Effect of Zinc Supplementation on the Serum Metabolites Profile at the Early Stage of Breast Cancer in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Zinc Micro- and Nanoparticles
2.2. Rodent Model
2.3. Preparation of Biological Material and Histopathological Examination
2.4. Solvents and Derivatizing Agents for GC/MS Analysis
2.5. Extraction of Metabolites from Blood Serum and Derivatization
2.6. GC/MS System and Analysis Method
2.7. Processing of Obtained Profiles
2.8. Statistical and Semiquantitative Analysis
3. Results
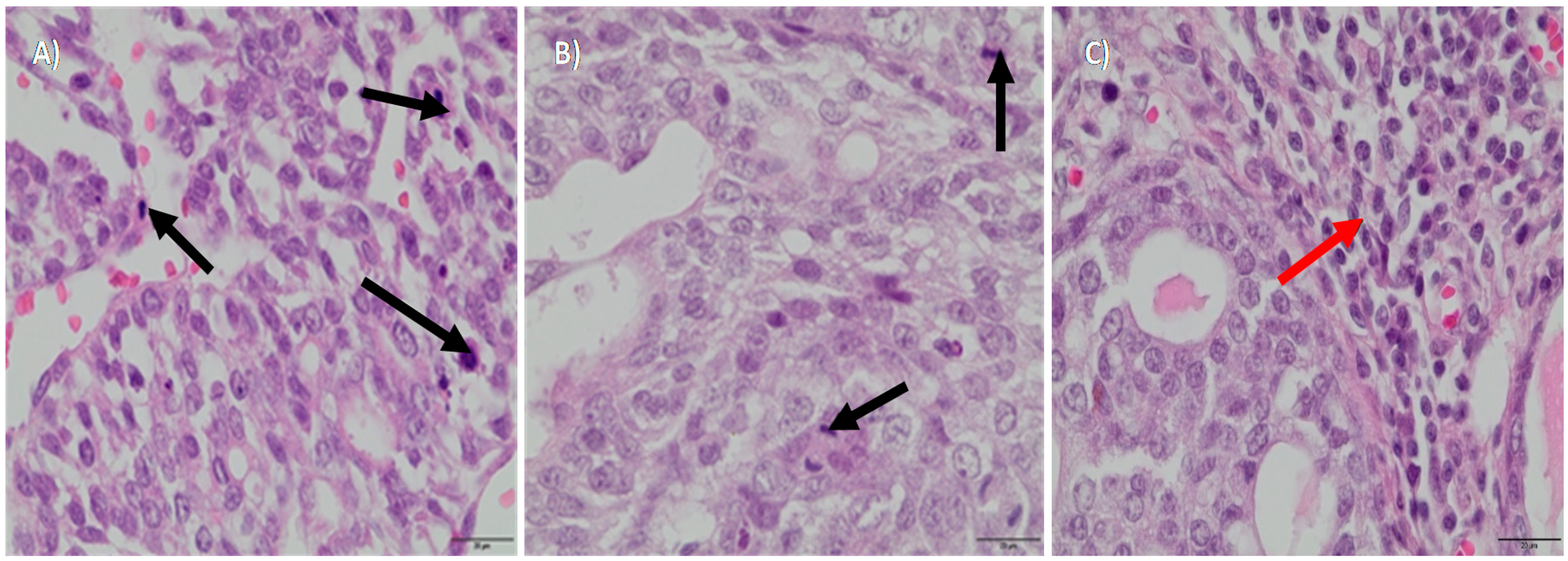
3.1. Histopathological Examination of Tumors after Treatment with Zn NP
3.2. The Characterization of Metabolome Components by GC-TOF MS Approach
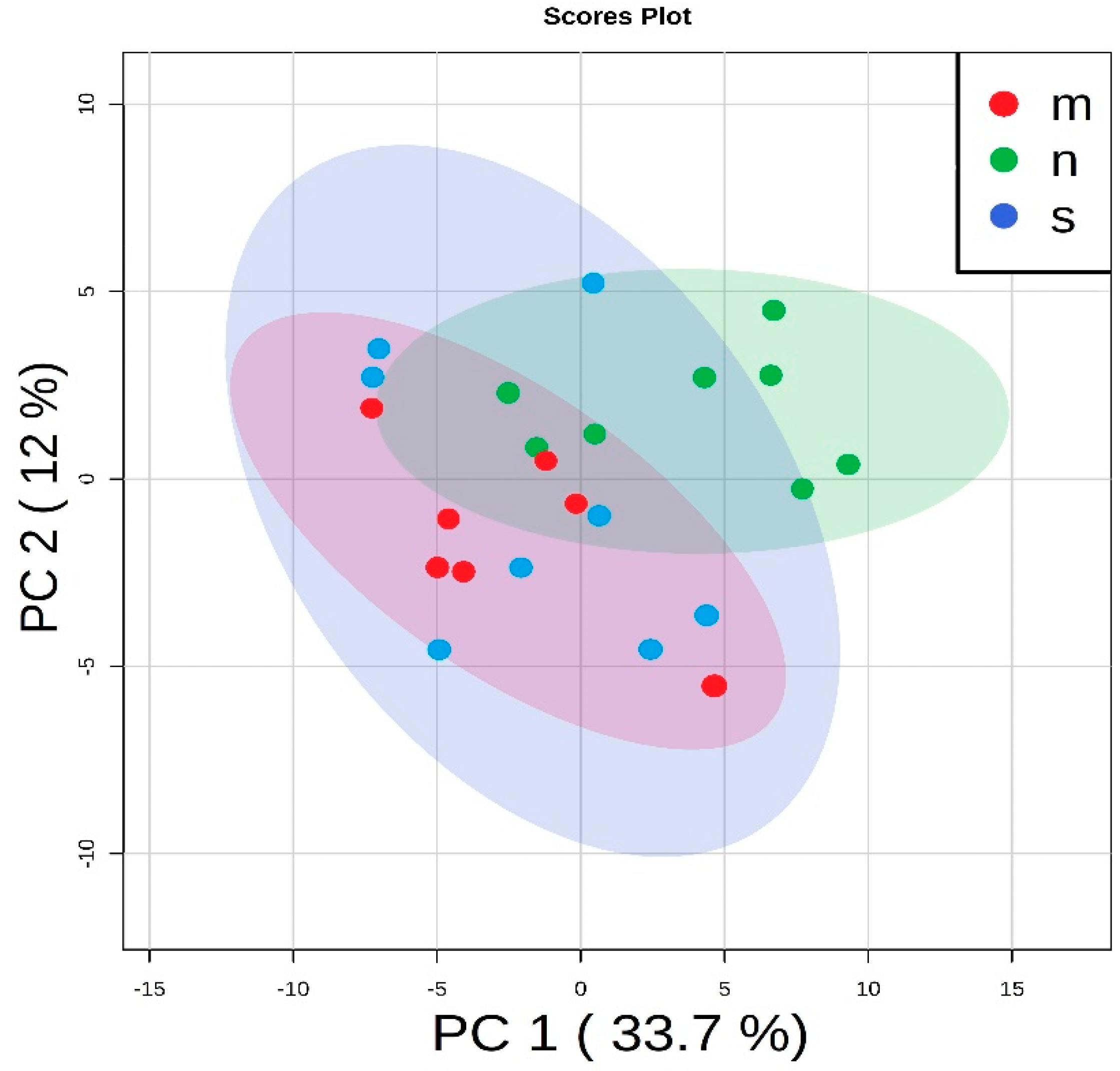
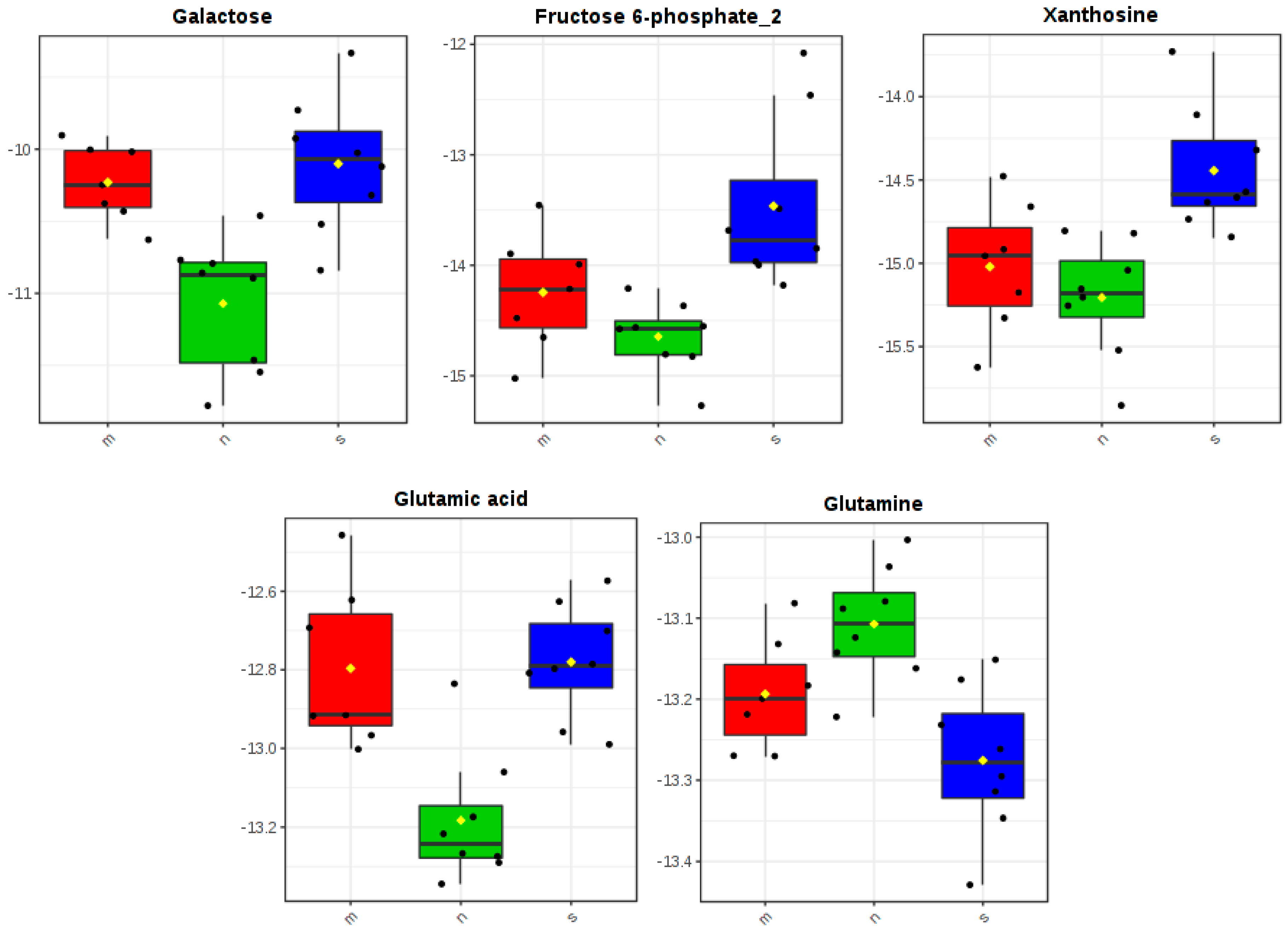
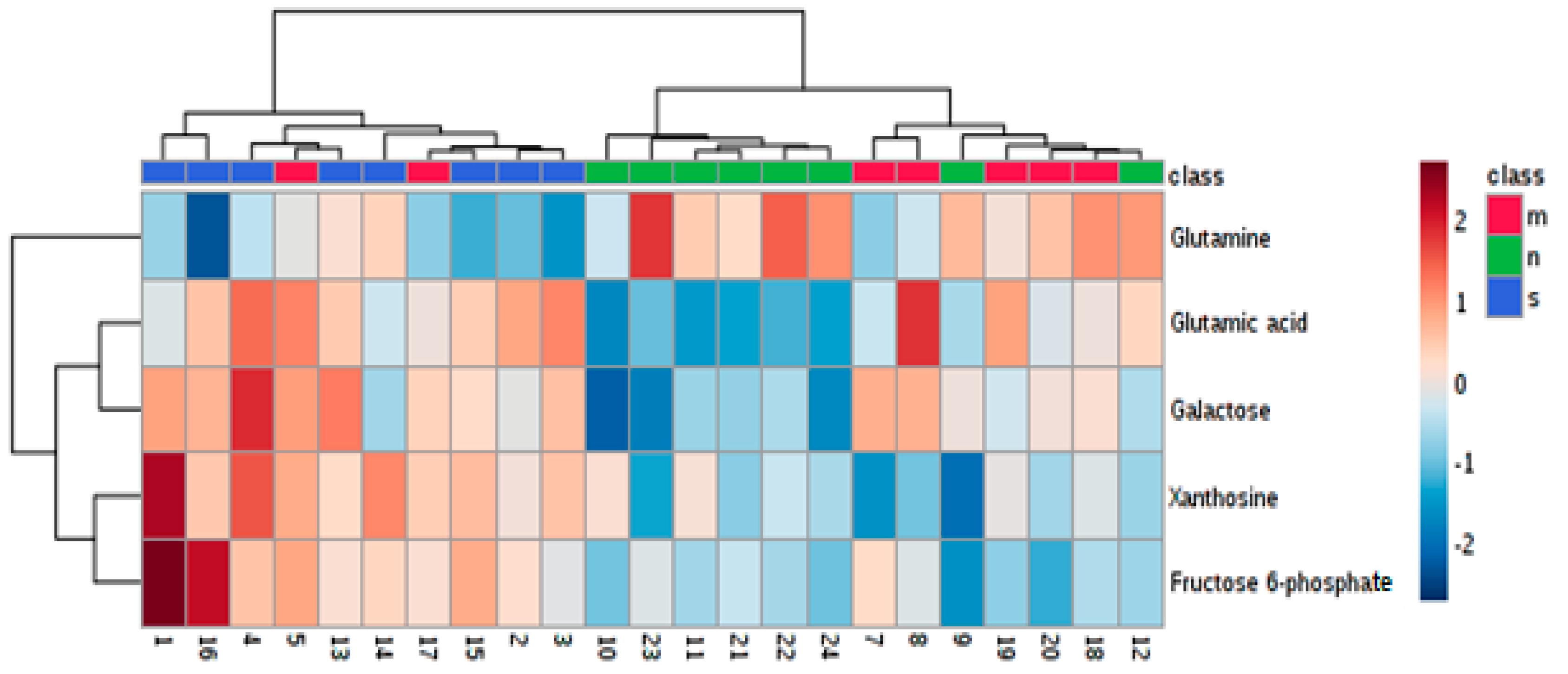
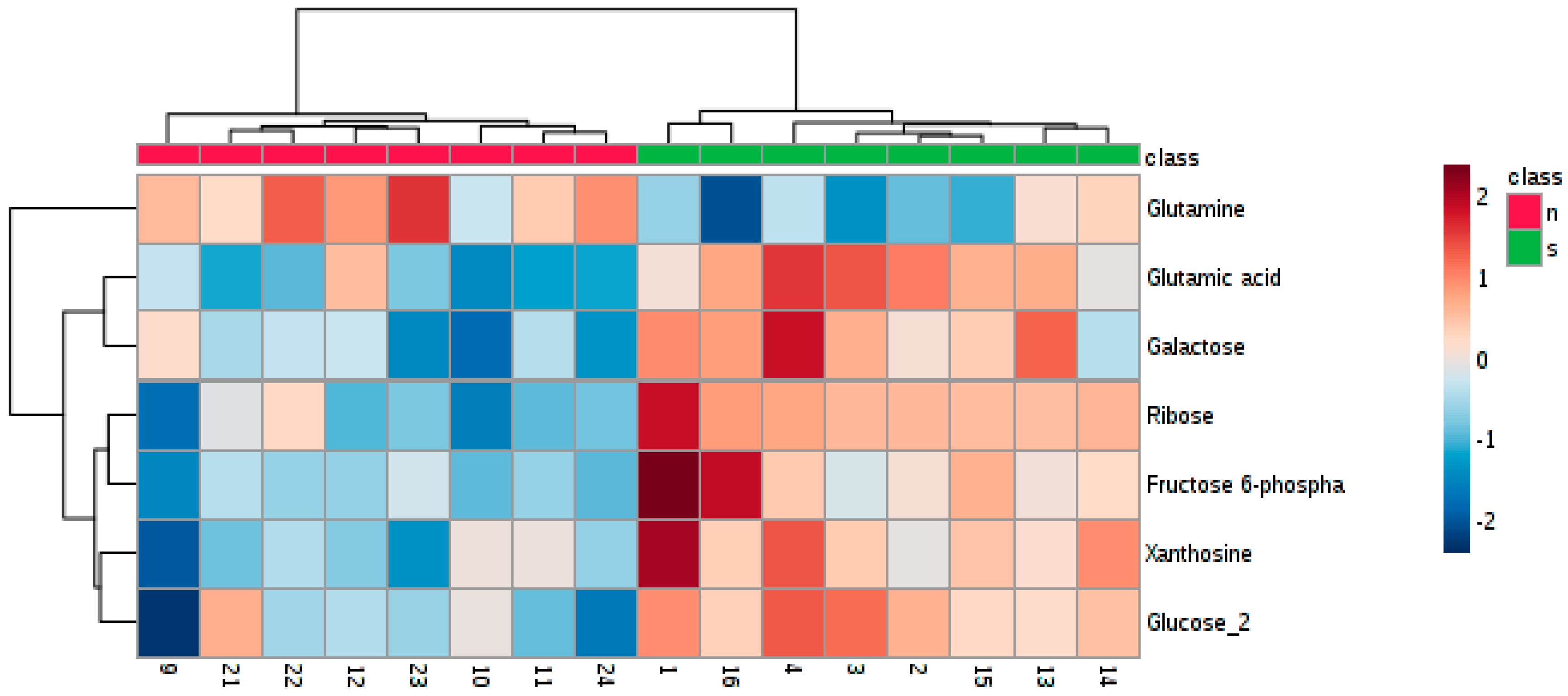
3.3. Determination of Differences in Profiles of Treated and Diseased Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Cancer Research Fund, Breast Cancer Statistics. Breast Cancer Is the Most Common Cancer in Women Worldwide. Available online: https://www.wcrf.org/dietandcancer/cancer-trends/breast-cancer-statistics (accessed on 2 November 2019).
- Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; Meyers, C.; Jiang, Y.; Adair, J.H. The use of nanoparticulates to treat breast cancer. Nanomedicine 2017, 12, 2367–2388. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Krol, A.; Pomastowski, P.; Rafinska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Dwivedi, S.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Shin, H.-S. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B Biointerfaces 2014, 117, 267–276. [Google Scholar] [CrossRef]
- Hassan, H.F.; Mansour, A.M.; Abo-Youssef, A.M.H.; Elsadek, B.E.M.; Massiha, B.A.S. Zinc oxide nanoparticles as a novel anticancer approach; in vitro and in vivo evidence. Clin. Exp. Pharmacol. Physiol. 2017, 44, 235–243. [Google Scholar] [CrossRef]
- Pandurangan, M.; Enkhtaivan, G.; Kim, D.H. Anticancer studies of synthesized ZnO nanoparticles against human cervical carcinoma cells. J. Photochem. Photobiol. B Biol. 2016, 158, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.P.; Zhang, X.F.; Zhang, G.L.; Huang, Y.F.; Gurunathan, S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int. J. Nanomed. 2017, 12, 6521–6535. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.; Ahmad, J.; Alrokayan, S. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845–857. [Google Scholar] [CrossRef]
- Kadhem, H.A.; Ibraheem, S.A.; Jabir, M.S.; Kadhim, A.A.; Taqi, Z.J.; Florin, M.D. Zinc Oxide Nanoparticles Induces Apoptosis in Human Breast Cancer Cells via Caspase-8 and P53 Pathway. Nano Biomed. Eng. 2019, 11, 35–43. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Novaderi, M.; Mohamad, R. Eco-Friendly Formulated Zinc Oxide Nanoparticles: Induction of Cell Cycle Arrest and Apoptosis in the MCF-7 Cancer Cell Line. Genes 2017, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Taccola, L.; Raffa, V.; Riggio, C.; Vittorio, O.; Iorio, M.C.; Vanacore, R.; Pietrabissa, A.; Cuschieri, A. Zinc oxide nanoparticles as selective killers of proliferating cells. Int. J. Nanomed. 2011, 6, 1129–1140. [Google Scholar] [CrossRef]
- Raajshree, R.K.; Brindha, D. In Vivo Anticancer Activity of Biosynthesized Zinc Oxide Nanoparticle using Turbinaria conoides on a Dalton’s Lymphoma Ascites Mice Model. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 103–115. [Google Scholar] [CrossRef]
- Tanino, R.; Amano, Y.; Tong, X.; Sun, R.; Tsubata, Y.; Harada, M.; Fujita, Y.; Isobe, T. Anticancer Activity of ZnO Nanoparticles against Human Small-Cell Lung Cancer in an Orthotopic Mouse Model. Mol. Cancer Ther. 2020, 19, 502–512. [Google Scholar] [CrossRef]
- Ghant, S.R.; Rao, M.H.; Muralidharan, K. Single-pot synthesis of zinc nanoparticles, borane (BH3) and closo-dodecaborate (B12H12)2- using LiBH4 under mild conditions. Dalton Trans. 2013, 42, 8420–8425. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30.4.1–30.4.32. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; Van Der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Oqiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Zgura, A.; Galesa, L.; Bratila, E.; Anghel, R. Relationship between Tumor Infiltrating Lymphocytes and Progression in Breast Cancer. Maedica 2018, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, H.J.; Ryu, H.J.; Ryu, W.I.; Park, Y.H.; Bae, H.C.; Jang, Y.S.; Son, S.W. ZnO nanoparticles induce TNF-α expression via ROS-ERK-Egr-1 pathway in human keratinocytes. J. Dermatol. Sci. 2013, 72, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.K.; Ghosh, T.; Roy, S.; Chattopadhyay, S.; Das, D. Zinc sulfide nanoparticles selectively induce cytotoxic and genotoxic effects on leukemic cells: Involvement of reactive oxygen species and tumor necrosis factor alpha. J. Appl. Toxicol. 2014, 34, 1130–1144. [Google Scholar] [CrossRef]
- Sayes, C.M.; Reed, K.L.; Warheit, D.B. Assessing Toxicity of Fine and Nanoparticles: Comparing In Vitro Measurements to In Vivo Pulmonary Toxicity Profiles. Toxicol. Sci. 2007, 97, 163–180. [Google Scholar] [CrossRef]
- Hanley, C.; Thurber, A.; Hanna, C.; Punnoose, A.; Zhang, J.; Wingett, D.G. The Influences of Cell Type and ZnO Nanoparticle Size on Immune Cell Cytotoxicity and Cytokine Induction. Nanoscale Res. Lett. 2009, 4, 1409. [Google Scholar] [CrossRef]
- Gojova, A.; Guo, B.; Kota, R.S.; Rutledge, J.C.; Kennedy, I.M.; Barakat, A.I. Induction of Inflammation in Vascular Endothelial Cells by Metal Oxide Nanoparticles: Effect of Particle Composition. Environ. Health Perspect. 2007, 115, 403–409. [Google Scholar] [CrossRef]
- Beyerle, A.; Schulz, H.; Kissel, T.; Stoeger, T. Screening strategy to avoid toxicological hazards of inhaled nanoparticles for drug delivery: The use of alpha-quartz and nano zinc oxide particles as benchmark. J. Phys. Conf. Ser. 2009, 151, 012034. [Google Scholar] [CrossRef]
- Cardoso, M.R.; Santos, J.C.; Ribeiro, M.L.; Talarico, M.C.R.; Viana, L.R.; Derchain, S.F.M. A Metabolomic Approach to Predict Breast Cancer Behavior and Chemotherapy Response. Int. J. Mol. Sci. 2018, 19, 617. [Google Scholar] [CrossRef]
- Grasmann, G.; Smolle, E.; Olschewski, H.; Leihner, K. Gluconeogenesis in cancer cells – Repurposing of a starvation-induced metabolic pathway? Biochim. Biophys. Acta (BBA) Rev. Cancer 2019, 1872, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Schwartsburd, P. Cancer-Induced Reprogramming of Host Glucose Metabolism: “Vicious Cycle” Supporting Cancer Progression. Front. Oncol. 2019, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Bott, A.J.; Maimouni, S.; Zong, W.X. The Pleiotropic Effects of Glutamine Metabolism in Cancer. Cancers 2019, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Makowski, G.S. Advances in Clinical Chemistry; Academic Press: Cambridge, MA, USA, 2018; Volume 83. [Google Scholar]
- Yin, J.; Ren, W.; Huang, X.; Deng, J.; Li, T.; Yin, Y. Potential Mechanisms Connecting Purine Metabolism and Cancer Therapy. Front. Immunol. 2018, 9, 1697. [Google Scholar] [CrossRef]


| DLS Parameter | Size (d.nm) a | Z-average (d.nm) | Zeta potential b (mV) | D c |
|---|---|---|---|---|
| Zn nanoparticles | 99 ± 18 | 321 | +37.1± 7.61 | 1000 |
| Zn microparticles | 342 ± 6.6 | 3058 | +26.7 ± 7.12 | 1000 |
| Experimental Group | Number of Tumors in One Rat | Tumor Incidence (%) | Tumor Weight (g) | Tumor Weight Mean (g) | Tumor Grade |
|---|---|---|---|---|---|
| Standard diet (no supplementation) | 2–9 | 100% | 0.10–7.80 | 0.89 ± 0.52 * | Adenocarcinoma 2 grade |
| Supplementation with zinc microparticles | 1–6 | 100% | 0.06–7.41 | 0.68 ± 0.66 | Adenocarcinoma 2 grade |
| Supplementation with zinc nanoparticles | 0–3 | 88% | 0.01–1.79 | 0.40 ± 0.34 * | Adenocarcinoma 1 grade |
| Results of Tukey’s HSD Post Hoc Test | |||||
|---|---|---|---|---|---|
| No | Identification | ANOVA Significance FDR Corrected p-Value < 0.05 | Standard Diet vs. Zinc Nanoparticles Supplementation (S vs. N) | Standard Diet vs. Zinc Microparticles Supplementation (S vs. M) | Zinc Microparticles Supplementation vs. Zinc Nanoparticles Supplementation (M vs. N) |
| 1. | Glutamic acid | 0.01497 | + | + | |
| 2. | Galactose | 0.01497 | + | + | |
| 3. | Glutamine | 0.04854 | + | ||
| 4. | Xanthosine | 0.04854 | + | + | |
| 6. | Fructose 6-phosphate | 0.04869 | + | + | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobrowska-Korczak, B.; Gątarek, P.; Skrajnowska, D.; Bielecki, W.; Wyrebiak, R.; Kovalczuk, T.; Wrzesień, R.; Kałużna-Czaplińska, J. Effect of Zinc Supplementation on the Serum Metabolites Profile at the Early Stage of Breast Cancer in Rats. Nutrients 2020, 12, 3457. https://doi.org/10.3390/nu12113457
Bobrowska-Korczak B, Gątarek P, Skrajnowska D, Bielecki W, Wyrebiak R, Kovalczuk T, Wrzesień R, Kałużna-Czaplińska J. Effect of Zinc Supplementation on the Serum Metabolites Profile at the Early Stage of Breast Cancer in Rats. Nutrients. 2020; 12(11):3457. https://doi.org/10.3390/nu12113457
Chicago/Turabian StyleBobrowska-Korczak, Barbara, Paulina Gątarek, Dorota Skrajnowska, Wojciech Bielecki, Rafal Wyrebiak, Tomas Kovalczuk, Robert Wrzesień, and Joanna Kałużna-Czaplińska. 2020. "Effect of Zinc Supplementation on the Serum Metabolites Profile at the Early Stage of Breast Cancer in Rats" Nutrients 12, no. 11: 3457. https://doi.org/10.3390/nu12113457
APA StyleBobrowska-Korczak, B., Gątarek, P., Skrajnowska, D., Bielecki, W., Wyrebiak, R., Kovalczuk, T., Wrzesień, R., & Kałużna-Czaplińska, J. (2020). Effect of Zinc Supplementation on the Serum Metabolites Profile at the Early Stage of Breast Cancer in Rats. Nutrients, 12(11), 3457. https://doi.org/10.3390/nu12113457





