The Role of Omega-3 Polyunsaturated Fatty Acids from Different Sources in Bone Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diet Preparation and Composition
2.3. Lipid Extraction and Analysis by Gas Chromatography (GC)
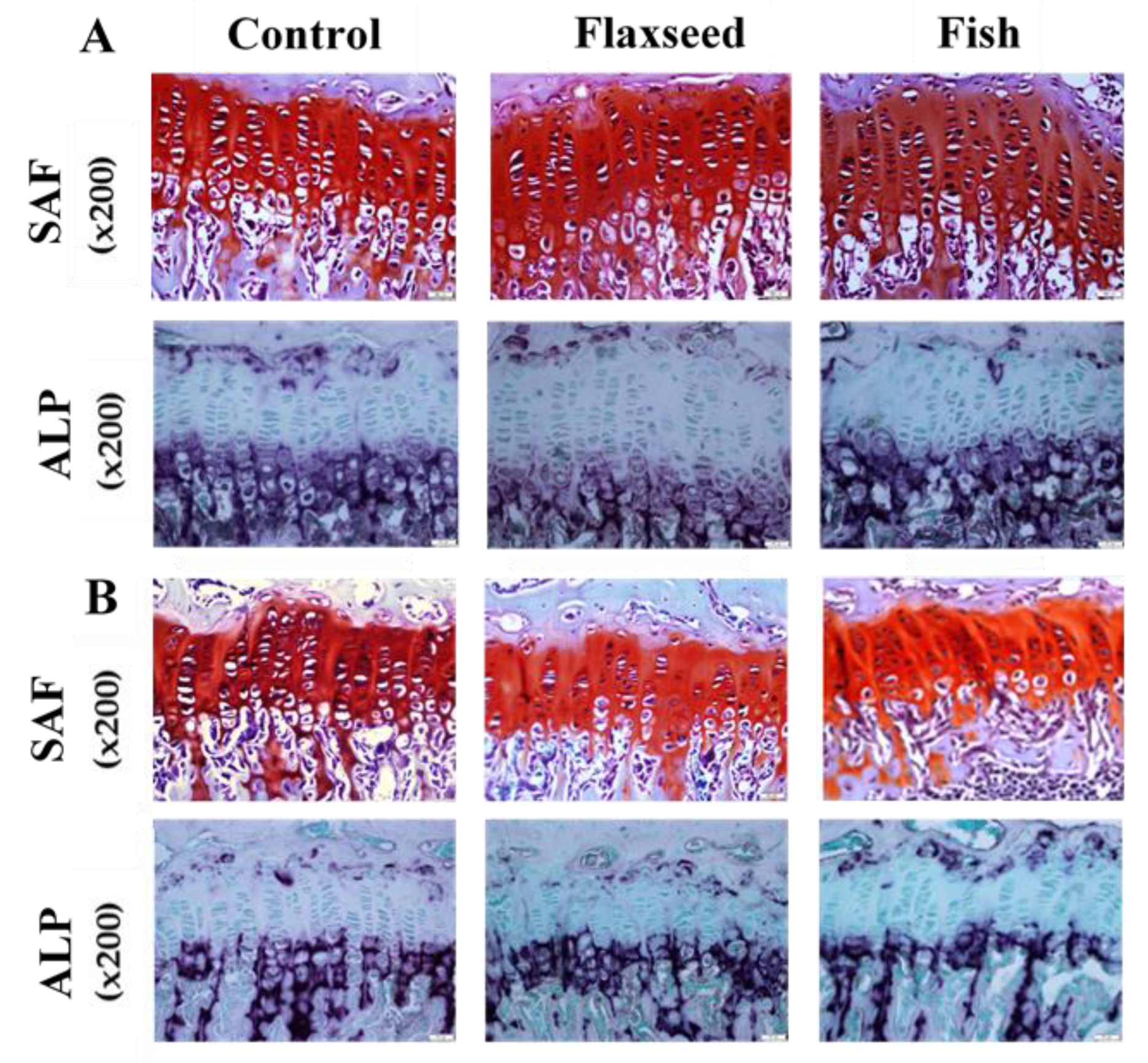
2.4. Histological Staining of Growth-Plate (GP) Sections
2.5. Imaging and Measurement of GPs
2.6. Micro-CT
2.7. Mechanical Testing
2.8. Bone RNA Extraction and RNA-Sequencing
2.8.1. Bioinformatics
2.8.2. Gene-Set Enrichment Analysis
2.9. Statistical Analysis
3. Results
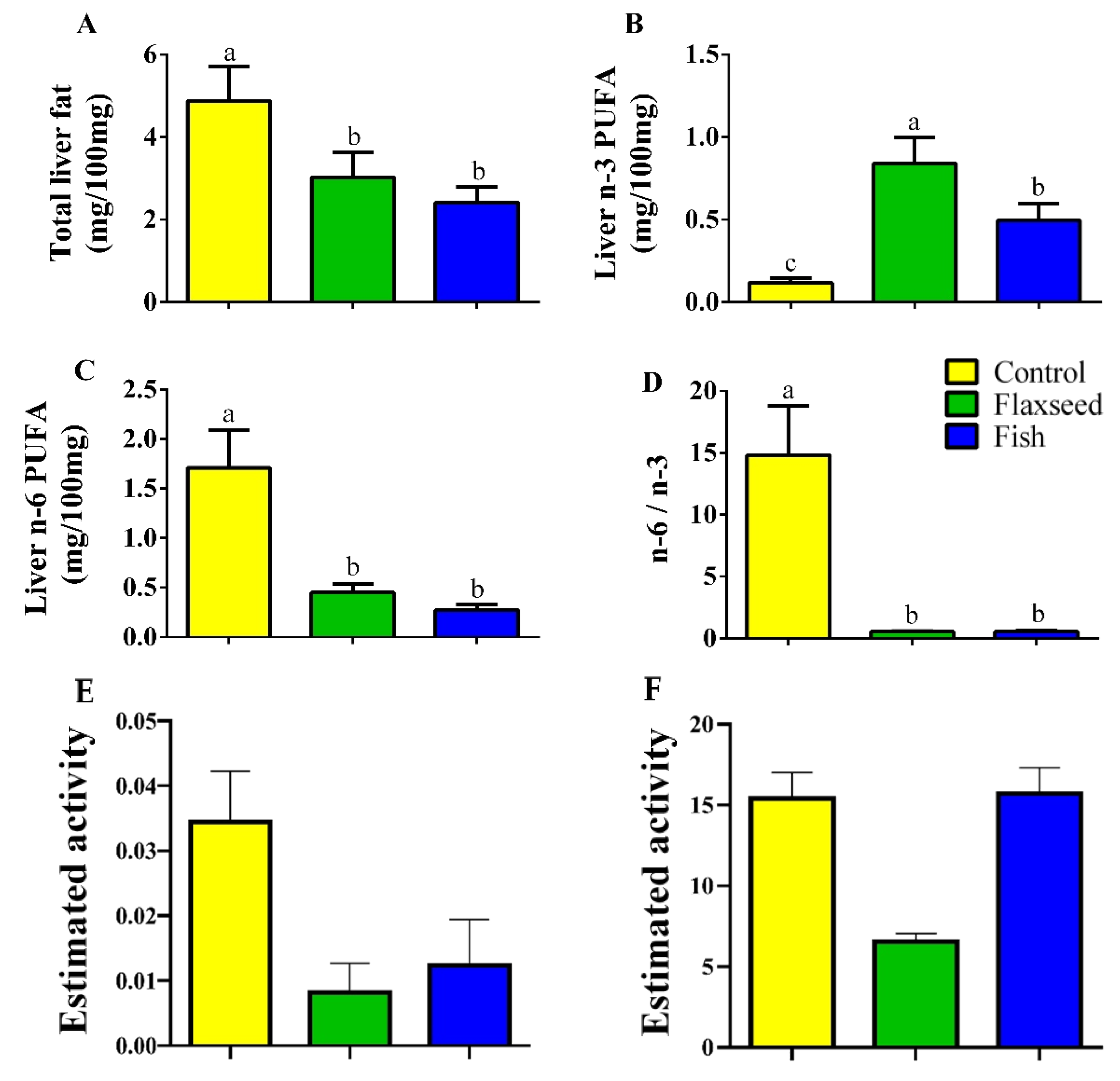
3.1. Dietary n-3 PUFAs Decrease Fat Accumulation and Alter FA Content in Liver and Serum
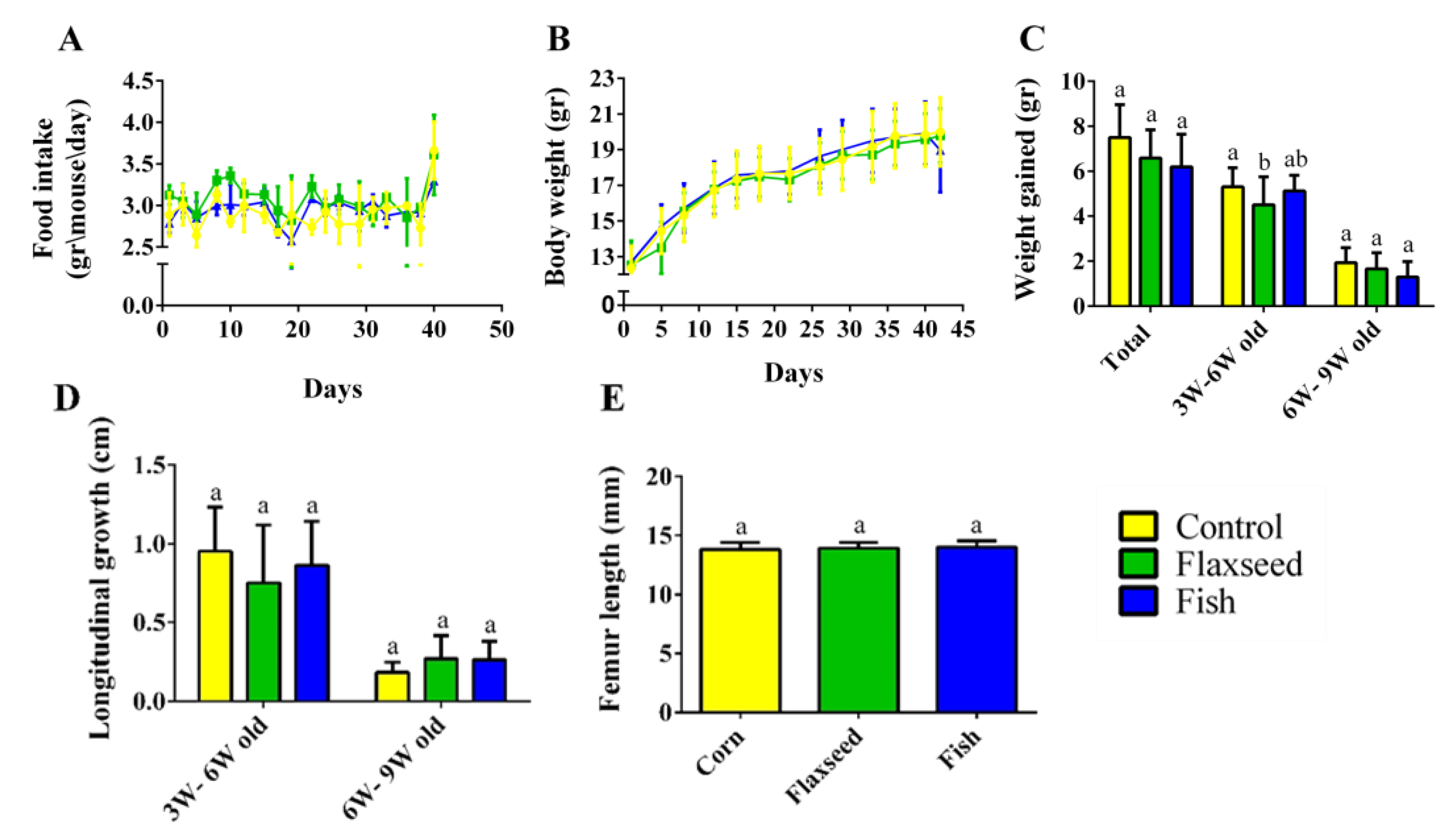
3.2. Effect of Omega-3 from Different Sources on Growth Pattern and Food Consumption
3.3. The Effect of Diets Rich in n-3 PUFAs on Bone Quality
3.3.1. Diets Rich in n-3 PUFAs Improve Bone Mechanical Properties
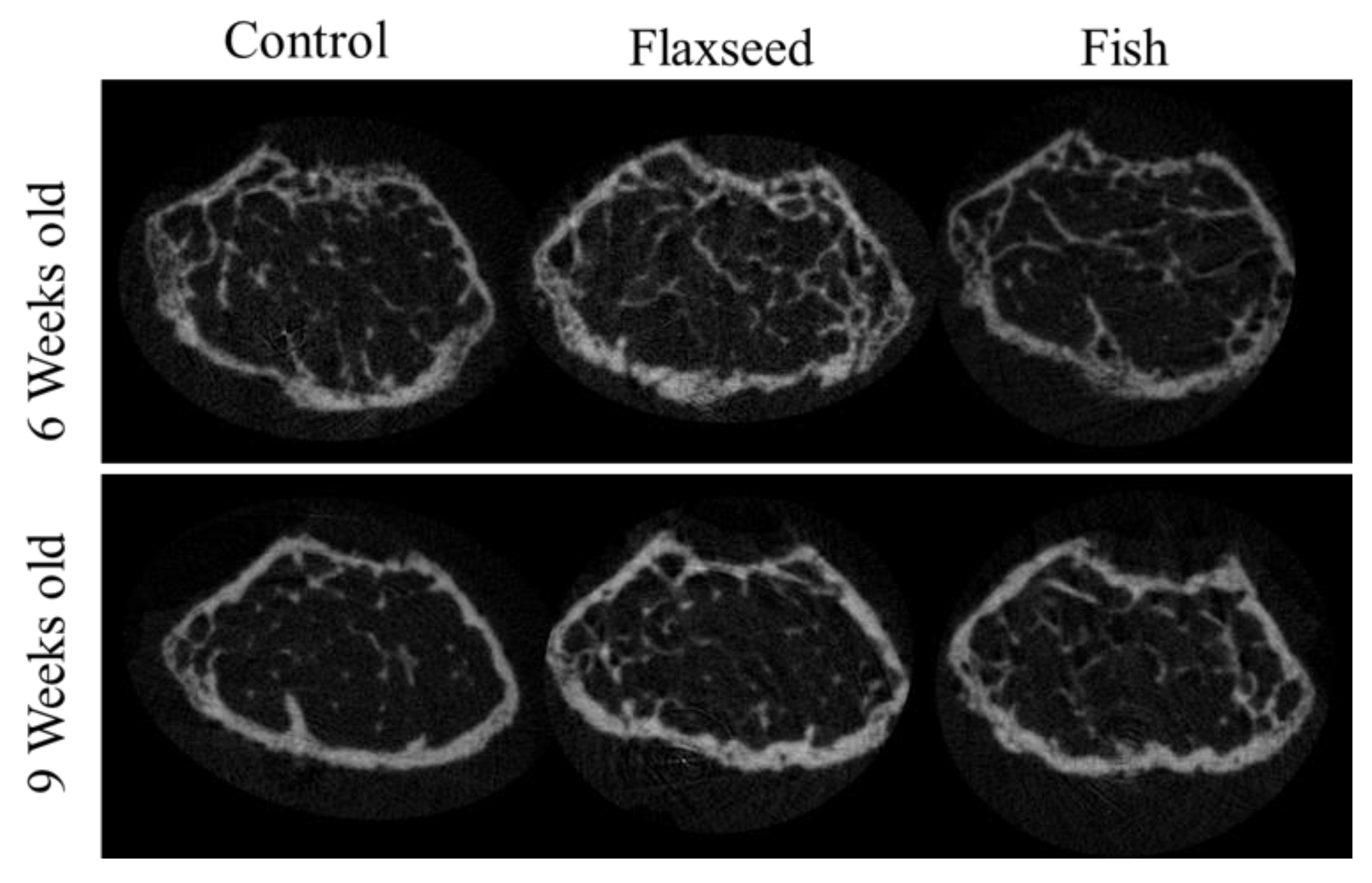
3.3.2. Diet Containing Plant Source of n-3 PUFAs (Flaxseed Oil) Improved Trabecular Bone’s Micro-Architecture
3.3.3. Diet Containing n-3 PUFAs from an Animal Source (Fish Oil) Increased BMD
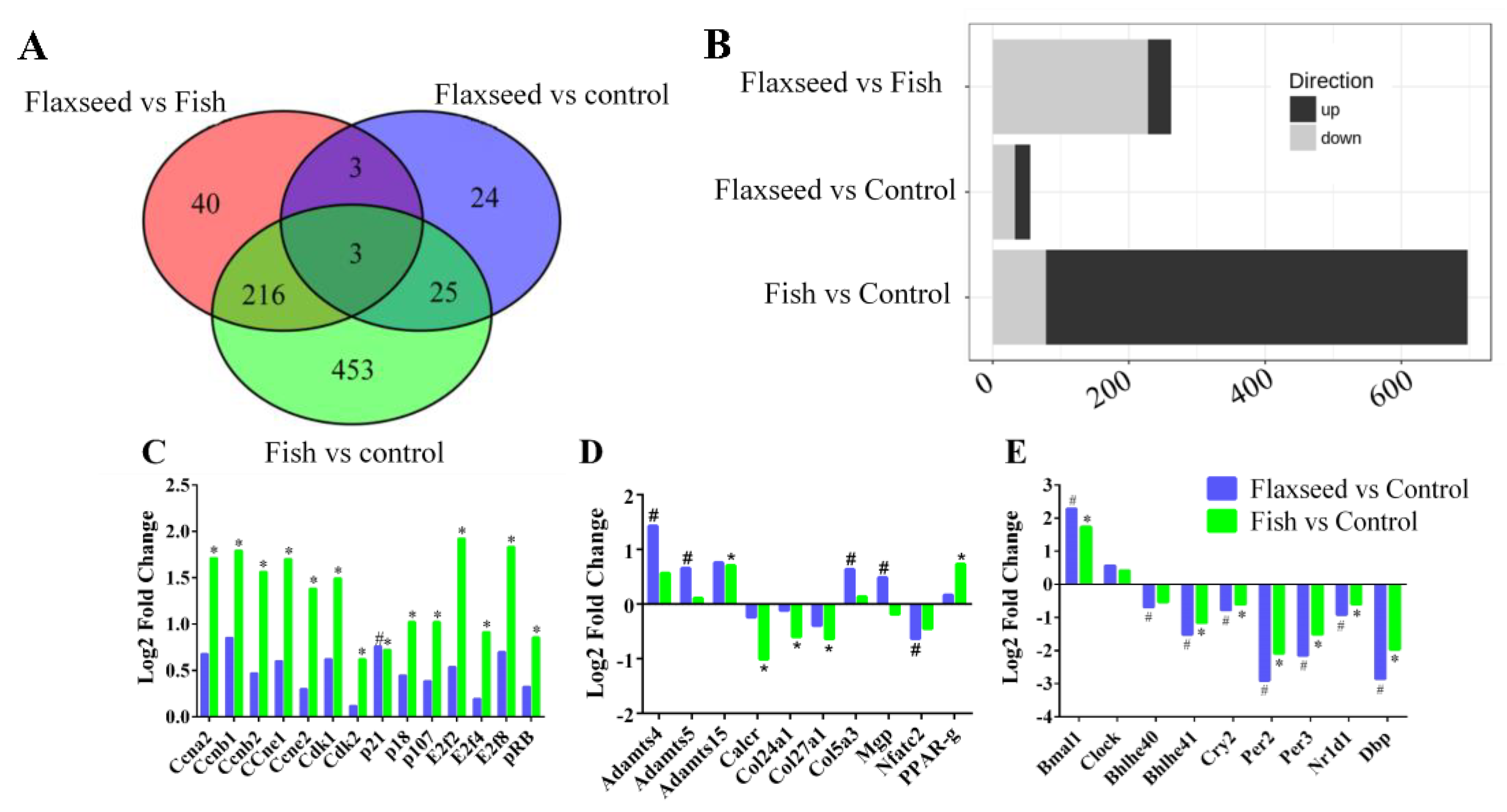
3.4. The Effect of Different Sources of n-3 PUFAs on Bone’s Transcriptional Regulation
IPA Pathway Enrichment Analysis of Genes Related to Dietary n-3 PUFAs
4. Discussion
4.1. Dietary n-3 PUFAs Decrease Hepatic and Serum Fat Levels
4.2. Diet Rich in n-3 PUFAs Affects Postnatal Skeletal Development and Bone Characteristics
4.3. Transcriptional Regulation of Bone by Different Sources n-3 PUFAs
4.4. Dietary n-3 PUFAs Alter Circadian Clock Gene Expression in Bone
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Karsenty, G. The complexities of skeletal biology. Nature 2003, 423, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.M.; Duckham, R.L.; Gianoudis, J. Evidence for an Interaction Between Exercise and Nutrition for Improving Bone and Muscle Health. Curr. Osteoporos. Rep. 2014, 12, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Longo, A.B.; Ward, W.E. PUFAs, Bone Mineral Density, and Fragility Fracture: Findings from Human Studies. Adv. Nutr. 2016, 7, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.; Ferrari, S.; Dempster, D.W. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2016, 8, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Genuis, S.J.; Schwalfenberg, G.K. Picking a bone with contemporary osteoporosis management: Nutrient strategies to enhance skeletal integrity. Clin. Nutr. 2007, 26, 193–207. [Google Scholar] [CrossRef]
- Högström, M.; Nordström, P.; Nordström, A. n-3 fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: The NO2 study. Am. J. Clin. Nutr. 2007, 85, 803–807. [Google Scholar] [CrossRef]
- Lukas, R.; Gigliotti, J.C.; Smith, B.J.; Altman, S.; Tou, J.C. Consumption of different sources of omega-3 polyunsaturated fatty acids by growing female rats affects long bone mass and microarchitecture. Bone 2011, 49, 455–462. [Google Scholar] [CrossRef]
- López-vicario, C.; González-périz, A.; Rius, B.; Morán-salvador, E.; García-alonso, V.; Lozano, J.J.; Bataller, R.; Kang, J.X.; Arroyo, V.; Clària, J.; et al. Molecular interplay between Δ5/Δ6 desaturases and long-chain fatty acids in the pathogenesis of non-alcoholic steatohepatitis. Gut 2014, 63, 344–355. [Google Scholar] [CrossRef]
- Poudyal, H.; Panchal, S.K.; Diwan, V.; Brown, L. Omega-3 fatty acids and metabolic syndrome: Effects and emerging mechanisms of action. Prog. Lipid Res. 2011, 50, 372–387. [Google Scholar] [CrossRef]
- Narayan, B.; Miyashita, K.; Hosokawa, M. Physiological effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—A review. Food Rev. Int. 2006, 22, 291–307. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.C.; Coetzee, M.; Haag, M.; Weiler, H. Long-chain polyunsaturated fatty acids: Selected mechanisms of action on bone. Prog. Lipid Res. 2010, 49, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. Oléagineux Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Tur, J.A.; Bibiloni, M.M.; Sureda, A.; Pons, A. Dietary sources of omega 3 fatty acids: Public health risks and benefits. Br. J. Nutr. 2012, 107, S23–S52. [Google Scholar] [CrossRef] [PubMed]
- Koren, N.; Simsa-Maziel, S.; Shahar, R.; Schwartz, B.; Monsonego-Ornan, E. Exposure to omega-3 fatty acids at early age accelerate bone growth and improve bone quality. J. Nutr. Biochem. 2014, 25, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G. Committee Report AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Mesilati-stahy, R.; Mida, K.; Argov-argaman, N. Size-Dependent Lipid Content of Bovine Milk Fat Globule and Membrane Phospholipids. J. Agric. Food Chem. 2011, 59, 7427–7435. [Google Scholar] [CrossRef]
- Simsa-maziel, S.; Zaretsky, J.; Reich, A.; Koren, Y.; Shahar, R.; Monsonego-ornan, E. IL-1RI participates in normal growth plate development and bone modeling. Am. J. Physiol. Metab. 2013, 305, E15–E21. [Google Scholar] [CrossRef]
- Idelevich, A.; Kerschnitzki, M.; Shahar, R.; Monsonego-ornan, E. 1,25(OH)2D3 Alters Growth Plate Maturation and Bone Architecture in Young Rats with Normal Renal Function. PLoS ONE 2011, 6, e20772. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Mu, R. Guidelines for Assessment of Bone Microstructure in Rodents Using Micro—Computed Tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Sharir, A.; Max, M.; Shahar, R. Whole bone mechanics and mechanical testing. Veter. J. 2008, 177, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ayturk, U.M.; Jacobsen, C.M.; Christodoulou, D.C.; Gorham, J.; Seidman, J.G.; Seidman, C.E.; Robling, G.; Warman, M.L. An RNA-seq Protocol to Identify mRNA Expression Changes in Mouse Diaphyseal Bone: Applications in Mice with Bone Property Altering Lrp5 Mutations. J. Bone Miner. 2013, 28, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Saeidipour, B.; Bakhshi, S. The relationship between organizational culture and knowledge management and their simultaneous effects on customer relation management. Adv. Environ. Biol. 2013, 7, 2803–2809. [Google Scholar]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Köster, J.; Rahmann, S. Snakemake—A scalable bioinformatics workflow engine. Bioinformatics 2012, 28, 2520–2522. [Google Scholar] [CrossRef]
- Awada, M.; Soulage, C.O.; Meynier, A.; Debard, C.; Plaisancié, P.; Benoit, B.; Picard, G.; Loizon, E.; Chauvin, M.; Estienne, M.; et al. Dietary oxidized n-3 PUFA induce oxidative stress and infl ammation: Role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J. Lipid Res. 2012, 53, 2069–2080. [Google Scholar] [CrossRef]
- Cho, H.P.; Nakamura, M.T.; Clarke, S.D. Cloning, Expression, and Nutritional Regulation of the Mammalian Δ-6 desaturase. J. Biol. Chem. 1999, 274, 471–477. [Google Scholar] [CrossRef]
- Wit, J.M.; Camacho-Hübner, C. Endocrine regulation of longitudinal bone growth. Endocr. Dev. 2011, 21, 30–41. [Google Scholar] [CrossRef]
- Golub, E.E.; Boesze-battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Glatt, V.; Canalis, E.; Stadmeyer, L.; Bouxsein, M.L. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Miner. Res. 2007, 22, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.Y.Y.; Ward, W.E.; Kang, J.X.; Ma, D.W.L. Femur EPA and DHA are correlated with femur biomechanical strength in young fat-1 mice. J. Nutr. Biochem. 2009, 20, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Dodin, S.; Lemay, A.; Jacques, H.; Le, F. The Effects of Flaxseed Dietary Supplement on Lipid Profile, Bone Mineral Density, and Symptoms in Menopausal Women: A Randomized, Double-Blind, Wheat Germ Placebo-Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 2015, 90, 1390–1397. [Google Scholar] [CrossRef]
- Lau, B.Y.Y.; Cohen, D.J.A.; Ward, W.E.; Ma, D.W.L. Investigating the Role of Polyunsaturated Fatty Acids in Bone Development Using Animal Models. Molecules 2013, 18, 14203–14227. [Google Scholar] [CrossRef]
- Khadge, S.; Graham, J.; Thiele, G.M.; Mcguire, T.R.; Klassen, L.W.; Duryee, M.J.; Britton, H.C.; Dafferner, A.J.; Beck, J.; Black, P.N.; et al. ScienceDirect Dietary omega-3 and omega-6 polyunsaturated fatty acids modulate hepatic pathology. J. Nutr. Biochem. 2018, 52, 92–102. [Google Scholar] [CrossRef]
- Huang, W. Endogenously elevated n-3 polyunsaturated fatty acids alleviate acute ethanol-induced liver steatosis. BioFactors 2015, 453–462. [Google Scholar] [CrossRef]
- Tou, J.C.; Altman, S.N.; Gigliotti, J.C.; Benedito, V.A.; Cordonier, E.L. Different sources of omega-3 polyunsaturated fatty acids affects apparent digestibility, tissue deposition, and tissue oxidative stability in growing female rats. Lipids Health Dis. 2011, 10, 179. [Google Scholar] [CrossRef]
- Food and Nutrition Board; Institute of Medicine; National Academy of Science. Dietary Reference Intakes (DRIs): Recommended Dietary Allowances and Adequate Intakes, Vitamins; The National Academic Press: Washington, DC, USA, 2015; ISBN 9780323340755. [Google Scholar]
- Wang, M.; Zhang, X.; Ma, L.; Feng, R.; Yan, C.; Su, H.; He, C.; Kang, J.X.; Liu, B.; Wan, J. Omega-3 polyunsaturated fatty acids ameliorate ethanol-induced adipose hyperlipolysis: A mechanism for hepatoprotective e ff ect against alcoholic liver disease. BBA Mol. Basis Dis. 2017, 1863, 3190–3201. [Google Scholar] [CrossRef]
- Peltier, S.; Portois, L.; Malaisse, W.J.; Carpentier, Y.A. Fatty acid profile of plasma and liver lipids in mice depleted in long-chain polyunsaturated (n-3) fatty acids. Int. J. Mol. Med. 2008, 22, 559–563. [Google Scholar] [CrossRef]
- Oh, D.Y.; Walenta, E. Omega-3 fatty acids and FFAR4. Front. Endocrinol. 2014, 5, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Park, S.Y.; Baek, J.E.; Lee, S.Y.; Baek, W.Y.; Lee, S.Y.; Lee, Y.S.; Yoo, H.J.; Kim, H.; Lee, S.H.; et al. Free fatty acid receptor 4 (GPR120) stimulates bone formation and suppresses bone resorption in the presence of elevated n-3 fatty acid levels. Endocrinology 2016, 157, 2621–2635. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H.; Van Der Meulen, M.C.H. Whole bone mechanics and bone quality. Clin. Orthop. Relat. Res. 2011, 469, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, R.A.; Nowlan, N.C.; Kenny, E.M.; Cormican, P.; Morris, D.W.; Prendergast, P.J.; Kelly, D.; Murphy, P. Identification of mechanosensitive genes during skeletal development: Alteration of genes associated with cytoskeletal rearrangement and cell signalling pathways. BMC Genom. 2014, 15, 48. [Google Scholar] [CrossRef]
- Zhao, X. Identification of key genes and pathways associated with osteogenic differentiation of adipose stem cells. J. Cell. Physiol. 2018, 233, 9777–9785. [Google Scholar] [CrossRef]
- Ho, X.D.; Phung, P.; Le, V.Q.; Nguyen, V.H.; Reimann, E.; Prans, E.; Kõks, G.; Maasalu, K.; Le, N.T.; Trinh, L.H.; et al. Whole transcriptome analysis identifies differentially regulated networks between osteosarcoma and normal bone samples. Exp. Biol. Med. 2017, 242, 1802–1811. [Google Scholar] [CrossRef]
- Articles, R. Osteoblast Differentiation at a Glance. Med Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and in fl ammation. BBA Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Nakanishi, A.; Tsukamoto, I. N-3 polyunsaturated fatty acids stimulate osteoclastogenesis through PPARγ-mediated enhancement of c-Fos expression, and suppress osteoclastogenesis through PPARγ-dependent inhibition of NFkB activation. J. Nutr. Biochem. 2015, 26, 1317–1327. [Google Scholar] [CrossRef]
- Turner, A.G.; Tjahyono, F.; Chiu, W.S.M.; Skinner, J.; Sawyer, R.; Moore, A.J.; Morris, H.A.; Findlay, D.M.; Zajac, J.D.; Davey, R.A. The role of the calcitonin receptor in protecting against induced hypercalcemia is mediated via its actions in osteoclasts to inhibit bone resorption. Bone 2011, 48, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.; Pearson, S.; Whittingham-Dowd, J.; Allen, J. PPARγ as a molecular target of EPA anti-inflammatory activity during TNF-α-impaired skeletal muscle cell differentiation. J. Nutr. Biochem. 2012, 23, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T. Circadian clock and bone biology. J. Oral Biosci. 2017, 59, 179–183. [Google Scholar] [CrossRef]
- Oosterman, J.E.; Kalsbeek, A.; Fleur, S.E.; Belsham, D.D. Impact of nutrients on circadian rhythmicity. Am. J. Physiol. Integr. Comp. Physiol. 2015, 308, R337–R350. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ochi, H.; Fukuda, T.; Sato, S.; Sunamura, S.; Takarada, T.; Hinoi, E.; Okawa, A.; Takeda, S. Circadian Clock Regulates Bone Resorption in Mice. J. Bone Miner. Res. 2016, 31, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Takarada, T.; Xu, C.; Ochi, H.; Nakazato, R.; Yamada, D.; Nakamura, S.; Kodama, A.; Shimba, S.; Mieda, M.; Fukasawa, K.; et al. Bone Resorption Is Regulated by Circadian Clock in Osteoblasts. J. Bone Miner. Res. 2017, 32, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Andreani, T.S.; Itoh, T.Q.; Yildirim, E.; Hwangbo, D.-S.; Allada, R. Genetics of Circadian Rhythms. Sleep Med. Clin. 2015, 10, 413–421. [Google Scholar] [CrossRef]
- Fu, L.; Patel, M.S.; Bradley, A.; Wagner, E.F.; Karsenty, G. Leptin-Regulated Bone Formation Bone Disease Program of Texas. Cell 2005, 122, 803–815. [Google Scholar] [CrossRef]
- Goldman, A.L.; Donlon, C.M.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Copeland, T.; Yu, C.Y.; Leboff, M.S. VIT amin D and OmegA-3 TriaL (VITAL) bone health ancillary study: Clinical factors associated with trabecular bone score in women and men. Osteoporos. Int. 2018, 29, 2505–2515. [Google Scholar] [CrossRef]
- Kawai, M.; Rosen, C.J. PPARγ: A circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 2010, 6, 629–636. [Google Scholar] [CrossRef]
- Bunney, P.E.; Zink, A.N.; Holm, A.A.; Billington, C.J.; Kotz, C.M. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kriebs, A.; Jordan, S.D.; Soto, E.; Henriksson, E.; Sandate, C.R.; Vaughan, M.E.; Chan, A.B.; Duglan, D.; Papp, S.J.; Huber, A.L.; et al. Circadian repressors CRY1 and CRY2 broadly interact with nuclear receptors and modulate transcriptional activity. Proc. Natl. Acad. Sci. USA 2017, 114, 8776–8781. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Goldbeter, A. Entrainment of the mammalian cell cycle by the circadian clock: Modeling two coupled cellular rhythms. PLoS Comput. Biol. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cho, H.; Yu, R.T.; Atkins, A.R.; Downes, M.; Evans, R.M. Nuclear receptors rock around the clock. EMBO Rep. 2014, 15, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, T. Cell cycle control factors and skeletal development. Jpn. Dent. Sci. Rev. 2013, 49, 79–87. [Google Scholar] [CrossRef]
- Berman, S.D.; Yuan, T.L.; Miller, E.S.; Lee, E.Y.; Caron, A.; Lees, J.A. The Retinoblastoma Protein Tumor Suppressor Is Important for Appropriate Osteoblast Differentiation and Bone Development. Mol. Cancer Res. 2008, 6, 1440–1452. [Google Scholar] [CrossRef]
- Verlinden, L.; Eelen, G.; Beullens, I.; Van Camp, M.; Van Hummelen, P.; Engelen, K.; Van Hellemont, R.; Marchal, K.; De Moor, B.; Foijer, F.; et al. Characterization of the Condensin Component Cnap1 and Protein Kinase Melk as Novel E2F Target Genes Down-regulated by 1, 25-Dihydroxyvitamin D3. J. Biol. Chem. 2005, 280, 37319–37330. [Google Scholar] [CrossRef]
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A Systemic Review of the Roles of n-3 Fatty Acids in Health and Disease. J. Am. Diet. Assoc. 2009, 109, 668–679. [Google Scholar] [CrossRef]
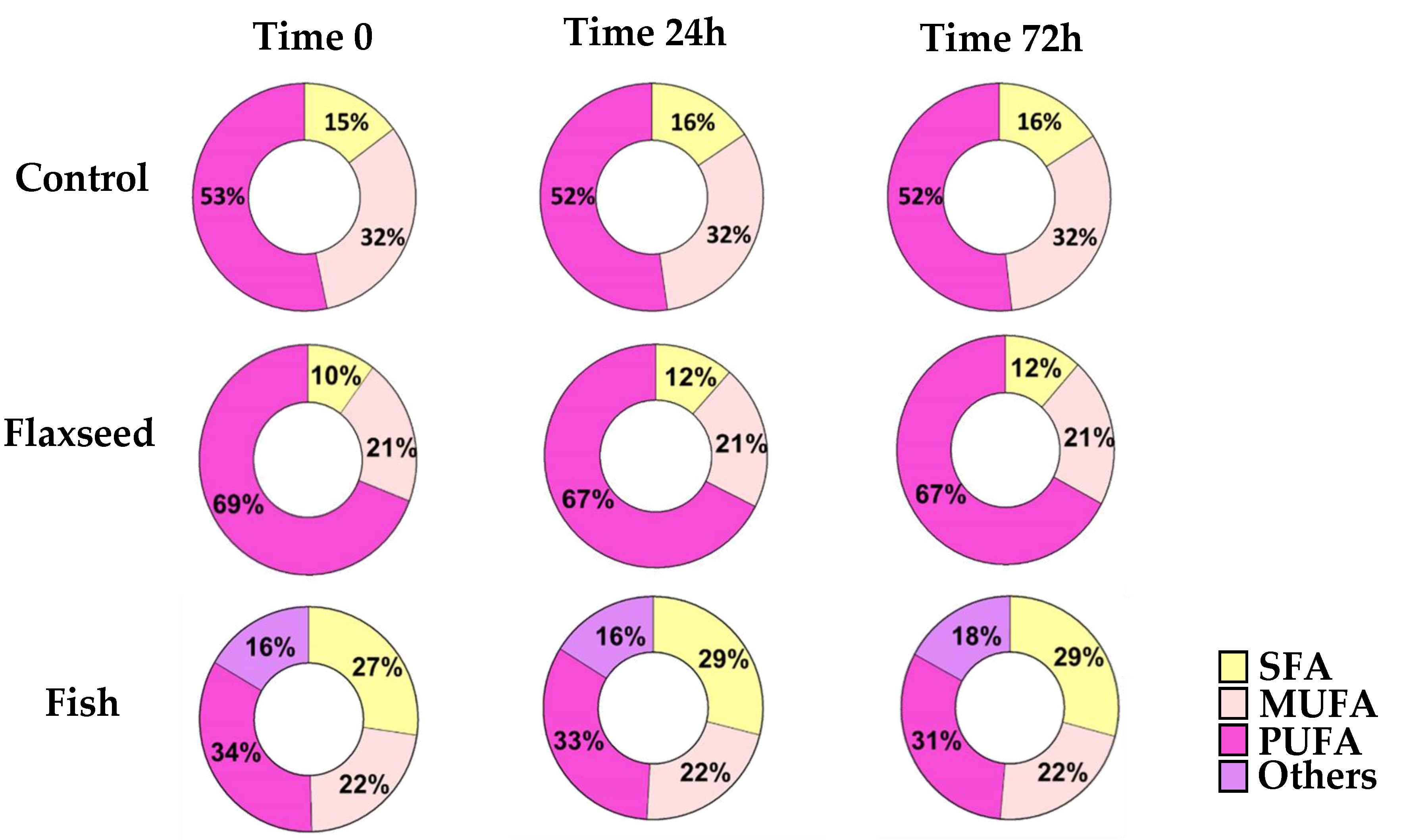

| Ingredient | g/kg diet | Kcal/kg |
|---|---|---|
| Cornstarch | 397.486 | 1590 |
| Casein (≥85% protein) | 200 | 800 |
| Dextrinized cornstarch (90–94% tetrasaccharides) | 132 | 528 |
| Sucrose | 100 | 400 |
| Corn oil\flaxseed oil\fish oil | 70 | 630 |
| Fiber | 50 | |
| Mineral mix (AIN-93G-MX) | 35 | |
| Vitamin mix (AIN-93-VX) | 10 | |
| L-Cystine | 3 | 12 |
| Choline bitartrate (41.1% choline) | 2.5 | |
| Tert- butylhydroquinone | 0.014 |
| Fatty Acids (gr\mouse) | Diet | ||
|---|---|---|---|
| Control | Flaxseed | Fish | |
| SFAs | 1.68 | 1.05 | 3.49 |
| MUFAs | 3.21 | 1.88 | 2.65 |
| PUFAs | 5.87 | 7.69 | 3.54 |
| Total n-6 PUFAs | 5.74 | 1.77 | 0.5 |
| Total n-3 PUFAs | 0.12 | 5.89 | 3.01 |
| Fatty Acid | Liver | Serum | ||||
|---|---|---|---|---|---|---|
| Control (mg/100 mg) | Flaxseed (mg/100 mg) | Fish (mg/100 mg) | Control (mg/mL) | Flaxseed (mg/mL) | Fish (mg/mL) | |
| Total n-3 | 0.118 (0.02) b | 0.839 (0.15) a | 0.494 (0.10) b | 0.100 (0.03) b | 0.535 (0.07) a | 0.522 (0.09) a |
| 18:3 ALA (n-3) | 0.011 (0) b | 0.539 (0.12) a | 0.015 (0) b | 0.005 (0.00) b | 0.232 (0.03) a | 0.014 (0.00) b |
| 20:5 EPA (n-3) | 0.002 (0) b | 0.158 (0.02) a | 0.157 (0.04) a | 0.003 (0.00) c | 0.184 (0.03) b | 0.241 (0.03) a |
| 22:6 DHA (n-3) | 0.082 (0.02) c | 0.132 (0.02) b | 0.316 (0.05) a | 0.066 (0.03) b | 0.102 (0.01) b | 0.252 (0.05) a |
| Total n-6 | 1.710 (0.38) a | 0.449 (0.08) b | 0.274 (0.05) b | 1.381 (0.36) a | 0.587 (0.08) b | 0.430 (0.09) b |
| 18:2 LA (n-6) | 1.282 (0.34) a | 0.376 (0.07) b | 0.176 (0.04) b | 0.820 (0.31) a | 0.441 (0.07) b | 0.240 (0.06) b |
| 20:4 AA (n-6) | 0.343 (0.05) a | 0.059 (0.01) b | 0.086 (0) b | 0.480 (0.04) a | 0.101 (0.01) c | 0.147 (0.03) b |
| SFA | 1.434 (0.27) a | 0.921 (0.16) b | 1.038 (0.12) b | 0.984 (0.22) a | 0.737 (0.09) b | 0.787 (0.14) ab |
| 14:0 Mytistic | 0.030 (0.01) a | 0.016 (0) b | 0.025 (0) ab | 0.016 (0) a | 0.013 (0) a | 0.016 (0) a |
| 16:0 Palmitic | 1.104 (0.24) a | 0.640 (0.11) b | 0.747 (0.10) b | 0.658 (0.18) a | 0.433 (0.06) b | 0.541 (0.10) ab |
| 18:0 Stearic | 0.294 (0.03) a | 0.261 (0.04) a | 0.261 (0.01) a | 0.300 (0.03) a | 0.270 (0.03) a | 0.220 (0.03) b |
| 20:0 Arachidic | 0.001 (0) a | 0 (0) b | 0.001 (0) ab | 0.002 (0) a | 0 (0) a | 0 (0) a |
| MUFA | 1.619 (0.29) a | 0.815 (0.23) b | 0.615 (0.12)b | 0.582 (0.21) a | 0.372 (0.05) b | 0.389 (0.09) b |
| 16:1 Palmitoleic | 0.187 (0.04) a | 0.108 (0.03) b | 0.132 (0.04) b | 0.061 (0.01) ab | 0.046 (0.01) b | 0.079 (0.02) a |
| 18:1 Vaccenic | 0.003 (0) a | 0 (0) b | 0.002 (0) b | 0.008 (0.01) a | 0.004 (0.01) a | 0.002 (0) a |
| 18:1 Oleic (n-9) | 1.315 (0.24) a | 0.659 (0.18) b | 0.432 (0.06) b | 0.455 (0.18) a | 0.284 (0.03) b | 0.267 (0.06) b |
| Total lipids | 4.873 (0.82) a | 3.020 (0.60) b | 2.418 (0.37) b | 3.023 (0.82) a | 2.216 (0.28) b | 2.116 (0.41) b |
| Control | ||
| 18:3 ALA (n-3) | 1.70 | 0.60 |
| 20:5 EPA (n-3) | 0.05 | 0.11 |
| 22:6 DHA (n-3) | 0.37 | 4.55 |
| 18:2 LA (n-6) | 97.45 | 70.47 |
| 20:4 AA (n-6) | 0.00 | 18.89 |
| Flaxseed | ||
| 18:3 ALA (n-3) | 76.55 | 41.89 |
| 20:5 EPA (n-3) | 0.04 | 12.32 |
| 22:6 DHA (n-3) | 0.09 | 10.25 |
| 18:2 LA (n-6) | 22.76 | 29.20 |
| 20:4 AA (n-6) | 0.01 | 4.63 |
| Fish | ||
| 18:3 ALA (n-3) | 3.07 | 1.98 |
| 20:5 EPA (n-3) | 56.44 | 20.65 |
| 22:6 DHA (n-3) | 25.7 | 41.68 |
| 18:2 LA (n-6) | 8.61 | 23.16 |
| 20:4 AA (n-6) | 3.48 | 11.40 |
| 6 Weeks Old | 9 Weeks Old | |||||
| Width (µm) | Control | Flaxseed | Fish | Control | Flaxseed | Fish |
| Total | 119.9 (11.8) b | 134.0 (19) a | 128.2 (19.5) a | 89.8 (9.4) b | 101.0(7.4) a | 101.1 (10.6) a |
| PZ | 56.3 (9.9) b | 65.1 (13.1) a | 60.1 (13.8) ab | 44.4 (7.9) b | 51.8 (6.6) a | 51.2 (7.7) a |
| HZ | 63.6 (9.3) a | 68.9 (14.2) a | 68.0 (15.9) a | 45.4 (7.6) b | 49.2 (5.9) a | 49.9 (8.1) a |
| No. of cells | Control | Flaxseed | Fish | Control | Flaxseed | Fish |
| Total | 12.2 (0.4) a | 11.9 (0.7) a | 12.2 (1.2) a | 10.4 (0.8) a | 10.3 (0.7) a | 10.4 (0.5) a |
| PZ | 8.6 (0.6) a | 7.9 (0.6) a | 8.3 (0.6) a | 7.1 (0.6) a | 6.6 (0.7) a | 6.9 (0.1) a |
| HZ | 3.6 (0.2) a | 4.0 (0.5) a | 3.9 (0.8) a | 3.3 (0.3) a | 3.6 (0.2) a | 3.5 (0.5) a |
| 6 Weeks Old | 9 Weeks Old | |||||
| Bone Mechanical Properties | Control | Flaxseed | Fish | Control | Flaxseed | Fish |
| Stiffness (N/µ) | 0.031 (0.005) b | 0.038 (0.002) ab | 0.042 (0.009) a | 0.063 (0.013) | 0.062 (0.011) | 0.052 (0.01) |
| Max load (N) | 9.025 (0.82) b | 11.264 (1.566) a | 12.022 (1.137) a | 15.474 (1.826) | 15.082 (0.963) | 14.121 (2.905) |
| Yield point (N) | 5.07 (0.698) | 6.092 (1.537) | 7.228 (2.426) | 6.628 (1.668) | 7.05 (1.657) | 6.395 (1.037) |
| Young’s modulus | 0.405 (0.103) b | 0.522 (0.118) ab | 0.613 (0.178) a | 0.994(0.305) | 0.895(0.304) | 0.72(0.427) |
| Trabecular Bone Microarchitecture | Control | Flaxseed | Fish | Control | Flaxseed | Fish |
| BV/TV % | 8.04 (1.35) b | 10.28 (0.8) a | 8.81 (1.39) ab | 6.02 (1.06) b | 8.10 (1.32) a | 6.87 (1.82) ab |
| Tb.Th (mm) | 0.03 (0) | 0.04 (0) | 0.03 (0) | 0.04 (0) | 0.04 (0) | 0.04 (0) |
| Tb.Sp (mm) | 0.22 (0.01) | 0.20 (0.02) | 0.21 (0.03) | 0.27 (0.03) | 0.24 (0.03) | 0.26 (0.03) |
| Tb.N (1/mm) | 2.21 (0.33) b | 2.75 (0.41) a | 2.48 (0.46) ab | 1.54 (0.25) b | 2.00 (0.32) a | 1.76 (0.46) ab |
| Cortical Bone Microarchitecture | Control | Flaxseed | Fish | Control | Flaxseed | Fish |
| B.Ar/T.Ar (%) | 30.22 (1.88) | 31.67 (1.77) | 30.61 (2.79) | 33.18 (1.89) | 35.66 (2.06) | 34.52 (3.19) |
| M.Ar (mm2) | 1.39 (0.16) | 1.38 (0.11) | 1.36 (0.09) | 1.36 (0.1) | 1.26 (0.1) | 1.37 (0.11) |
| Cs.Th (mm) | 0.11 (0.01) | 0.12 (0) | 0.11 (0.01) | 0.12 (0.01) | 0.13 (0) | 0.13 (0.02) |
| BMD (gr/cm3) | 1.17 (0.07) | 1.21 (0.06) | 1.23 (0.08) | 1.33 (0.05) b | 1.30 (0.05) b | 1.41 (0.06) a |
| Comparison | Pathway | Molecules |
|---|---|---|
| Flaxseed vs. control | Circadian Rhythm Signaling | Bmal, bhlhe40, cry2, nr1d1, per2, per3, dpb |
| Glutathione Redox Reactions | Gstt1, gstt2/gstt2b | |
| Coagulation System | Plat, serpine1 | |
| Adipogenesis pathway | Bmal1, nr1d2, per2 | |
| Role of Osteoblasts, Osteoclasts and Chondrocytes | Adamts4, adamts5, nfatc2 | |
| Fish vs. control | Cell Cycle Control of Replication | Cdc6, cdc7, cdk1, cdk2, cdt1, dbf4, dna2, lig1, mcm2, mcm3, mcm4, mcm5, mcm6, mcm7, orc1, orc2, orc6, pcna, pold1, pole, prim1, top2a |
| Role of BRCA1 in DNA Damage Response | Bard1, blm, brca1, brca2, cdkn1a, e2f1, e2f2, e2f4, e2f7, e2f8, fancd2, fancl, gadd45a, mdc1, plk1, rad51, rb1, rbl1, rfc3, rfc4, topbp1 | |
| Heme Biosynthesis II | Alad, cpox, fech, hmbs, ppox, urod, uros | |
| Circadian Rhythm Signaling | Bmal1, cry2, nr1d1, per2, per3 | |
| Hedgehog Signaling | Ccnb1, cdk1, gli2, prkar2b | |
| Flaxseed vs. Fish | Heme Biosynthesis II | Alad, cpox, fech, ppox, urod, uros |
| Role of BRCA1 in DNA Damage Response | Brca1, brca2, e2f2, e2f4, fancd2, topbp1 | |
| Cell Cycle Control of Replication | Mcm3, mcm5, orc1, pole | |
| Cytokine Signaling | Blvrb, ccr5, il1rl1, socs3 | |
| Hypoxia Signaling | Ube2c, ube2l6, ube2o, ube2t |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozner, R.; Vernikov, J.; Griess-Fishheimer, S.; Travinsky, T.; Penn, S.; Schwartz, B.; Mesilati-Stahy, R.; Argov-Argaman, N.; Shahar, R.; Monsonego-Ornan, E. The Role of Omega-3 Polyunsaturated Fatty Acids from Different Sources in Bone Development. Nutrients 2020, 12, 3494. https://doi.org/10.3390/nu12113494
Rozner R, Vernikov J, Griess-Fishheimer S, Travinsky T, Penn S, Schwartz B, Mesilati-Stahy R, Argov-Argaman N, Shahar R, Monsonego-Ornan E. The Role of Omega-3 Polyunsaturated Fatty Acids from Different Sources in Bone Development. Nutrients. 2020; 12(11):3494. https://doi.org/10.3390/nu12113494
Chicago/Turabian StyleRozner, Reut, Janna Vernikov, Shelley Griess-Fishheimer, Tamar Travinsky, Svetlana Penn, Betty Schwartz, Ronit Mesilati-Stahy, Nurit Argov-Argaman, Ron Shahar, and Efrat Monsonego-Ornan. 2020. "The Role of Omega-3 Polyunsaturated Fatty Acids from Different Sources in Bone Development" Nutrients 12, no. 11: 3494. https://doi.org/10.3390/nu12113494
APA StyleRozner, R., Vernikov, J., Griess-Fishheimer, S., Travinsky, T., Penn, S., Schwartz, B., Mesilati-Stahy, R., Argov-Argaman, N., Shahar, R., & Monsonego-Ornan, E. (2020). The Role of Omega-3 Polyunsaturated Fatty Acids from Different Sources in Bone Development. Nutrients, 12(11), 3494. https://doi.org/10.3390/nu12113494





