, H. Children’s Depression Rating Scale–Revised; WPS: Los Angeles, CA, USA, 1996; Volume 12. [Google Scholar]
- Guo, Y.; Nilsson, M.E.; Heiligenstein, J.; Wilson, M.G.; Emslie, G. An Exploratory Factor Analysis of the Children’s Depression Rating Scale—Revised. J. Child Adolesc. Psychopharmacol. 2006, 16, 482–491. [Google Scholar] [CrossRef]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef]
- Mayes, T.L.; Bernstein, I.H.; Haley, C.L.; Kennard, B.D.; Emslie, G.J. Psychometric Properties of the Children’s Depression Rating Scale–Revised in Adolescents. J. Child Adolesc. Psychopharmacol. 2010, 20, 513–516. [Google Scholar] [CrossRef]
- Harris, W.S.; Von Schacky, C.; Park, Y. Standardizing Methods for Assessing Omega-3 Fatty Acid Biostatus. In The Omega-3 Fatty Acid Deficiency Syndrome: Opportunities for Disease Prevention; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 385–398. [Google Scholar]
- Helmstaedter, C.; Durwen, H.F. Vlmt: Verbaler Lern- Und Merkfahigkeitstest. Ein Praktikables und Differenziertes Instrumentarium zur Prufung der Verbalen Gedachtnisleistungen. Schweiz. Arch. Neurol. Psychiatry 1990, 141, 21–30. [Google Scholar]
- Schmidt, M. Auditory and Verbal Learning Test. A handbook; Western Psychological Association: Los Angeles, CA, USA, 1996. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; (WISC-IV); The Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Hagmann-Von, A.P.; Grob, A. Reynolds Intellectual Assessment Scales and Screening. Deutschsprachige Adaptation der Reynolds Intellectual Assessment Scales (RIAS) & des Reynolds Intellectual Screening Test (RIST) von Cecil R. Reynolds und Randy W. Kamphaus; Huber: Unterschleißheim, Germany, 2014. [Google Scholar]
- Gygi, J.T.; Arx, P.H.-V.; Schweizer, F.; Grob, A. The Predictive Validity of Four Intelligence Tests for School Grades: A Small Sample Longitudinal Study. Front. Psychol. 2017, 8, 8. [Google Scholar] [CrossRef]
- Krist, H.; Kunter, M.; Nückles, M.; Pinquart, M.; Seidel, T. Testbesprechung. Z. Entwickl. Padag. Psychol. 2016, 48, 50–55. [Google Scholar]
- Von Schacky, C. Verwirrung um die Wirkung von Omega-3-Fettsäuren. Internist (Berl.) 2019, 60, 1319–1327. [Google Scholar] [CrossRef]
- Lumley, T.; Diehr, P.; Emerson, S.; Chen, L. The Importance of the Normality Assumption in Large Public Health Data Sets. Annu. Rev. Public Health 2002, 23, 151–169. [Google Scholar] [CrossRef]
- Ateş, C.; Kaymaz, O.; Kale, H.E.; Tekindal, M.A. Comparison of Test Statistics of Nonnormal and Unbalanced Samples for Multivariate Analysis of Variance in terms of Type-I Error Rates. Comput. Math. Methods Med. 2019, 2019, 2173638. [Google Scholar] [CrossRef]
- Howell, D.C. Statistical Methods for Psychology, 7th ed.; Wadsworth Cengage Learning: Belmont, CA, USA, 2012. [Google Scholar]
- Bountziouka, V.; Polychronopoulos, E.; Zeimbekis, A.; Papavenetiou, E.; Ladoukaki, E.; Papairakleous, N.; Gotsis, E.; Metallinos, G.; Lionis, C.; Panagiotakos, D. Long-Term Fish Intake is Associated with Less Severe Depressive Symptoms Among Elderly Men and Women. J. Aging Health 2009, 21, 864–880. [Google Scholar] [CrossRef]
- McClintock, S.M.; Husain, M.M.; Greer, T.L.; Cullum, C.M. Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology 2010, 24, 9–34. [Google Scholar] [CrossRef]
- Bauer, I.E.; Hughes, M.; Rowsell, R.; Cockerell, R.; Pipingas, A.; Crewther, S.; Crewther, D. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Hum. Psychopharmacol. Clin. Exp. 2014, 29, 133–144. [Google Scholar] [CrossRef]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef]
| Sample Characteristics | Variable Specifics | n | M (SD) | Min | Max | Moderate Depression M (SD) | Severe Depression M (SD) | t/χ2/U | p |
|---|---|---|---|---|---|---|---|---|---|
| Sociodemographic information | Age | 107 | 15.50 (1.89) | 8.67 | 18.00 | 15.25 (2.09) | 15.85 (1.51) | −1.630 | 0.106 |
| Sex: %female | 107 | 67% | 57% | 82% | 7.166 | 0.007 ** | |||
| Physiological parameters | BMI | 99 | 22.35 (4.87) | 14.00 | 39.80 | 21.86 (4.35) | 23.06 (5.52) | 1285.0 | 0.454 |
| CRP | 105 | 0.79 (1.26) | 0 | 6.6 | 0.65 (0.81) | 0.99 (1.69) | 1220.0 | 0.410 | |
| IQ | |||||||||
| RIAS | VIX | 100 | 101.73 (9.93) | 79 | 129 | 102.58 (10.18) | 100.51(9.61) | 1.019 | 0.102 |
| NIX | 100 | 106.33 (8.93) | 70 | 123 | 107.71 (8.61) | 104.34 (9.12) | 1.879 | 0.310 | |
| GIX | 100 | 104.52 (9.35) | 76 | 128 | 105.78 (9.32) | 102.68 (9.21) | 1.651 | 0.102 | |
| Depression severity | |||||||||
| CDRS-R | Total score | 107 | 57.73 (7.76) | 42 | 79 | 52.37 (4.18) | 65.41 (4.54) | −15.331 | <0.001 *** |
| Severe % a | 44 | 41.1% | - | - | - | - | |||
| Course of illness | |||||||||
| Mean duration of depression (months) | Months | 104 | 14.71 (11.69) | 1 | 84 | 13.45 (9.76) | 16.43 (13.84) | 1503.0 | 0.228 |
| Total number of episodes | 105 | 1.46 (0.95) | 1 | 8 | 1.44 (1.10) | 1.48 (0.70) | 1490.0 | 0.230 | |
| Recurrent depression | Yes | 104 | 31% | 26% | 38% | 1.775 | 0.183 | ||
| Use of antidepressant medication | Yes | 102 | 37% | 35% | 40% | 0.317 | 0.573 |
| Total | Moderate MDD (n = 63) | Severe MDD (n = 44) | t | p | |
|---|---|---|---|---|---|
| Fatty acid | M (SD) | M (SD) | M (SD) | ||
| EPA | 0.49 (0.15) | 0.47 (0.14) | 0.51 (0.16) | −1.515 | 0.133 |
| DHA | 3.63 (0.79) | 3.50 (0.71) | 3.82 (0.87) | −2.077 | 0.040 * |
| All n-6 | 34.37 (1.32) | 34.45 (1.26) | 34.32 (1.40) | 0.512 | 0.609 |
| All n-3 | 6.45 (1.0) | 6.26 (0.93) | 6.71 (1.06) | −2.318 | 0.022 * |
| AA/EPA | 35.10 (11.27) | 36.08 (11.21) | 33.74 (11.60) | 1.045 | 0.298 |
| MANCOVA EPA status (EPA) Depression Severity (S) Interaction Severity * EPA Status (I) Covariate: Gender (G) | ||||||||
|---|---|---|---|---|---|---|---|---|
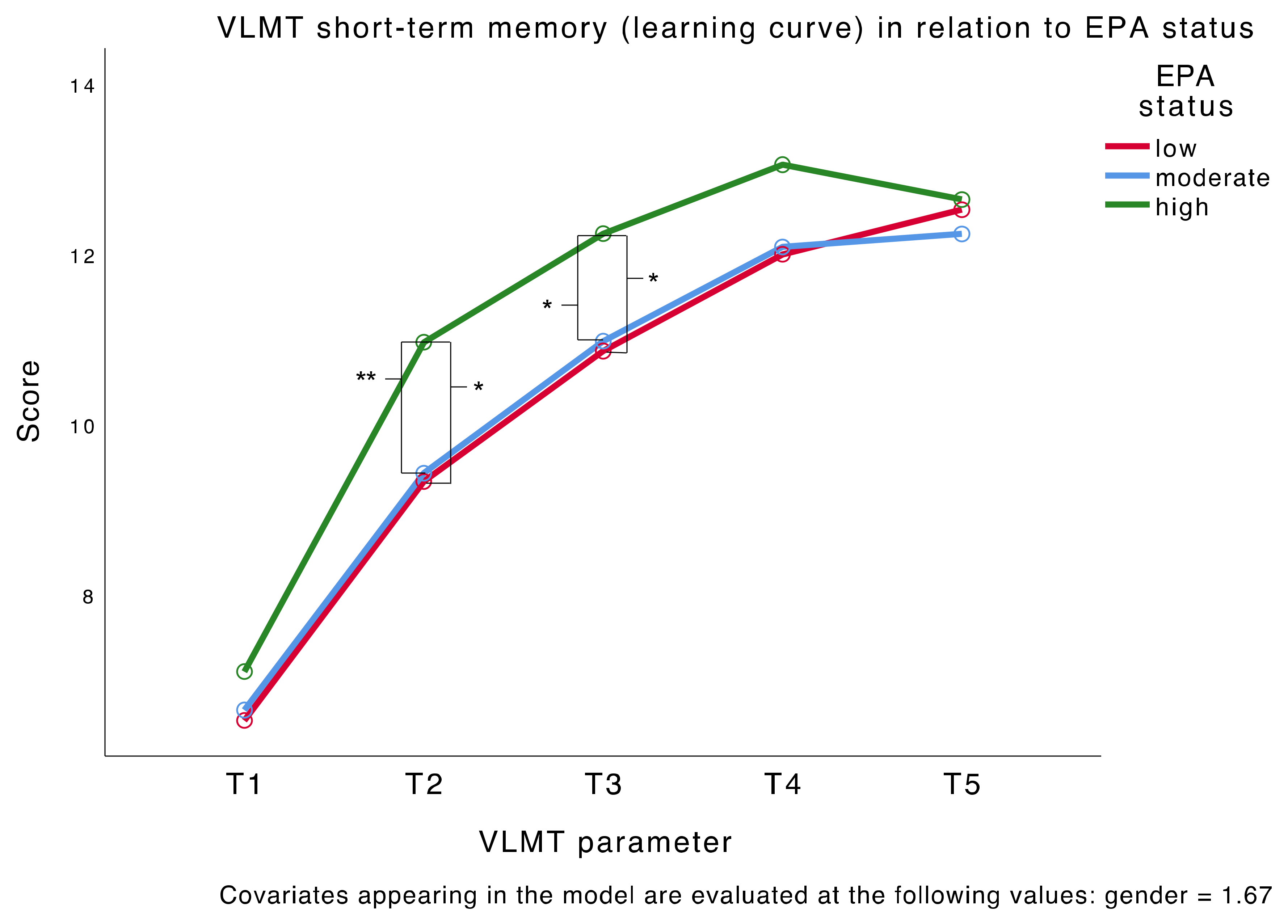
| VLMT short-term memory parameters | EPA: F(10,194) = 2.094, p = 0.027 *, ηp2 = 0.097 S: F(5,96) = 0.622, p = 0.684, ηp2 = 0.031 I: F(10,194) = 0.910, p = 0.525, ηp2 = 0.045 G: F(5,96) = 1.287, p = 0.276, ηp2 = 0.063 | |||||||
| Moderate depression N = 63 M (SD) | Severe depression N = 44 M (SD) | F p ηp2 | Pairwise | p | ||||
| EPA | S | I | G | |||||
| T1 score | ||||||||
| Low EPA | 6.52 (0.33) | 6.55 (0.53) | 1.044 | 0.816 | 0.415 | 0.6 | NS | NS |
| Moderate EPA | 7.00 (0.35) | 6.29 (0.46) | 0.356 | 9.369 | 0.661 | 0.44 | ||
| High EPA | 7.11 (0.40) | 7.06 (0.42) | 0.02 | 0.008 | 0.008 | 0.006 | ||
| T2 score | ||||||||
| Low EPA | 9.74 (0.52) | 8.91 (0.69) | 6.096 | 1.577 | 2.03 | 0 | EPA: h > m EPA: h > l | p = 0.009 ** p = 0.010 * |
| Moderate EPA | 10.14 (0.56) | 8.71 (0.46) | 0.003 * | 0.212 | 0.137 | 0.997 | ||
| High EPA | 10.67 (0.26) | 11.25 (0.44) | 0.109 | 0.016 | 0.039 | 0 | ||
| T3 score | ||||||||
| Low EPA | 11.43 (0.40) | 10.36 (0.64) | 4.825 | 1.674 | 1.366 | 2.47 | EPA: h > m EPA: h > l | p = 0.027 * p = 0.023 * |
| Moderate EPA | 11.27 (0.52) | 10.71 (0.45) | 0.010 ° | 0.199 | 0.26 | 0.119 | ||
| High EPA | 11.94 (0.38) | 12.50 (0.52) | 0.088 | 0.016 | 0.027 | 0.024 | ||
| T4 score | ||||||||
| Low EPA | 12.17 (0.38) | 10.91 (0.64) | 2.652 | 0.944 | 0.082 | 3.069 | NS | NS |
| Moderate EPA | 12.32 (0.50) | 11.88 (0.70) | 0.075 | 0.334 | 0.921 | 0.083 | ||
| High EPA | 13.00 (0.33) | 13.06 (0.42) | 0.05 | 0.009 | 0.002 | 0.03 | ||
| T5 score | ||||||||
| Low EPA | 12.57 (0.38) | 12.55 (0.51) | 0.41 | 0.103 | 0.059 | 2.101 | NS | NS |
| Moderate EPA | 12.32 (0.50) | 12.18 (0.58) | 0.665 | 0.749 | 0.942 | 0.15 | ||
| High EPA | 12.50 (0.41) | 12.75 (0.37) | 0.008 | 0.001 | 0.001 | 0.021 | ||
| VLMT interference parameters | EPA: F(6,198) = 1.426, p = 0.206, ηp2 = 0.041 S: F(3,98) = 1.056, p = 0.372, ηp2 = 0.031 I: F(6,198) = 0.102, p = 0.996, ηp2 = 0.003 G: F(3,98) = 0.869, p = 0.460, ηp2 = 0.026 | |||||||
| I | ||||||||
| Low EPA | 6.43 (0.40) | 6.18 (0.62) | 1.671 | 2.28 | 0.079 | 1.414 | NS | NS |
| Moderate EPA | 6.41 (0.44) | 5.71 (0.58) | 0.193 | 0.134 | 0.924 | 0.237 | ||
| High EPA | 7.33 (0.71) | 6.63 (0.52) | 0.032 | 0.022 | 0.002 | 0.014 | ||
| T6 | ||||||||
| Low EPA | 11.96 (0.59) | 11.55 (0.56) | 2.364 | 0.68 | 0.134 | 1.917 | NS | NS |
| Moderate EPA | 11.36 (0.56) | 10.94 (0.70) | 0.101 | 0.411 | 0.875 | 0.169 | ||
| High EPA | 12.28 (0.43) | 12.44 (0.48) | 0.045 | 0.007 | 0.003 | 0.019 | ||
| T5–6 | ||||||||
| Low EPA | 0.61 (0.43) | 1.00 (0.54) | 2.556 | 0.756 | 0.075 | 0.107 | NS | NS |
| Moderate EPA | 0.95 (0.24) | 1.24 (0.36) | 0.083 | 0.387 | 0.928 | 0.744 | ||
| High EPA | 0.22 (0.31) | 0.31 (0.37) | 0.049 | 0.008 | 0.001 | 0.001 | ||
| VLMT long-term memory parameters | EPA: F(4,200) = 0.604, p = 0.660, ηp2 = 0.012 S: F(2,99) = 0.055, p = 0.946, ηp2 = 0.001 I: F(4,200) = 0.093, p = 0.984, ηp2 = 0.002 G: F(2,99) = 1.123, p = 0.330, ηp2 = 0.022 | |||||||
| T7 | ||||||||
| Low EPA | 11.78 (0.54) | 11.82 (0.48) | 1.1 | 0.091 | 0.157 | 1.832 | NS | NS |
| Moderate EPA | 11.55 (0.67) | 11.18 (0.69) | 0.337 | 0.763 | 0.855 | 0.179 | ||
| High EPA | 12.00 (0.46) | 12.44 (0.58) | 0.022 | 0.001 | 0.003 | 0.001 | ||
| T5–7 | ||||||||
| Low EPA | 0.78 (0.36) | 0.73 (0.41) | 0.774 | 0.007 | 0.123 | 0.128 | NS | NS |
| Moderate EPA | 0.77 (0.44) | 1.00 (0.39) | 0.464 | 0.934 | 0.884 | 0.721 | ||
| High EPA | 0.50 (0.31) | 0.31 (0.44) | 0.015 | 0 | 0.002 | 0.001 | ||
| Moderate depression N = 62 M (SD) | Severe depression N = 44 M (SD) | |||||||
| Digits forward | ||||||||
| Low EPA | 8.68 (0.44) | 8.64 (0.41) | 0.654 | 0.018 | 0.019 | 0.043 | NS | NS |
| Moderate EPA | 8.45 (0.37) | 8.29 (0.49) | 0.522 | 0.894 | 0.981 | 0.837 | ||
| High EPA | 8.89 (0.40) | 8.88 (0.52) | 0.013 | 0 | 0 | 0 | ||
| Digits backward | ||||||||
| Low EPA | 8.09 (0.41) | 7.64 (0.49) | 0.556 | 1.016 | 0.188 | 0.151 | NS | NS |
| Moderate EPA | 8.41 (0.47) | 7.88 (0.36) | 0.575 | 0.316 | 0.829 | 0.699 | ||
| High EPA | 8.33 (0.43) | 8.31 (0.27) | 0.011 | 0.01 | 0.004 | 0.002 | ||
| MANCOVA DHA Status (DHA) Depression Severity (S) Interaction Severity * DHA Status (I) Covariates: Gender (G), GIX (IQ) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| VLMT short-term memory parameters | DHA: F(10,178) = 1.323, p = 0.221, ηp2 = 0.069 S: F(5,88) = 0.524, p = 0.758, ηp2 = 0.029 I: F(10,178) = 0.686, p = 0.736, ηp2 = 0.037 G: F(5,88) = 1.218, p = 0.308, ηp2 = 0.065 IQ: F(5,88) = 6.113, p < 0.001 ***, ηp2 = 0.258 | ||||||||
| Moderate depression N = 59 M (SD) | Severe depression N = 41 M (SD) | F p ηp2 | Pairwise | p | |||||
| DHA | S | I | G | IQ | |||||
| T1 score | |||||||||
| Low DHA | 6.65 (1.61) | 6.40 (2.07) | 1.57 | 0.057 | 0.115 | 0.073 | 7.401 | NS | NS |
| Moderate DHA | 7.25 (1.68) | 7.00 (1.41) | 0.214 | 0.812 | 0.892 | 0.788 | 0.008 * | ||
| High DHA | 6.94 (1.61) | 6.78 (1.90) | 0.033 | 0.001 | 0.002 | 0.001 | 0.074 | ||
| T2 score | |||||||||
| Low DHA | 9.87 (2.97) | 8.91 (0.69) | 1.733 | 0.923 | 0.323 | 0 | 14.652 | NS | NS |
| Moderate DHA | 10.85 (2.87) | 9.10 (2.13) | 0.182 | 0.339 | 0.725 | 0.982 | <0.001 * | ||
| High DHA | 10.25 (1.18) | 10.28 (2.63) | 0.036 | 0.01 | 0.007 | 0 | 0.137 | ||
| T3 score | |||||||||
| Low DHA | 11.09 (2.17) | 11.50 (2.72) | 1.096 | 0.184 | 0.641 | 3.039 | 15.105 | NS | NS |
| Moderate DHA | 11.70 (2.06) | 10.38 (2.06) | 0.338 | 0.669 | 0.529 | 0.085 | <0.001 * | ||
| High DHA | 11.75 (2.05) | 11.94 (1.86) | 0.023 | 0.002 | 0.014 | 0.032 | 0.141 | ||
| T4 score | |||||||||
| Low DHA | 11.96 (2.35) | 12.20 (2.20) | 1.769 | 0.1 | 0.913 | 2.062 | 31.252 | NS | NS |
| Moderate DHA | 13.15 (1.73) | 11.54 (3.05) | 0.176 | 0.752 | 0.405 | 0.154 | <0.001 * | ||
| High DHA | 12.63 (1.09) | 13.06 (1.51) | 0.037 | 0.001 | 0.019 | 0.022 | 0.254 | ||
| T5 score | |||||||||
| Low DHA | 12.09 (2.28) | 13.10 (1.60) | 0.038 | 0.366 | 1.816 | 0.881 | 19.51 | NS | NS |
| Moderate DHA | 13.05 (1.79) | 11.77 (1.88) | 0.963 | 0.547 | 0.169 | 0.35 | <0.001 * | ||
| High DHA | 12.44 (1.63) | 12.89 (1.64) | 0.001 | 0.004 | 0.038 | 0.009 | 0.175 | ||
| VLMT interference parameters | DHA: F(6,182) = 0.933, p = 0.472, ηp2 = 0.039 S: F(3,90) = 0.716, p = 0.545, ηp2 = 0.023 I: F(6,182) = 2.219, p = 0.052 °, ηp2 = 0.066 G: F(3,90) = 0.480, p = 0.697, ηp2 = 0.016 IQ: F(3,90) = 8.593, p = < 0.001 ***, ηp2 = 0.223 | ||||||||
| I | |||||||||
| Low DHA | 7.13 (2.70) | 5.90 (2.38) | 0.066 | 0.804 | 0.753 | 1.131 | 16.398 | NS | NS |
| Moderate DHA | 6.60 (1.76) | 5.92 (2.63) | 0.936 | 0.372 | 0.474 | 0.29 | <0.001 * | ||
| High DHA | 6.25 (2.46) | 6.39 (1.91) | 0.001 | 0.009 | 0.016 | 0.012 | 0.151 | ||
| T6 | |||||||||
| Low DHA | 11.43 (2.76) | 12.70 (2.36) | 1.405 | 0.006 | 4.337 | 0.445 | 12.435 | I: s/h > s/m I: m/m > s/m | p = 0.036 * p = 0.021 * |
| Moderate DHA | 12.45 (2.21) | 10.00 (2.35) | 0.251 | 0.936 | 0.016 * | 0.506 | 0.001 * | ||
| High DHA | 12.06 (1.95) | 12.61 (1.29) | 0.03 | 0 | 0.086 | 0.005 | 0.119 | ||
| T5–6 | |||||||||
| Low DHA | 0.65 (1.56) | 0.40 (1.58) | 2.621 | 0.613 | 1.959 | 0.017 | 0.006 | NS | NS |
| Moderate DHA | 0.60 (1.60) | 1.77 (1.64) | 0.078 | 0.436 | 0.147 | 0.897 | 0.936 | ||
| High DHA | 0.38 (1.41) | 0.28 (1.32) | 0.054 | 0.007 | 0.041 | 0 | 0 | ||
| VLMT Long-term memory parameters | DHA: F(4,184) = 0.599, p = 0.664, ηp2 = 0.013 S: F(2,91) = 0.181, p = 0.835, ηp2 = 0.004 I: F(4,184) = 1.805, p = 0.130, ηp2 = 0.038 G: F(2,91) = 0.488, p = 0.615, ηp2 = 0.011 IQ: F(2,91) = 11.721, p < 0.001 ***, ηp2 = 0.205 | ||||||||
| T7 | |||||||||
| Low DHA | 11.70 (2.79) | 11.90 (2.51) | 0.804 | 0.151 | 1.598 | 0.744 | 19.794 | NS | NS |
| Moderate DHA | 12.25 (2.95) | 10.62 (2.18) | 0.45 | 0.698 | 0.208 | 0.391 | <0.001 * | ||
| High DHA | 11.69 (2.02) | 13.00 (1.33) | 0.017 | 0.002 | 0.034 | 0.008 | 0.177 | ||
| T5–7 | |||||||||
| Low DHA | 0.39 (1.64) | 1.20 (1.75) | 1.11 | 0.011 | 1.86 | 0.031 | 1.802 | NS | NS |
| Moderate DHA | 0.80 (2.12) | 1.15 (1.46) | 0.334 | 0.916 | 0.161 | 0.86 | 0.183 | ||
| High DHA | 0.75 (1.44) | −0.11 (1.41) | 0.024 | 0 | 0.039 | 0 | 0.019 | ||
| Moderate depression N = 58 M (SD) | Severe depression N = 41 M (SD) | ||||||||
| Digits forward | |||||||||
| Low DHA | 9.09 (2.18) | 9.90 (1.37) | 3.342 | 0.956 | 1.523 | 0.23 | 25.145 | NS | NS |
| Moderate DHA | 8.45 (1.50) | 7.85 (1.41) | 0.040 * | 0.543 | 0.224 | 0.633 | <0.001 * | ||
| High DHA | 8.69 (1.66) | 8.28 (2.08) | 0.068 | 0.004 | 0.032 | 0.003 | 0.216 | ||
| Digits backward | |||||||||
| Low DHA | 8.64 (2.36) | 8.60 (1.17) | 0.78 | 0.005 | 0.192 | 0.058 | 17.851 | NS | NS |
| Moderate DHA | 8.15 (1.81) | 7.62 (1.26) | 0.461 | 0.942 | 0.826 | 0.81 | <0.001 * | ||
| High DHA | 8.19 (1.64) | 7.89 (1.61) | 0.017 | 0 | 0.004 | 0.001 | 0.164 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emery, S.; Häberling, I.; Berger, G.; Baumgartner, N.; Strumberger, M.; Albermann, M.; Nalani, K.; Schmeck, K.; Erb, S.; Bachmann, S.; et al. Verbal Memory Performance in Depressed Children and Adolescents: Associations with EPA but Not DHA and Depression Severity. Nutrients 2020, 12, 3630. https://doi.org/10.3390/nu12123630
Emery S, Häberling I, Berger G, Baumgartner N, Strumberger M, Albermann M, Nalani K, Schmeck K, Erb S, Bachmann S, et al. Verbal Memory Performance in Depressed Children and Adolescents: Associations with EPA but Not DHA and Depression Severity. Nutrients. 2020; 12(12):3630. https://doi.org/10.3390/nu12123630
Chicago/Turabian StyleEmery, Sophie, Isabelle Häberling, Gregor Berger, Noemi Baumgartner, Michael Strumberger, Mona Albermann, Kristin Nalani, Klaus Schmeck, Suzanne Erb, Silke Bachmann, and et al. 2020. "Verbal Memory Performance in Depressed Children and Adolescents: Associations with EPA but Not DHA and Depression Severity" Nutrients 12, no. 12: 3630. https://doi.org/10.3390/nu12123630
APA StyleEmery, S., Häberling, I., Berger, G., Baumgartner, N., Strumberger, M., Albermann, M., Nalani, K., Schmeck, K., Erb, S., Bachmann, S., Wöckel, L., Müller-Knapp, U., Contin-Waldvogel, B., Rhiner, B., Walitza, S., Hersberger, M., & Drechsler, R. (2020). Verbal Memory Performance in Depressed Children and Adolescents: Associations with EPA but Not DHA and Depression Severity. Nutrients, 12(12), 3630. https://doi.org/10.3390/nu12123630





