High Glycemic Load Is Associated with Cognitive Decline in Apolipoprotein E ε4 Allele Carriers
Abstract
:1. Introduction
2. Materials and Methods
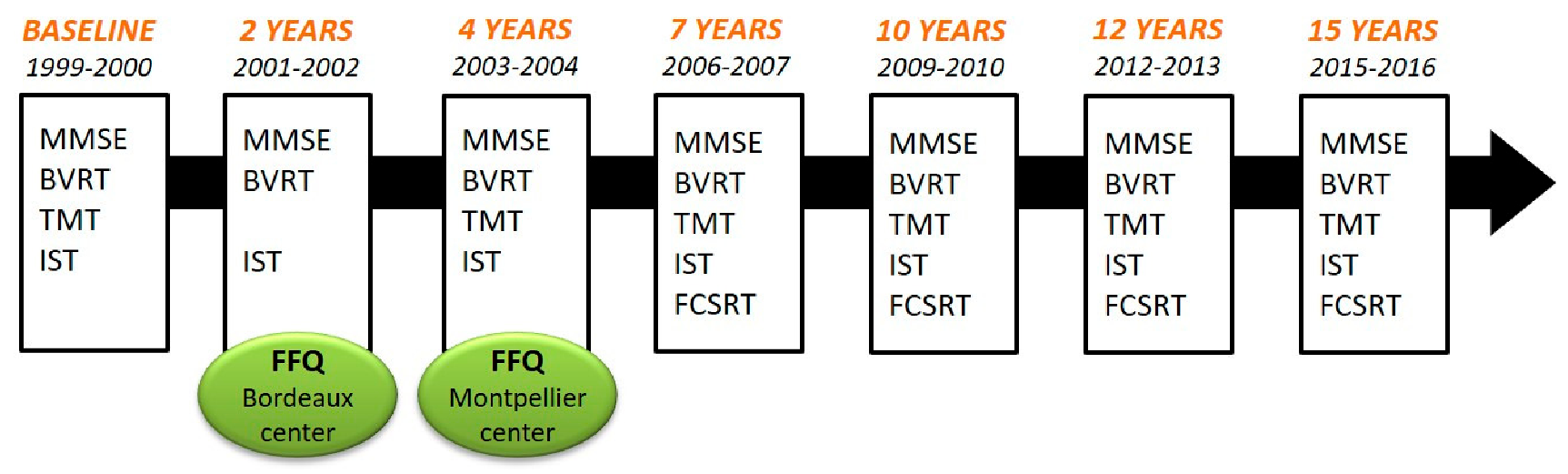
2.1. Study Sample
2.2. Dietary Data
2.3. Neuropsychological Evaluation and Dementia Diagnosis
2.4. Covariates
2.5. Statistical Analyses
2.5.1. Main Analyses
2.5.2. Sensitivity Analyses
2.5.3. Imputation of Missing Values
3. Results
3.1. Population Characteristics
3.2. Main Analyses
3.2.1. Association between GL and Cognitive Changes in APOE4 Non-Carriers (12-Year Follow-up)
3.2.2. Association between Glycemic Load and Cognitive Changes in APOE4 Carriers (12-Year Follow-up)
3.3. Sensitivity Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shah, H.; Albanese, E.; Duggan, C.; Rudan, I.; Langa, K.M.; Carrillo, M.C.; Chan, K.Y.; Joanette, Y.; Prince, M.; Rossor, M.; et al. Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol. 2016, 15, 1285–1294. [Google Scholar] [CrossRef]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Barberger-Gateau, P.; Samieri, C.; Féart, C.; Plourde, M. Dietary omega 3 polyunsaturated fatty acids and Alzheimer’s disease: Interaction with apolipoprotein E genotype. Curr. Alzheimer Res. 2011, 8, 479–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivipelto, M.; Rovio, S.; Ngandu, T.; Kåreholt, I.; Eskelinen, M.; Winblad, B.; Hachinski, V.; Cedazo-Minguez, A.; Soininen, H.; Tuomilehto, J.; et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: A population-based study. J. Cell. Mol. Med. 2008, 12, 2762–2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipper, H.M. Apolipoprotein E: Implications for AD neurobiology, epidemiology and risk assessment. Neurobiol. Aging 2011, 32, 778–790. [Google Scholar] [CrossRef]
- Kern, S.; Mehlig, K.; Kern, J.; Zetterberg, H.; Thelle, D.; Skoog, I.; Lissner, L.; Blennow, K.; Börjesson-Hanson, A. The Distribution of Apolipoprotein E Genotype over the Adult Lifespan and in Relation to Country of Birth. Am. J. Epidemiol. 2015, 181, 214–217. [Google Scholar] [CrossRef] [Green Version]
- Hersi, M.; Irvine, B.; Gupta, P.; Gomes, J.; Birkett, N.; Krewski, D. Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. NeuroToxicology 2017, 61, 143–187. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.; Zhao, L. Human ApoE Isoforms Differentially Modulate Brain Glucose and Ketone Body Metabolism: Implications for Alzheimer’s Disease Risk Reduction and Early Intervention. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 6665–6681. [Google Scholar] [CrossRef]
- Safieh, M.; Korczyn, A.D.; Michaelson, D.M. ApoE4: An emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019, 17, 64. [Google Scholar] [CrossRef] [Green Version]
- Farmer, B.C.; Johnson, L.A.; Hanson, A.J. Effects of apolipoprotein E on nutritional metabolism in dementia. Curr. Opin. Lipidol. 2019, 30, 10–15. [Google Scholar] [CrossRef]
- Reiman, E.M.; Chen, K.; Alexander, G.E.; Caselli, R.J.; Bandy, D.; Osborne, D.; Saunders, A.M.; Hardy, J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc. Natl. Acad. Sci. USA 2004, 101, 284–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandon, J.A.; Farmer, B.C.; Williams, H.C.; Johnson, L.A. APOE and Alzheimer’s Disease: Neuroimaging of Metabolic and Cerebrovascular Dysfunction. Front. Aging Neurosci. 2018, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Liu, C.-C.; Van Ingelgom, A.J.; Martens, Y.A.; Linares, C.; Knight, J.A.; Painter, M.M.; Sullivan, P.M.; Bu, G. Apolipoprotein E4 Impairs Neuronal Insulin Signaling by Trapping Insulin Receptor in the Endosomes. Neuron 2017, 96, 115–129.e5. [Google Scholar] [CrossRef] [Green Version]
- Rönnemaa, E.; Zethelius, B.; Sundelöf, J.; Sundström, J.; Degerman-Gunnarsson, M.; Berne, C.; Lannfelt, L.; Kilander, L. Impaired insulin secretion increases the risk of Alzheimer disease. Neurology 2008, 71, 1065–1071. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Gentreau, M.; Chuy, V.; Féart, C.; Samieri, C.; Ritchie, K.; Raymond, M.; Berticat, C.; Artero, S. Refined carbohydrate-rich diet is associated with long-term risk of dementia and Alzheimer’s disease in apolipoprotein E ε4 allele carriers. Alzheimers Dement. J. Alzheimers Assoc. 2020, 16, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Andel, R.; McEvoy, C.; Dahl Aslan, A.K.; Finkel, D.; Pedersen, N.L. Blood Glucose, Diet-Based Glycemic Load and Cognitive Aging Among Dementia-Free Older Adults. J. Gerontol. Ser. A 2015, 70, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Goh, H.J.; Govindharajulu, P.; Leow, M.K.-S.; Henry, C.J. Postprandial glucose, insulin and incretin responses differ by test meal macronutrient ingestion sequence (PATTERN study). Clin. Nutr. 2019, 39, 950–957. [Google Scholar] [CrossRef]
- Post, R.E.; Mainous, A.G.; King, D.E.; Simpson, K.N. Dietary fiber for the treatment of type 2 diabetes mellitus: A meta-analysis. J. Am. Board Fam. Med. JABFM 2012, 25, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Hätönen, K.A.; Virtamo, J.; Eriksson, J.G.; Sinkko, H.K.; Sundvall, J.E.; Valsta, L.M. Protein and fat modify the glycaemic and insulinaemic responses to a mashed potato-based meal. Br. J. Nutr. 2011, 106, 248–253. [Google Scholar] [CrossRef] [Green Version]
- 3C Study Group. Vascular factors and risk of dementia: Design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology 2003, 22, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Monro, J.A.; Shaw, M. Glycemic impact, glycemic glucose equivalents, glycemic index, and glycemic load: Definitions, distinctions, and implications. Am. J. Clin. Nutr. 2008, 87, 237S–243S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster-Powell, K.; Holt, S.H.A.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glycemic Index. Available online: www.glycemicindex.com (accessed on 7 November 2018).
- Ciqual, Table de Composition Nutritionnelle des Aliments. Available online: www.anses.ciqual.fr (accessed on 7 November 2018).
- Isaacs, B.; Kennie, A.T. The Set test as an aid to the detection of dementia in old people. Br. J. Psychiatry J. Ment. Sci. 1973, 123, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Benton, A.L. Manuel du Test de Rétention Visuelle. Applications Cliniques et Expérimentales; Centre de Psychologie Appliquée: Paris, France, 1965. [Google Scholar]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Skills 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Van der Linden, M.; Coyette, F.; Poitrenaud, J.; Kalafat, M.; Calicis, F.; Wyns, C.; Adam, S. L’épreuve de rappel libre/rappel indice à 16 items (RL/RI-16). In L’évaluation des Troubles de la Mémoire: Présentation de Quatre Tests de Mémoire Épisodique (avec leur Étalonnage); Van der Linden, M., Adam, S., Agniel, A., Baisset Mouly, C., les membres du GREMEM, Eds.; Solal Editeur: Marseille, France, 2004; pp. 25–47. [Google Scholar]
- Grober, E.; Buschke, H.; Crystal, H.; Bang, S.; Dresner, R. Screening for dementia by memory testing. Neurology 1988, 38, 900–903. [Google Scholar] [CrossRef]
- Mura, T.; Baramova, M.; Gabelle, A.; Artero, S.; Dartigues, J.-F.; Amieva, H.; Berr, C. Predicting dementia using socio-demographic characteristics and the Free and Cued Selective Reminding Test in the general population. Alzheimers Res. Ther. 2017, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text Revision (DSM-IV-TR), 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000; ISBN 978-0-89042-062-1. [Google Scholar]
- Dufouil, C.; Richard, F.; Fiévet, N.; Dartigues, J.F.; Ritchie, K.; Tzourio, C.; Amouyel, P.; Alpérovitch, A. APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: The Three-City Study. Neurology 2005, 64, 1531–1538. [Google Scholar] [CrossRef]
- Brindel, P.; Hanon, O.; Dartigues, J.-F.; Ritchie, K.; Lacombe, J.-M.; Ducimetière, P.; Alpérovitch, A.; Tzourio, C.; 3C Study Investigators. Prevalence, awareness, treatment, and control of hypertension in the elderly: The Three City study. J. Hypertens. 2006, 24, 51–58. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Fuhrer, R.; Rouillon, F. La version française de l’échelle CES-D (Center for Epidemiologic Studies-Depression Scale). Description et traduction de l’échelle d’autoévaluation. Eur. Psychiatry 1989, 4, 163–166. [Google Scholar] [CrossRef]
- Letois, F.; Mura, T.; Scali, J.; Gutierrez, L.-A.; Féart, C.; Berr, C. Nutrition and mortality in the elderly over 10 years of follow-up: The Three-City study. Br. J. Nutr. 2016, 116, 882–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Buuren, S. Flexible Imputation of Missing Data, 2nd ed.; Chapman & Hall/CRC: New York, NY, USA, 2018. [Google Scholar]
- R: The R Project for Statistical Computing. Available online: www.r-project.org (accessed on 30 September 2018).
- Chong, C.P.; Shahar, S.; Haron, H.; Din, N.C. Habitual sugar intake and cognitive impairment among multi-ethnic Malaysian older adults. Clin. Interv. Aging 2019, 14, 1331–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, M.K.; Sullivan, D.K.; Swerdlow, R.H.; Vidoni, E.D.; Morris, J.K.; Mahnken, J.D.; Burns, J.M. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am. J. Clin. Nutr. 2017, 106, 1463–1470. [Google Scholar] [CrossRef]
- Power, S.E.; O’Connor, E.M.; Ross, R.P.; Stanton, C.; O’Toole, P.W.; Fitzgerald, G.F.; Jeffery, I.B. Dietary glycaemic load associated with cognitive performance in elderly subjects. Eur. J. Nutr. 2015, 54, 557–568. [Google Scholar] [CrossRef]
- Pase, M.P.; Himali, J.J.; Jacques, P.F.; DeCarli, C.; Satizabal, C.L.; Aparicio, H.; Vasan, R.S.; Beiser, A.S.; Seshadri, S. Sugary beverage intake and preclinical Alzheimer’s disease in the community. Alzheimers Dement. 2017, 13, 955–964. [Google Scholar] [CrossRef]
- Philippou, E.; Pot, G.K.; Heraclides, A.; Richards, M.; Bendayan, R. Dietary glycaemic index and cognitive function: Prospective associations in adults of the 1946 British birth cohort. Public Health Nutr. 2019, 22, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- An, R.; Liu, G.; Khan, N.; Yan, H.; Wang, Y. Dietary Habits and Cognitive Impairment Risk Among Oldest-Old Chinese. J. Gerontol. B Psychol. Sci. Soc. Sci. 2019, 74, 474–483. [Google Scholar] [CrossRef] [Green Version]
- Gardener, S.L.; Rainey-Smith, S.R.; Sohrabi, H.R.; Weinborn, M.; Verdile, G.; Fernando, W.M.A.D.B.; Lim, Y.Y.; Harrington, K.; Burnham, S.; Taddei, K.; et al. Increased Carbohydrate Intake is Associated with Poorer Performance in Verbal Memory and Attention in an APOE Genotype-Dependent Manner. J. Alzheimers Dis. JAD 2017, 58, 193–201. [Google Scholar] [CrossRef]
- Hanson, A.J.; Bayer, J.L.; Baker, L.D.; Cholerton, B.; VanFossen, B.; Trittschuh, E.; Rissman, R.A.; Donohue, M.C.; Moghadam, S.H.; Plymate, S.R.; et al. Differential Effects of Meal Challenges on Cognition, Metabolism, and Biomarkers for Apolipoprotein E ε4 Carriers and Adults with Mild Cognitive Impairment. J. Alzheimers Dis. JAD 2015, 48, 205–218. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.M.; Meigs, J.B.; Liu, S.; Saltzman, E.; Wilson, P.W.F.; Jacques, P.F. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 2004, 27, 538–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Lu, H.; Lewis, T.L.; Li, L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J. Biol. Chem. 2007, 282, 36275–36282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, C.J.; Ruderman, N.B.; Kahn, S.E.; Pedersen, O.; Prentki, M. Insulin resistance as a physiological defense against metabolic stress: Implications for the management of subsets of type 2 diabetes. Diabetes 2015, 64, 673–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterfield, D.A.; Di Domenico, F.; Barone, E. Elevated Risk of Type 2 Diabetes for Development of Alzheimer Disease: A Key Role for Oxidative Stress in Brain. Biochim. Biophys. Acta 2014, 1842, 1693–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garber, A.; Csizmadi, I.; Friedenreich, C.M.; Sajobi, T.T.; Longman, R.S.; Tyndall, A.V.; Drogos, L.L.; Davenport, M.H.; Poulin, M.J. Association between glycemic load and cognitive function in community-dwelling older adults: Results from the Brain in Motion study. Clin. Nutr. Edinb. Scotl. 2018, 37, 1690–1699. [Google Scholar] [CrossRef] [Green Version]
- Bangen, K.J.; Himali, J.J.; Beiser, A.S.; Nation, D.A.; Libon, D.J.; Fox, C.S.; Seshadri, S.; Wolf, P.A.; McKee, A.C.; Au, R.; et al. Interaction Between Midlife Blood Glucose and APOE Genotype Predicts Later Alzheimer’s Disease Pathology. J. Alzheimers Dis. JAD 2016, 53, 1553–1562. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, R.J.; Greenwood, C.E.; Winocur, G.; Wolever, T.M. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. Am. J. Clin. Nutr. 2000, 72, 825–836. [Google Scholar] [CrossRef]

| Characteristics Mean (SD) or n (%) | Daily Glycemic Load Tertiles | |||
|---|---|---|---|---|
| Low (<93) n = 846 | Middle (93 to <119) n = 845 | High (≥119) n = 848 | p Value 1 | |
| Montpellier center | 342 (40.4) | 390 (46.2) | 527 (62.1) | <0.0001 |
| Age (years) | 75.9 (4.8) | 75.5 (4.8) | 76.4 (4.9) | 0.038 |
| Women | 572 (67.6) | 512 (60.6) | 515 (60.7) | 0.003 |
| Education level | 0.19 | |||
| No school | 231 (27.3) | 216 (25.6) | 208 (24.5) | |
| Primary school | 237 (28) | 265 (31.4) | 224 (26.4) | |
| High school | 192 (22.7) | 183 (21.7) | 210 (24.8) | |
| Graduated | 185 (21.9) | 178 (21.1) | 204 (24.1) | |
| MMSE (IQR) | 28 (27-29) | 28 (27-29) | 28 (27–29) | 0.805 |
| APOE4 carriers | 142 (16.8) | 156 (18.5) | 144 (17) | 0.539 |
| BMI (kg/m2) | 26.1 (4.1) | 25.6 (3.9) | 24.8 (3.4) | <0.0001 |
| Hypertension | 521 (61.6) | 487 (57.6) | 465 (54.8) | 0.017 |
| Cardiovascular history | 90 (10.6) | 82 (9.7) | 77 (9.1) | 0.555 |
| Stroke history | 37 (4.4) | 25 (3) | 39 (4.6) | 0.173 |
| Hypercholesterolemia | 464 (54.8) | 475 (56.2) | 452 (53.3) | 0.486 |
| Depressive symptomatology 2 | 74 (8.7) | 56 (6.6) | 70 (8.3) | 0.238 |
| Tobacco use (current or past) | 337 (39.8) | 310 (36.7) | 300 (35.4) | 0.15 |
| Mediterranean-like diet | 4.3 (1.6) | 5 (1.6) | 5.5 (1.5) | <0.0001 |
| Physical activity | 251 (29.7) | 274 (32.4) | 317 (37.4) | 0.002 |
| Energy intake (kJ/day) | 3553.2 (698.8) | 4694.5 (656.2) | 6379.4 (1476.5) | <0.0001 |
| Glycemic Load | IST n = 1815 | BVRT n = 1794 | log(TMTA) n = 1612 | log(TMTB) n = 1593 | FIRS n = 1351 | FDRS n = 1348 | MMSE n = 1831 |
|---|---|---|---|---|---|---|---|
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | |
| Daily | |||||||
| Middle | −0.159 (0.654) | −0.1 (0.12) | 0.003 (0.026) | 0.01 (0.029) | 0.649 (0.654) | 0.304 (0.285) | −0.096 (0.119) |
| High | −0.103 (0.82) | −0.129 (0.146) | 0.023 (0.032) | −0.004 (0.035) | 0.944 (0.757) | 0.411 (0.326) | 0.03 (0.147) |
| Middle × time | 0.102 (0.056) | 0.008 (0.012) | −0.006 * (0.003) | −0.003 (0.003) | 0.015 (0.075) | 0.003 (0.034) | 0.022 (0.024) |
| High × time | 0.086 (0.055) | 0.01 (0.012) | −0.004 (0.003) | −0.001 (0.003) | 0.045 (0.073) | 0.007 (0.033) | −0.015 (0.024) |
| Breakfast | |||||||
| Middle | 0.608 (0.579) | 0.073 (0.109) | -0.033 (0.024) | −0.031 (0.026) | −0.965 (0.604) | 0.136 (0.267) | 0.058 (0.105) |
| High | 0.136 (0.67) | 0.033 (0.122) | 0.015 (0.027) | −0.036 (0.029) | −0.31 (0.644) | 0.026 (0.28) | 0.07 (0.121) |
| Middle × time | 0.031 (0.057) | −0.005 (0.012) | 0 (0.003) | 0.002 (0.003) | 0.136 (0.076) | −0.014 (0.035) | 0.023 (0.024) |
| High × time | 0.08 (0.054) | 0.015 (0.012) | −0.003 (0.003) | 0 (0.003) | 0.09 (0.071) | 0.01 (0.032) | 0.023 (0.023) |
| Lunch | |||||||
| Middle | −1.312 * (0.6) | −0.07 (0.114) | 0.027 (0.025) | 0.067 (0.027) | 0.292 (0.627) | −0.279 (0.274) | 0.017 (0.11) |
| High | −1.904 ** (0.732) | −0.324 * (0.133) | 0.074 * (0.029) | 0.066 (0.032) | 0.124 (0.687) | −0.1 (0.297) | −0.054 (0.132) |
| Middle × time | 0.021 (0.057) | −0.008 (0.012) | −0.001 (0.003) | −0.008 ** (0.003) | −0.055 (0.077) | 0.026 (0.035) | −0.021 (0.024) |
| High × time | 0.102 (0.054) | 0.011 (0.012) | −0.006 * (0.003) | −0.006 * (0.003) | 0.064 (0.072) | 0.025 (0.033) | −0.012 (0.023) |
| Afternoon snack | |||||||
| Middle | −2.125 (5.936) | −0.552 (0.964) | −0.003 (0.216) | −0.07 (0.225) | −2.922 (4.169) | −0.029 (1.732) | −0.328 (1.035) |
| High | −2.924 (5.978) | −0.61 (0.972) | 0.019 (0.217) | −0.047 (0.227) | −3.165 (4.205) | 0.16 (1.747) | −0.44 (1.043) |
| Middle × time | −0.054 (0.054) | −0.006 (0.012) | −0.002 (0.003) | −0.002 (0.003) | 0.026 (0.072) | −0.016 (0.033) | −0.016 (0.023) |
| High × time | 0.038 (0.056) | −0.006 (0.012) | −0.002 (0.003) | 0.001 (0.003) | 0.106 (0.074) | 0.001 (0.034) | −0.035 (0.024) |
| Dinner | |||||||
| Middle | 0.565 (0.601) | −0.065 (0.113) | 0.033 (0.025) | 0.054 (0.027) | −0.056 (0.616) | −0.19 (0.269) | −0.178 (0.109) |
| High | 0.194 (0.778) | −0.204 (0.138) | 0.064 * (0.03) | 0.072 * (0.033) | 0.212 (0.71) | −0.03 (0.305) | −0.066 (0.139) |
| Middle × time | 0.027 (0.056) | 0.017 (0.012) | 0.002 (0.003) | 0.002 (0.003) | 0.046 (0.075) | 0.039 (0.034) | 0.045 (0.024) |
| High × time | −0.015 (0.053) | 0.019 (0.012) | −0.003 (0.003) | −0.001 (0.003) | −0.02 (0.071) | 0.015 (0.032) | 0.001 (0.023) |
| Glycemic Load | IST n = 401 | BVRT n = 401 | log(TMTA) n = 348 | log(TMTB) n = 348 | FIRS n = 278 | FDRS n = 275 | MMSE n = 409 |
|---|---|---|---|---|---|---|---|
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | |
| Daily | |||||||
| Middle | −0.719 (1.402) | −0.049 (0.259) | 0.04 (0.059) | −0.024 (0.065) | −0.018 (1.58) | −0.037 (0.706) | 0.183 (0.266) |
| High | −1.476 (1.741) | 0.115 (0.316) | 0.034 (0.071) | −0.034 (0.077) | 0.428 (1.811) | 0.747 (0.799) | 0.141 (0.332) |
| Middle × time | 0.086 (0.134) | −0.009 (0.033) | 0.002 (0.006) | −0.002 (0.007) | −0.033 (0.2) | 0.03 (0.093) | −0.013 (0.062) |
| High × time | 0.042 (0.131) | −0.031 (0.032) | 0.007 (0.006) | 0.001 (0.006) | −0.167 (0.196) | −0.103 (0.09) | −0.028 (0.062) |
| Breakfast | |||||||
| Middle | −1.571 (1.237) | −0.055 (0.234) | 0.037 (0.054) | −0.022 (0.058) | −0.771 (1.475) | −0.649 (0.654) | 0.088 (0.238) |
| High | −1.647 (1.444) | −0.201 (0.267) | 0.065 (0.059) | −0.06 (0.064) | 0.192 (1.534) | −0.133 (0.677) | 0.165 (0.279) |
| Middle × time | −0.11 (0.134) | −0.041 (0.034) | 0.003 (0.007) | 0.002 (0.007) | −0.095 (0.2) | −0.007 (0.092) | −0.143 * (0.061) |
| High × time | 0.018 (0.126) | −0.053 (0.031) | 0.002 (0.006) | 0.004 (0.006) | −0.269 (0.18) | −0.076 (0.083) | −0.051 (0.057) |
| Lunch | |||||||
| Middle | −0.772 (1.27) | 0.006 (0.242) | 0.114 * (0.055) | −0.005 (0.06) | 1.738 (1.521) | 0.325 (0.683) | 0.032 (0.246) |
| High | −0.202 (1.58) | 0.219 (0.293) | 0.085 (0.065) | −0.066 (0.07) | 0.545 (1.674) | −0.452 (0.76) | −0.162 (0.302) |
| Middle × time | 0.038 (0.132) | 0.021 (0.033) | −0.01 (0.006) | −0.001 (0.006) | −0.09 (0.195) | −0.007 (0.089) | 0.039 (0.06) |
| High × time | 0.011 (0.131) | −0.024 (0.032) | −0.006 (0.006) | 0.002 (0.006) | −0.082 (0.188) | −0.055 (0.087) | −0.024 (0.06) |
| Afternoon snack | |||||||
| Middle | 15.27 (8.8) | 0.896 (1.499) | −0.032 (0.322) | 0.082 (0.345) | −0.888 (6.569) | −1.579 (2.938) | −2.218 (1.747) |
| High | 14.429 (8.908) | 0.421 (1.524) | 0.071 (0.326) | 0.277 (0.349) | 0.386 (6.723) | −1.213 (3.01) | −2.522 (1.77) |
| Middle × time | −0.069 (0.133) | −0.071 * (0.033) | 0.004 (0.006) | 0.005 (0.007) | 0.081 (0.198) | −0.005 (0.091) | −0.04 (0.06) |
| High × time | −0.183 (0.129) | −0.04 (0.032) | 0.011 (0.006) | 0.002 (0.006) | −0.343 (0.188) | −0.175 * (0.087) | −0.158 ** (0.059) |
| Dinner | |||||||
| Middle | 0.962 (1.343) | −0.012 (0.255) | −0.026 (0.058) | −0.051 (0.063) | 1.418 (1.589) | 0.351 (0.711) | −0.002 (0.255) |
| High | 2.361 (1.765) | 0.281 (0.31) | −0.011 (0.071) | −0.058 (0.077) | 0.943 (1.81) | −0.116 (0.805) | −0.184 (0.32) |
| Middle × time | −0.067 (0.139) | −0.031 (0.035) | 0.006 (0.007) | 0.006 (0.007) | −0.141 (0.204) | −0.054 (0.094) | −0.034 (0.063) |
| High × time | 0.029 (0.128) | −0.02 (0.032) | 0.005 (0.006) | −0.004 (0.006) | −0.129 (0.193) | −0.033 (0.087) | 0.012 (0.058) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentreau, M.; Raymond, M.; Chuy, V.; Samieri, C.; Féart, C.; Berticat, C.; Artero, S. High Glycemic Load Is Associated with Cognitive Decline in Apolipoprotein E ε4 Allele Carriers. Nutrients 2020, 12, 3619. https://doi.org/10.3390/nu12123619
Gentreau M, Raymond M, Chuy V, Samieri C, Féart C, Berticat C, Artero S. High Glycemic Load Is Associated with Cognitive Decline in Apolipoprotein E ε4 Allele Carriers. Nutrients. 2020; 12(12):3619. https://doi.org/10.3390/nu12123619
Chicago/Turabian StyleGentreau, Mélissa, Michel Raymond, Virginie Chuy, Cécilia Samieri, Catherine Féart, Claire Berticat, and Sylvaine Artero. 2020. "High Glycemic Load Is Associated with Cognitive Decline in Apolipoprotein E ε4 Allele Carriers" Nutrients 12, no. 12: 3619. https://doi.org/10.3390/nu12123619






