Protein Requirements of Pre-Menopausal Female Athletes: Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Management
2.4. Quality Assessment
3. Results
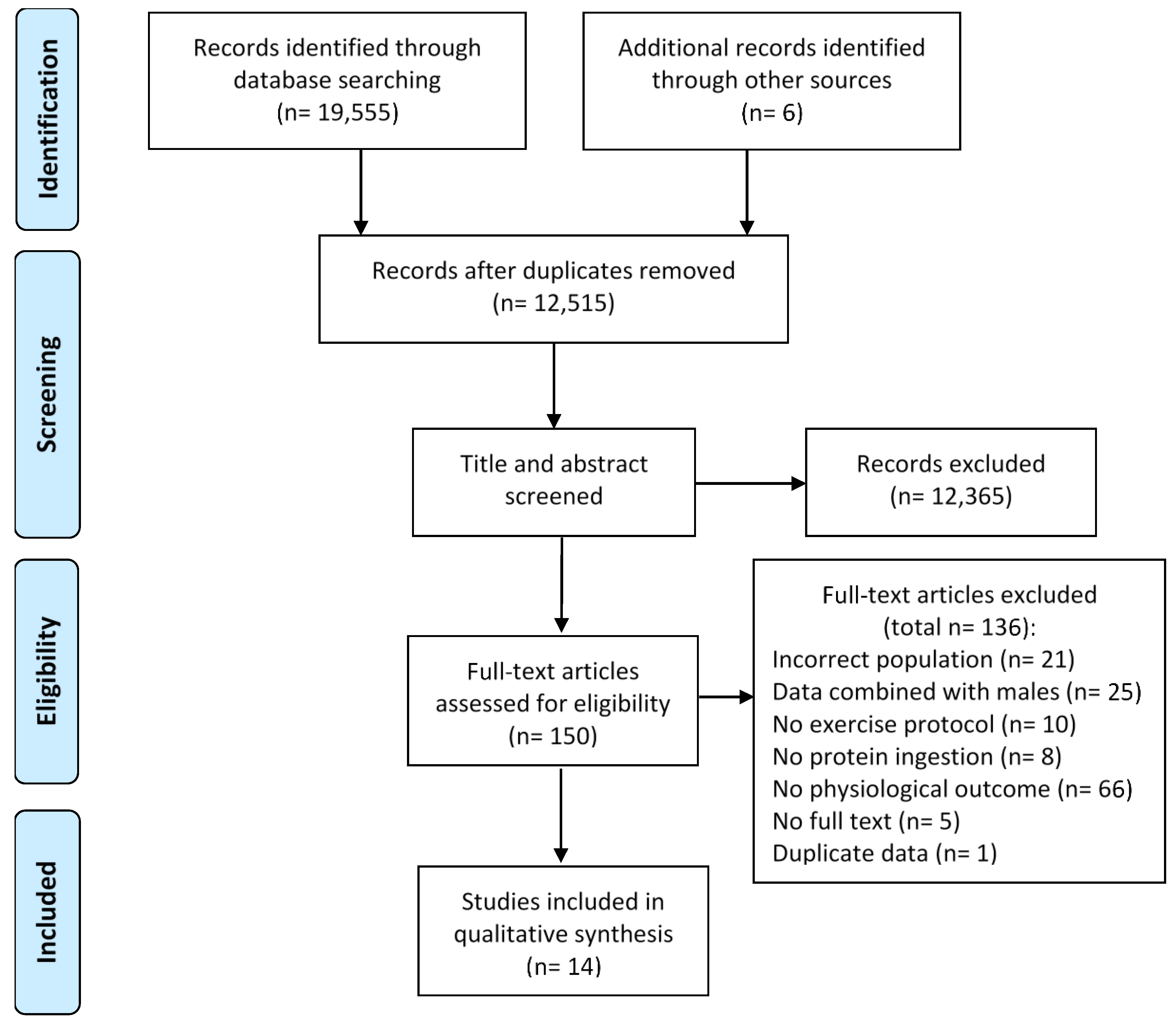
3.1. Screening and Study Selection
3.2. Quality Assessment of Included Studies
3.3. Participant Characteristics, Menstrual Cycle and Hormonal Contraceptive Use
3.4. Aerobic Endurance Exercise
3.5. Resistance Exercise
3.6. Intermittent Exercise
4. Discussion
4.1. Aerobic Endurance Exercise
4.2. Resistance Exercise
4.3. Intermittent Exercise
4.4. Influence of Menstrual Cycle and Hormonal Contraceptives on the Protein Requirements of Pre-Menopausal Female Athletes
4.5. Protein Type, Timing and Distribution
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Phillips, S.M.; Moore, D.R.; Tang, J.E. A critical examination of dietary protein requirements, benefits, and excesses in athletes. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, S58–S76. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Erdman, K.; Burke, L. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543. [Google Scholar]
- Department of Health. Dietary Reference Values for Food Energy and Nutrients for the United Kingdom; TSO: London, UK, 1991. [Google Scholar]
- National Health and Medical Research Council; Australian Government Department of Health and Ageing; New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; National Health and Medical Research Council: Canberra, Austrialia, 2006. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Witard, O.C. Protein Requirements and Recommendations for Athletes: Relevance of Ivory Tower Arguments for Practical Recommendations. Clin. Sports Med. 2007, 26, 17–36. [Google Scholar] [CrossRef]
- Tinline-Goodfellow, C.T.; West, D.W.D.; Malowany, J.M.; Gillen, J.B.; Moore, D.R. An Acute Reduction in Habitual Protein Intake Attenuates Post Exercise Anabolism and May Bias Oxidation-Derived Protein Requirements in Resistance Trained Men. Front. Nutr. 2020, 7, 55. [Google Scholar] [CrossRef]
- West, D.W.D.; Burd, N.A.; Churchward-Venne, T.A.; Mitchell, C.J.; Phillips, S.M.; Camera, D.M.; Hawley, J.A.; Coffey, V.G.; Baker, S.K. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. J. Appl. Physiol. 2012, 112, 1805–1813. [Google Scholar] [CrossRef]
- Moore, D.R. Maximizing Post-exercise Anabolism: The Case for Relative Protein Intakes. Front. Nutr. 2019, 6, 147. [Google Scholar] [CrossRef]
- Phillips, S.M. Sex-Based Differences in Muscle Protein Turnover and Metabolism In Aging: Feeding and Exercise Responses. Sports Sci. Exch. 2014, 27, 1–5. [Google Scholar]
- Tarnopolsky, M.A. Sex differences in exercise metabolism and the role of 17-beta estradiol. Med. Sci. Sports Exerc. 2008, 40, 648–654. [Google Scholar] [CrossRef]
- Lamont, L.S.; Lemon, P.W.R.; Bruot, B.C. Menstrual cycle and exercise effects on protein catabolism. Med. Sci. Sports Exerc. 1987, 19, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Lamont, L.S.; McCullough, A.J.; Kalhan, S.C. Gender differences in leucine, but not lysine, kinetics. J. Appl. Physiol. 2001, 91, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Atkinson, S.A.; Tarnopolsky, M.A.; MacDougall, J.D. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. J. Appl. Physiol. 1993, 75, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M. Protein requirements for endurance athletes. Eur. J. Sport Sci. 2004, 4, 1–15. [Google Scholar] [CrossRef]
- Webb, P. 24-Hour energy expenditure and the menstrual cycle. Am. J. Clin. Nutr. 1986, 44, 614–619. [Google Scholar] [CrossRef]
- Benton, M.J.; Hutchins, A.M.; Dawes, J.J. Effect of menstrual cycle on resting metabolism: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0236025. [Google Scholar] [CrossRef]
- Desbrow, B.; Burd, N.A.; Tarnopolsky, M.; Moore, D.R.; Elliott-Sale, K.J. Nutrition for Special Populations: Young, Female, and Masters Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 220. [Google Scholar] [CrossRef]
- Reilly, T. The Menstrual Cycle and Human Performance: An Overview. Biol. Rhythm Res. 2000, 31, 29–40. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. The effect of the menstrual cycle on exercise metabolism: Implications for exercise performance in eumenorrhoeic women. Sports Med. 2010, 207, 207–227. [Google Scholar] [CrossRef]
- Allen, S.V.; Hopkins, W.G. Age of Peak Competitive Performance of Elite Athletes: A Systematic Review. Sports Med. 2015, 45, 1431–1441. [Google Scholar] [CrossRef]
- Verrilli, L.; Landry, M.; Blanchard, H. Contraceptive choices and menstrual patterns in high level female athletes. Fertil. Steril. 2017, 108, e122. [Google Scholar] [CrossRef]
- Martin, D.; Sale, C.; Cooper, S.B.; Elliott-Sale, K.J. Period Prevalence and Perceived Side Effects of Hormonal Contraceptive Use and the Menstrual Cycle in Elite Athletes. Int. J. Sports Physiol. Perform. 2018, 13, 926. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Sale, K.J.; McNulty, K.L.; Ansdell, P.; Goodall, S.; Hicks, K.M.; Thomas, K.; Swinton, P.A.; Dolan, E. The Effects of Oral Contraceptives on Exercise Performance in Women: A Systematic Review and Meta-analysis. Sports Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The Effects of Menstrual Cycle Phase on Exercise Performance in Eumenorrheic Women: A Systematic Review and Meta-Analysis. Sports Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Landau, R.L.; Lugibihl, K. The effect of dietary protein on the catabolic influence of progesterone. J. Clin. Endocrinol. Metab. 1961, 21, 1345–1354. [Google Scholar] [CrossRef]
- Calloway, D.H.; Kurzer, M.S. Menstrual cycle and protein requirements of women. J. Nutr. 1982, 112, 356. [Google Scholar] [CrossRef]
- Miller, B.F.; Hansen, M.; Olesen, J.L.; Flyvbjerg, A.; Schwarz, P.; Babraj, J.A.; Smith, K.; Rennie, M.J.; Kjaer, M. No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E163–E168. [Google Scholar] [CrossRef]
- Hansen, M.; Langberg, H.; Holm, L.; Miller, B.; Petersen, S.; Doessing, S.; Skovgaard, D.; Trappe, T.; Kjaer, M. Effect of administration of oral contraceptives on the synthesis and breakdown of myofibrillar proteins in young women. Scand. J. Med. Sci. Sports 2011, 21, 62–72. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Tipton, K.D.; Ferrando, A.A.; Williams, B.D.; Wolfe, R.R. Muscle protein metabolism in female swimmers after a combination of resistance and endurance exercise. J. Appl. Physiol. 1996, 81, 2034–2038. [Google Scholar] [CrossRef]
- Poulios, A.; Georgakouli, K.; Draganidis, D.; Deli, C.K.; Tsimeas, P.D.; Chatzinikolaou, A.; Papanikolaou, K.; Batrakoulis, A.; Mohr, M.; Jamurtas, A.Z.; et al. Protein-Based Supplementation to Enhance Recovery in Team Sports: What is the Evidence? J. Sports Sci. Med. 2019, 18, 523–536. [Google Scholar] [PubMed]
- Rand, W.M.; Pellett, P.L.; Young, V.R. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am. J. Clin. Nutr. 2003, 77, 109. [Google Scholar] [CrossRef] [PubMed]
- Academy of Nutrition and Dietetics. Quality Criteria Checklist: Primary Research. In Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process; Academy of Nutrition and Dietetics: Chicago, IL, USA, 2016. [Google Scholar]
- Brown, M.A.; Stevenson, E.J.; Howatson, G. Whey protein hydrolysate supplementation accelerates recovery from exercise-induced muscle damage in females. Appl. Physiol. Nutr. Metab. 2018, 43, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.I.; Aguilar, D.; Conlin, L.; Vargas, A.; Schoenfeld, B.J.; Corson, A.; Gai, C.; Best, S.; Galvan, E.; Couvillion, K. Effects of High Versus Low Protein Intake on Body Composition and Maximal Strength in Aspiring Female Physique Athletes Engaging in an 8-Week Resistance Training Program. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Houltham, S.D.; Rowlands, D.S. A snapshot of nitrogen balance in endurance-trained women. Appl. Physiol. Nutr. Metab. 2014, 39, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Malowany, J.M.; West, D.W.D.; Williamson, E.; Volterman, K.A.; Abou Sawan, S.; Mazzulla, M.; Moore, D.R. Protein to Maximize Whole-Body Anabolism in Resistance-trained Females after Exercise. Med. Sci. Sports Exerc. 2019, 51, 798–804. [Google Scholar] [CrossRef]
- Pihoker, A.A.; Peterjohn, A.M.; Trexler, E.T.; Hirsch, K.R.; Blue, M.N.M.; Anderson, K.C.; Ryan, E.D.; Smith-Ryan, A.E. The effects of nutrient timing on training adaptations in resistance-trained females. J. Sci. Med. Sport 2019, 22, 472–477. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Wadsworth, D.P. Effect of high-protein feeding on performance and nitrogen balance in female cyclists. Med. Sci. Sports Exerc. 2011, 43, 44–53. [Google Scholar] [CrossRef]
- Roy, B.D.; Luttmer, K.; Bosman, M.J.; Tarnopolsky, M.A. The Influence of Post-exercise Macronutrient Intake on Energy Balance and Protein Metabolism in Active Females Participating in Endurance Training. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 172. [Google Scholar] [CrossRef]
- Taylor, L.W.; Wilborn, C.; Roberts, M.D.; White, A.; Dugan, K. Eight weeks of pre- and postexercise whey protein supplementation increases lean body mass and improves performance in Division III collegiate female basketball players. Appl. Physiol. Nutr. Metab. 2016, 41, 249–254. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Wilborn, C.D.; Taylor, L.W.; Outlaw, J.; Williams, L.; Campbell, B.; Foster, C.A.; Smith-Ryan, A.; Urbina, S.; Hayward, S. The Effects of Pre- and Post-Exercise Whey vs. Casein Protein Consumption on Body Composition and Performance Measures in Collegiate Female Athletes. J. Sports Sci. Med. 2013, 12, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Wilborn Colin, D.; Outlaw Jordan, J.; Mumford Petey, W.; Urbina Stacie, L.; Hayward, S.; Roberts, M.D.; Taylor, L.W.; Foster, C.A. A Pilot Study Examining the Effects of 8-Week Whey Protein versus Whey Protein Plus Creatine Supplementation on Body Composition and Performance Variables in Resistance-Trained Women. Ann. Nutr. Metab. 2016, 69, 190. [Google Scholar] [CrossRef] [PubMed]
- Wooding, D.J.; Packer, J.E.; Kato, H.; West, D.W.D.; Courtney-Martin, G.; Moore, D.R.; Pencharz, P.B. Increased Protein Requirements in Female Athletes after Variable-Intensity Exercise. Med. Sci. Sports Exerc. 2017, 49, 2297–2304. [Google Scholar] [CrossRef]
- Kato, H.; Suzuki, K.; Bannai, M.; Moore, D.R. Protein Requirements Are Elevated in Endurance Athletes according to the Indicator Amino Acid Oxidation Method. Med. Sci. Sports Exerc. 2016, 48, 5–6. [Google Scholar] [CrossRef]
- Tremblay, J.; Peronnet, F.; Massicotte, D.; Lavoie, C. Carbohydrate supplementation and sex differences in fuel selection during exercise. Med. Sci. Sports Exerc. 2010, 42, 1314–1323. [Google Scholar] [CrossRef]
- Deldicque, L.; Francaux, M. Recommendations for Healthy Nutrition in Female Endurance Runners: An Update. Front. Nutr. 2015. [Google Scholar] [CrossRef]
- Slater, J.; McLay-Cooke, R.; Brown, R.; Black, K. Female Recreational Exercisers at Risk for Low Energy Availability. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 421–427. [Google Scholar] [CrossRef]
- Lemon, P.W.R. Effects of Exercise on Dietary Protein Requirements. Int. J. Sport Nutr. 1998, 8, 426. [Google Scholar] [CrossRef]
- Moore, D.R.; Camera, D.M.; Areta, J.L.; Hawley, J.A. Beyond muscle hypertrophy: Why dietary protein is important for endurance athletes. Appl. Physiol. Nutr. Metab. 2014, 39, 987–997. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; Smeets, J.S.J.; Betz, M.W.; Senden, J.M.; Goessens, J.P.B.; Gijsen, A.P.; Rollo, I.; Verdijk, L.B.; van Loon, L.J.C. Dose-response effects of dietary protein on muscle protein synthesis during recovery from endurance exercise in young men: A double-blind randomized trial. Am. J. Clin. Nutr. 2020, 112, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R. One size doesn’t fit all: Postexercise protein requirements for the endurance athlete. Am. J. Clin. Nutr. 2020, 112, 249. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Van Loon, L.J.C. Dietary protein for athletes: From requirements to optimum adaptation. J. Sports Sci. 2011, 29 (Suppl. 1), S29–S38. [Google Scholar] [CrossRef]
- Burd, N.A.; Holwerda, A.M.; Selby, K.C.; West, D.W.D.; Staples, A.W.; Cain, N.E.; Phillips, S.M.; Cashaback, J.G.A.; Potvin, J.R.; Baker, S.K. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J. Physiol. 2010, 588, 3119–3130. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.J.; McGlory, C.; Damas, F.; Mazara, N.; Baker, S.K.; Phillips, S.M. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. 2018, 32, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef]
- Macnaughton, L.S.; Wardle, S.L.; Witard, O.C.; McGlory, C.; Hamilton, D.L.; Jeromson, S.; Lawrence, C.E.; Wallis, G.A.; Tipton, K.D. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol. Rep. 2016, 4, e12893. [Google Scholar] [CrossRef] [PubMed]
- Areta, J.L.; Burke, L.M.; Camera, D.M.; West, D.W.; Crawshay, S.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; Hawley, J.A.; Coffey, V.G. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am. J. Physiol. Endocrinol. Metab. 2014. [Google Scholar] [CrossRef] [PubMed]
- Slater, G.J.; Dieter, B.P.; Marsh, D.J.; Helms, E.R.; Shaw, G.; Iraki, J. Is an energy surplus required to maximize skeletal muscle hypertrophy associated with resistance training. Front. Nutr. 2019, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Perez-Schindler, J.; Hamilton, D.L.; Moore, D.R.; Baar, K.; Philp, A. Nutritional strategies to support concurrent training. Eur. J. Sport Sci. 2015, 15, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Faustmann, G.; Meinitzer, A.; Magnes, C.; Tiran, B.; Obermayer-Pietsch, B.; Gruber, H.-J.; Ribalta, J.; Rock, E.; Roob, J.M.; Winklhofer-Roob, B.M. Progesterone-associated arginine decline at luteal phase of menstrual cycle and associations with related amino acids and nuclear factor kB activation. PLoS ONE 2018, 13, e0200489. [Google Scholar] [CrossRef] [PubMed]
- Sawai, A.; Tsushima, T.; Sugiyama, K.; Tsuzuki, K.; Yamauchi, M.; Kimura, N.; Ota, Y.; Sawai, S.; Tochikubo, O. The effects of estrogen and progesterone on plasma amino acids levels: Evidence from change plasma amino acids levels during the menstrual cycle in women. Biol. Rhythm Res. 2020, 51, 151–164. [Google Scholar] [CrossRef]
- Wallace, M.; Brennan, L.; Hashim, Y.Z.H.Y.; Gibney, M.J.; Wingfield, M.; Culliton, M.; McAuliffe, F. Effects of menstrual cycle phase on metabolomic profiles in premenopausal women. Hum. Reprod. 2010, 25, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Draper, C.F.; Weger, B.; Chakrabarti, A.; Goulet, L.; Konz, T.; Martin, F.P.; Moco, S.; Duisters, K.; Harms, A.C.; Hankemeier, T.; et al. Menstrual cycle rhythmicity: Metabolic patterns in healthy women. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Kriengsinyos, W.; Wykes, L.J.; Goonewardene, L.A.; Ball, R.O.; Pencharz, P.B. Phase of menstrual cycle affects lysine requirement in healthy women. Am. J. Physiol. 2004, 489, E489–E496. [Google Scholar] [CrossRef]
- Ouyang, Y.; Tong, H.; Luo, P.; Kong, H.; Xu, Z.; Yin, P.; Xu, G. A high throughput metabolomics method and its application in female serum samples in a normal menstrual cycle based on liquid chromatography-mass spectrometry. Talanta 2018, 185, 483–490. [Google Scholar] [CrossRef]
- Brennan, L.; Gibbons, H. Sex matters: A focus on the impact of biological sex on metabolomic profiles and dietary interventions. Proc. Nutr. Soc. 2020, 79, 205–209. [Google Scholar] [CrossRef]
- Bailey, S.P.; Zacher, C.M.; Mittleman, K.D. Effect of menstrual cycle phase on carbohydrate supplementation during prolonged exercise to fatigue. J. Appl. Physiol. 2000, 88, 690–697. [Google Scholar] [CrossRef]
- Janse De Jonge, X.; Thompson, B.; Han, A. Methodological Recommendations for Menstrual Cycle Research in Sports and Exercise. Med. Sci. Sports Exerc. 2019, 51, 2610–2617. [Google Scholar] [CrossRef]
- Wang, Q.; Würtz, P.; Auro, K.; Morin-Papunen, L.; Kangas, A.J.; Soininen, P.; Tiainen, M.; Tynkkynen, T.; Joensuu, A.; Havulinna, A.S.; et al. Effects of hormonal contraception on systemic metabolism: Cross-sectional and longitudinal evidence. Int. J. Epidemiol. 2016, 45, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Møller, S.E.; Maach-Møller, B.; Olesen, M.; Fjalland, B. Effects of oral contraceptives on plasma neutral amino acids and cholesterol during a menstrual cycle. Eur. J. Clin. Pharmacol. 1996, 50, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Ruoppolo, M.; Pecce, R.; Scolamiero, E.; Campesi, I.; Franconi, F.; Caterino, M.; Cherchi, S.; Tonolo, G.; Mercuro, G. Serum metabolomic profiles suggest influence of sex and oral contraceptive use. Am. J. Transl. Res. 2014, 6, 614–624. [Google Scholar]
- Dalgaard, L.B.; Dalgas, U.; Andersen, J.L.; Rossen, N.B.; Møller, A.B.; Stødkilde-Jørgensen, H.; Jørgensen, J.O.; Kovanen, V.; Couppé, C.; Langberg, H.; et al. Influence of Oral Contraceptive Use on Adaptations to Resistance Training. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.B.; Tarnopolsky, M.A.; MacDonald, M.J.; MacDonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [CrossRef]
- Hartman, J.W.; Tang, J.E.; Wilkinson, S.B.; Tarnopolsky, M.A.; Lawrence, R.L.; Fullerton, A.V.; Phillips, S.M. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am. J. Clin. Nutr. 2007, 86, 373–381. [Google Scholar] [CrossRef]
- Phillips, S.M.; Tang, J.E.; Moore, D.R. The Role of Milk- and Soy-Based Protein in Support of Muscle Protein Synthesis and Muscle Protein Accretion in Young and Elderly Persons. J. Am. Coll. Nutr. 2009, 28, 343–354. [Google Scholar] [CrossRef]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef]
- Gwin, J.A.; Church, D.D.; Wolfe, R.R.; Ferrando, A.A.; Pasiakos, S.M. Muscle Protein Synthesis and Whole-Body Protein Turnover Responses to Ingesting Essential Amino Acids, Intact Protein, and Protein-Containing Mixed Meals with Considerations for Energy Deficit. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Wilson, P.B. Nutrition behaviors, perceptions, and beliefs of recent marathon finishers. Phys. Sportsmed. 2016, 44, 242–251. [Google Scholar] [CrossRef]
- Wirnitzer, K.; Boldt, P.; Lechleitner, C.; Wirnitzer, G.; Leitzmann, C.; Rosemann, T.; Knechtle, B. Health Status of Female and Male Vegetarian and Vegan Endurance Runners Compared to Omnivores—Results from the NURMI Study (Step 2). Nutrients 2019, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Areta, J.L.; Burke, L.M.; Ross, M.L.; Camera, D.M.; West, D.W.D.; Broad, E.M.; Jeacocke, N.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 2013, 591, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.D.; Burd, N.A.; Coffey, V.G.; Baker, S.K.; Burke, L.M.; Hawley, J.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am. J. Clin. Nutr. 2011, 94, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Aragon, A.A.; Krieger, J.W. The effect of protein timing on muscle strength and hypertrophy: A meta-analysis. J. Int. Soc. Sports Nutr. 2013, 10, 1–23. [Google Scholar] [CrossRef]
- Wirth, J.; Hillesheim, E.; Brennan, L. The Role of Protein Intake and its Timing on Body Composition and Muscle Function in Healthy Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2020, 150, 1443–1460. [Google Scholar] [CrossRef]
- Madzima, T.A.; Melanson, J.T.; Black, J.R.; Nepocatych, S. Pre-sleep consumption of casein and whey protein: Effects on morning metabolism and resistance exercise performance in active women. Nutrients 2018, 10, 1273. [Google Scholar] [CrossRef]
- Ormsbee, M.J.; Gorman, K.A.; Miller, E.A.; Baur, D.A.; Eckel, L.A.; Contreras, R.J.; Panton, L.B.; Spicer, M.T. Nighttime feeding likely alters morning metabolism but not exercise performance in female athletes. Appl. Physiol. Nutr. Metab. 2016, 41, 719–727. [Google Scholar] [CrossRef]
- Yasuda, J.; Tomita, T.; Arimitsu, T.; Fujita, S. Evenly Distributed Protein Intake over 3 Meals Augments Resistance Exercise-Induced Muscle Hypertrophy in Healthy Young Men. J. Nutr. 2020, 150, 1845–1851. [Google Scholar] [CrossRef]
- MacKenzie-Shalders, K.L.; King, N.A.; Byrne, N.M.; Slater, G.J. Increasing Protein Distribution Has No Effect on Changes in Lean Mass During a Rugby Preseason. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 1–7. [Google Scholar] [CrossRef]
- Burke, L.M. Energy Needs of Athletes. Can. J. Appl. Physiol. 2001, 26, S202–S219. [Google Scholar] [CrossRef]
- Heydenreich, J.; Kayser, B.; Schutz, Y.; Melzer, K. Total Energy Expenditure, Energy Intake, and Body Composition in Endurance Athletes Across the Training Season: A Systematic Review. Sports Med. Open 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Black, K.E.; Baker, D.F.; Sims, S.T. Nutritional Needs of the Female Athlete: Risk and Prevention of Low Energy Availability. Strength Cond. J. 2020, 42, 77–81. [Google Scholar] [CrossRef]
- Gillen, J.B.; Trommelen, J.; Wardenaar, F.C.; Brinkmans, N.Y.J.; Versteegen, J.J.; Jonvik, K.L.; Kapp, C.; de Vries, J.; van den Borne, J.J.G.C.; Gibala, M.J.; et al. Dietary Protein Intake and Distribution Patterns of Well-Trained Dutch Athletes. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 105–114. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Overall Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brown et al., 2018 [37] | Y | Y | Y | N/A | Y | Y | Y | Y | Y | Y | Positive |
| Campbell et al., 2018 [38] | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Positive |
| Houltham and Rowlands 2014 [39] | Y | Y | Y | N/A | N | Y | Y | Y | Y | Y | Positive |
| Malowany et al., 2019 [40] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Positive |
| Phillips et al., 1993 [16] | Y | Y | N/A | N/A | N | Y | Y | Y | Y | Y | Positive |
| Pihoker et al., 2019 [41] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Positive |
| Rowlands and Wadsworth 2011 [42] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Positive |
| Roy et al., 2002 [43] | Y | Y | Y | N | Y | Y | Y | Y | Y | U | Positive |
| Taylor et al., 2016 [44] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Positive |
| Tinsley et al., 2019 [45] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Positive |
| West et al., 2012 [10] | Y | Y | N/A | N/A | N | Y | Y | Y | Y | Y | Positive |
| Wilborn et al., 2013 [46] | Y | Y | Y | N | Y | Y | Y | Y | N | U | Positive |
| Wilborn et al., 2016 [47] | Y | Y | N/A | N | Y | Y | Y | Y | Y | Y | Positive |
| Wooding et al., 2017 [48] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Positive |
| Author/Year (Study Design) | Female Athletes | Menstrual Cycle/Contraceptives | Exercise Protocol | Protein Intake | Control/Comparison Intake | Outcome(s) from Protein Intake 1 |
|---|---|---|---|---|---|---|
| Daily Protein Requirements | ||||||
| Houltham and Rowlands 2014 [39] (cross-over) | 10 competitive cyclists and triathletes 61.3 ± 5.4 kg Body fat % NR | Mid-follicular (day 4–11) | 90 min cycle intervals at 50–70% VO2max for 3 days | Daily protein intake 2.7 g/kg/day (includes mean 0.75 g/kg whey protein post-exercise) (daily energy: 32% protein, 45% CHO, 23% fat) | Daily protein intake 1.4 g/kg/day (habitual) (daily energy: 16% protein, 54% CHO, 30% fat) | Positive nitrogen balance vs. negative nitrogen balance in control EAR 1.63 g/kg/day RDI 2 2.02 g/kg/day |
| Phillips et al. 1993 [16] (single intervention) 3 | Six recreationally active students 58.1 ± 5.4 kg Body fat 18.8 ± 1.7% | Mid-follicular (day 4–11) | 90 min run at 65% VO2max | Daily protein intake 0.8 g/kg/day (breakfast energy: 4% protein, 82% CHO, 14% fat) | N/A | Negative nitrogen balance |
| Rowlands and Wadsworth 2011 [42] (cross-over) | 12 competitive cyclists 60.8 ± 3.4 kg Body fat 19 ± 3% | Six mid-follicular (day 3–7), six hormonal contraceptives | 150 min cycle intervals at 50–90% Wmax day 1, sprint performance test (10 × workload max sprints) days 2 and 4 | Protein blend 0.7 g/kg/h (with 1.4 g/kg/h CHO and 0.26 g/kg/h fat; energy 30% protein, 59% CHO, 11% fat) for 4 h post-exercise with high daily carbohydrate diet | Isocaloric control, protein 0.1 g/kg/h (with 2.1 g/kg/h CHO and 0.26 g/kg/h fat; energy 4% protein, 85% CHO, 11% fat) for 4 h post-exercise with high daily carbohydrate diet | Positive nitrogen balance vs. negative nitrogen balance in control EAR 1.28 g/kg/day RDI 2 1.59 g/kg/day |
| Acute Protein Requirements | ||||||
| Roy et al. 2002 [43] (cross-over) | 10 recreationally trained endurance athletes 61.6 ± 7.6 kg Body fat 21.9 ± 1.1% | Four mid-follicular (day 4–11), six triphasic OCP | 60 min cycle at 65% VO2peak on days 1, 3, 4 and for 90 min day 6, plus cycle to exhaustion (75% VO2peak) on day 7 | Post-exercise: mixed supplement 0.24 g/kg whey protein (energy 23% protein, 66% CHO, 12% fat) non-caloric placebo 10 h pre-exercise (daily energy: 16% protein, 58% CHO, 26% fat) | Pre-exercise: mixed supplement 0.24 g/kg whey protein 10 h pre-exercise (energy 23% protein, 66% CHO, 12% fat) non-caloric placebo post-exercise (daily energy: 16% protein, 58% CHO, 26% fat) | No differences in nitrogen balance (trend for improved balance on days 6 and 7 with post-exercise) ↓ body mass loss vs. pre-exercise |
| Author/Year (Study Design) | Female Athletes | Menstrual Cycle/Contraceptives | Exercise Protocol | Protein Intake | Control/Comparison Intake | Outcome(s) from Protein Intake 1 |
|---|---|---|---|---|---|---|
| Daily Protein Requirements | ||||||
| Malowany et al. 2019 [40] (cross-over) | Eight recreationally active RT athletes 67.0 ± 7.7 kg Body fat 24.4 ± 6.9% | Luteal (days NR) | Single whole-body RT session | Isocaloric meal with 0.2–2.9 g/kg/day crystalline amino acid based on egg protein provided in eight hourly doses post-exercise (% energy NR) | N/A | EAR 1.49 g/kg/day RDI 2 1.85 g/kg/day Nitrogen balance 1.53 g/kg/day |
| Campbell et al. 2018 [38] (cohort study) | 17 physique athletes (n = 8 intervention, n = 9 control) 61.0 ± 6.1 kg Body fat 22.7 ± 3.0% | NR | Eight-week whole-body RT program, two to four sessions/week | Daily protein intake 2.5 g/kg/day (includes mean 0.41 g/kg whey protein pre- and post-exercise. Daily energy: 41% protein, 41% CHO, 18% fat) | Daily protein intake 0.9 g/kg/day (includes acute mean 0.08 g/kg pre- and post-exercise. Daily energy: 19% protein, 62% CHO, 19% fat) | ↑ maximal strength in both groups ↑ FFM higher vs. control |
| Tinsley et al. 2019 [45] (cohort study) | 17 recreationally active RT athletes (n = 9 intervention, n = 8 control) 63.9 ± 7.8 kg Body fat < 33% | NR | Eight-week whole-body RT program, three sessions/week | Daily protein intake 1.6 g/kg/day (includes mean 0.39 g/kg whey protein post-exercise. Daily energy: 27% protein, 42% CHO, 34% fat) | Time-restricted (8 h) feeding with daily protein intake 1.6 g/kg/day (includes mean 0.39 g/kg whey protein post-exercise. Daily energy: 27% protein, 39% CHO, 32% fat) | ↑ maximal strength, endurance and FFM in both groups |
| Acute Protein Requirements | ||||||
| West et al. 2012. [10] (single intervention) 3 | Eight recreationally active 67.1 ± 5.6 kg Body fat 23.1 ± 4.1% | Four pre-menopausal (phase NR), four OCP | Single lower-body RT session | 0.37 g/kg whey protein post-exercise (daily energy: 15% protein, 55% CHO, 30% fat) | N/A | ↑ MPS early (1–5 h) and late (24–28 h) post-exercise |
| Pihoker et al. 2019 [41] (cohort study) | 43 recreationally active (n = 17 pre-exercise and n = 17 post-exercise, n = 9 control) 66.5 ± 11.4 kg Body fat % NR | NR | Six-week whole-body RT program, two sessions/week | Pre-exercise group: mixed supplement 0.38 g/kg whey and casein protein Post-exercise group: mixed supplement 0.38 g/kg whey and casein protein (supplement energy: 56% protein, 36% CHO, 8% fat) | No nutrition intake | ↑ maximal upper body strength vs. control No difference in lower body strength or body composition between groups |
| Author/Year (Study Design) | Female Athletes | Menstrual Cycle/Contraceptives | Exercise Protocol | Protein Dose, Type, Timing | Control/Comparison Intake | Outcome(s) from Protein Intake 1 |
|---|---|---|---|---|---|---|
| Daily Protein Requirements | ||||||
| Wooding et al. 2017 [48] (cross-over) | Six competitive rowing, ice hockey, volleyball athletes 68.8 ± 4.1 kg Body fat 21.8 ± 2.7% | Luteal (days NR) | Modified Loughborough test (4 × 15 min variable intensity shuttle run) | Isocaloric meal with 0.2–2.66 g/kg/day crystalline amino acids based on egg protein provided in eight hourly doses post-exercise (% energy NR) | N/A | EAR 1.41 g/kg/day RDI 2 1.75 g/kg/day |
| Acute Protein Requirements | ||||||
| Brown et al. 2018 [37] (cohort) | 20 competitive dancers (n = 10 intervention, n = 10 control) 61.8 ± 7.9 kg Body fat % NR | Six luteal, 14 hormonal contraceptives (groups NR) | 15 × 30 m repeated sprints | 0.32 g/kg whey protein immediately and 2 h post-exercise (energy 91% protein, 8% CHO, 1% fat) (average daily protein intake of 1.8 g/kg/day. Daily energy: 21% protein, 63% CHO, 24% fat) | 0.32 g/kg carbohydrate immediately and 2 h post-exercise (energy 0% protein, 99.5% CHO, 0.5% fat) (daily protein intake 1.3 g/kg/day. Daily energy: 15% protein, 61% CHO, 25% fat) | ↓ decline in reactive strength index during 72 h post-exercise ↓ CK levels at 24 h post-exercise |
| Wilborn et al. 2016 [47] (single intervention) 3 | Nine resistance-trained athletes 65.1 ± 8.4 kg Body fat 25.5 ± 7.2% | NR | Eight-week whole-body intermittent exercise program, four sessions/week | 0.38 g/kg whey protein post-exercise (energy 96% protein, 4% CHO, 0% fat) (average daily protein intake 1.1 g/kg/day) | N/A | ↑ maximal strength and agility |
| Taylor et al. 2016 [44] (cohort) | 14 competitive basketballers (n = 8 intervention 66.0 ± 3.1 kg, body fat 25.4 ± 4.2%; n = 6 control 68.2 ± 7.6 kg, body fat 25.1 ± 4.7%) | NR | Eight-week whole body anaerobic, agility and RT program, four sessions/week | 0.36 g/kg whey protein pre- and post-exercise (energy 96% protein, 0% CHO, 4% fat) (average daily protein intake 1.39 g/kg/day) | 0.35 g/kg maltodextrin pre- and post-exercise (energy 0% protein, 100% CHO, 0% fat) (daily protein intake of 1.08 g/kg/day) | ↑ maximal strength and agility scores vs. control |
| Wilborn et al. 2013 [46] (cohort) | 16 competitive basketballers (n = 8 intervention 66.0 ± 4.9 kg, body fat 27.0 ± 4.9%; n = 8 comparison 68.0 ± 2.9 kg, body fat 25.0 ± 5.7%) | NR | Eight-week whole body anaerobic, agility and RT program, four sessions/week | 0.36 g/kg whey protein pre- and post-exercise (energy 83% protein, 14% CHO, 3% fat) (daily protein intake NR) | 0.35 g/kg casein protein pre- and post-exercise (energy 86% protein, 11% CHO, 4% fat) (daily protein intake NR) | ↑ maximal strength, lean mass and anaerobic performance, and ↓ in fat mass in both groups |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercer, D.; Convit, L.; Condo, D.; Carr, A.J.; Hamilton, D.L.; Slater, G.; Snipe, R.M.J. Protein Requirements of Pre-Menopausal Female Athletes: Systematic Literature Review. Nutrients 2020, 12, 3527. https://doi.org/10.3390/nu12113527
Mercer D, Convit L, Condo D, Carr AJ, Hamilton DL, Slater G, Snipe RMJ. Protein Requirements of Pre-Menopausal Female Athletes: Systematic Literature Review. Nutrients. 2020; 12(11):3527. https://doi.org/10.3390/nu12113527
Chicago/Turabian StyleMercer, Drew, Lilia Convit, Dominique Condo, Amelia J. Carr, D. Lee Hamilton, Gary Slater, and Rhiannon M. J. Snipe. 2020. "Protein Requirements of Pre-Menopausal Female Athletes: Systematic Literature Review" Nutrients 12, no. 11: 3527. https://doi.org/10.3390/nu12113527
APA StyleMercer, D., Convit, L., Condo, D., Carr, A. J., Hamilton, D. L., Slater, G., & Snipe, R. M. J. (2020). Protein Requirements of Pre-Menopausal Female Athletes: Systematic Literature Review. Nutrients, 12(11), 3527. https://doi.org/10.3390/nu12113527








