Maternal Linoleic Acid Overconsumption Alters Offspring Gut and Adipose Tissue Homeostasis in Young but Not Older Adult Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Protocol
2.2. Microbiota Analysis
2.3. Intestinal Alkaline Phosphatase Activity (IAP) and Ussing Chamber Assay
2.4. Real-Time PCR
2.5. Adipose Tissue Histology
2.6. Fatty Acid Analysis and Conjugated Linoleic Acid Quantification
2.7. Statistical Analysis
3. Results
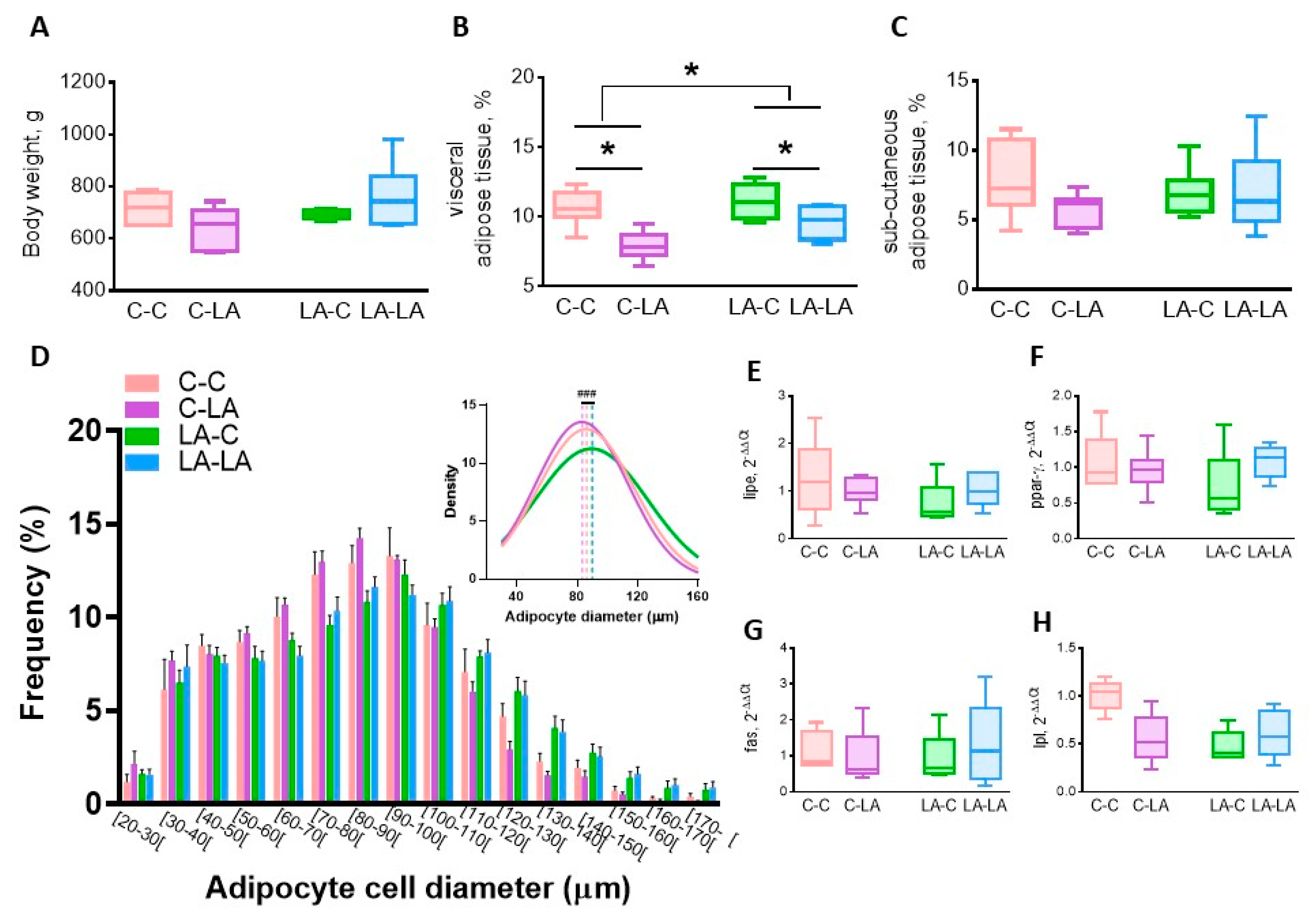
3.1. Maternal LA-Diet Impacted Dam and Offspring Tissue Composition at Weaning
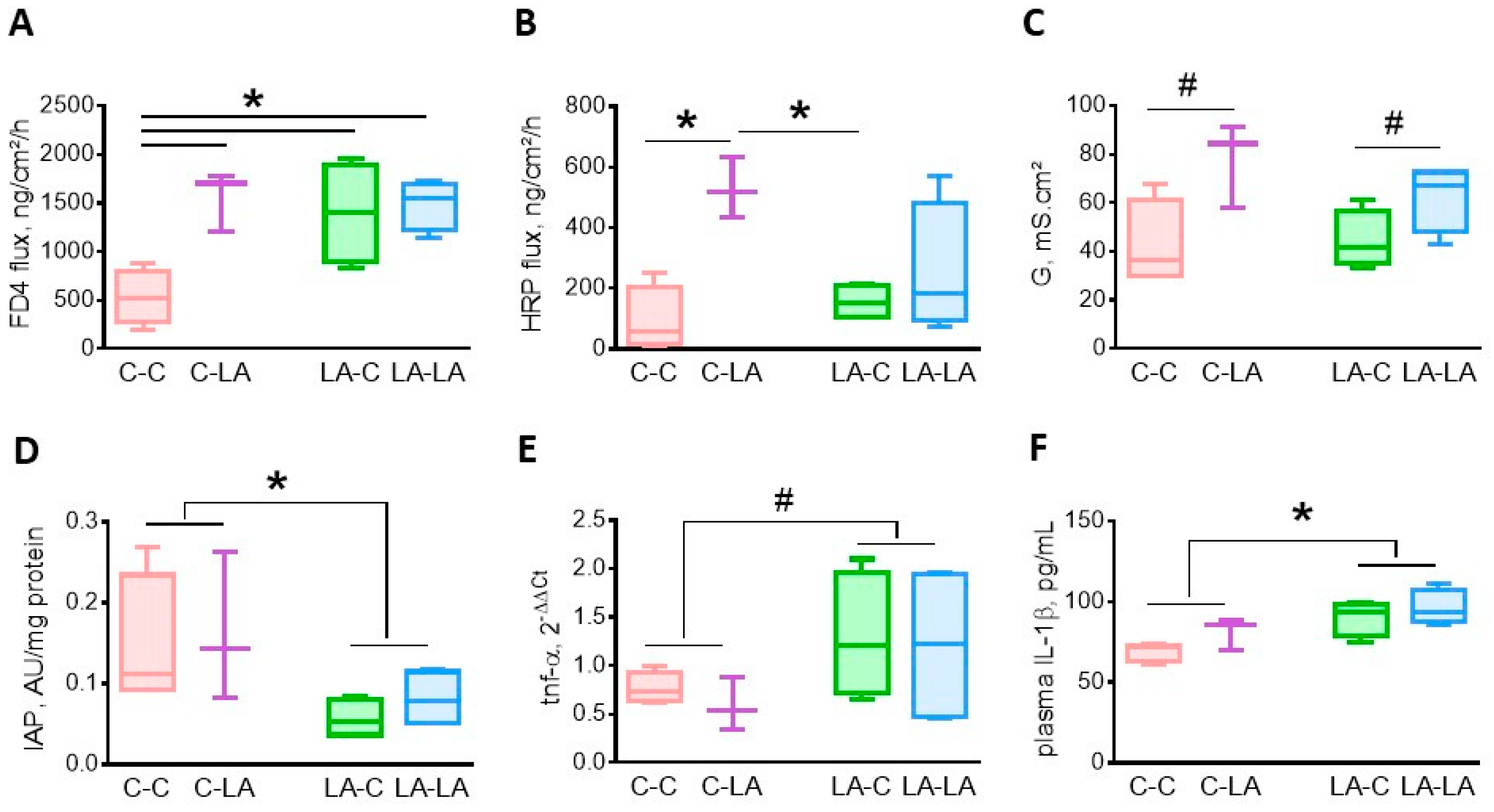
3.2. Maternal LA-Diet Impacted Gut Barrier Function and Adipose Tissue in Young Adult Offspring
3.2.1. Cecal Barrier Function and Inflammation
3.2.2. Epididymal Adipose Tissue
3.2.3. Conjugated-Linoleic Acids
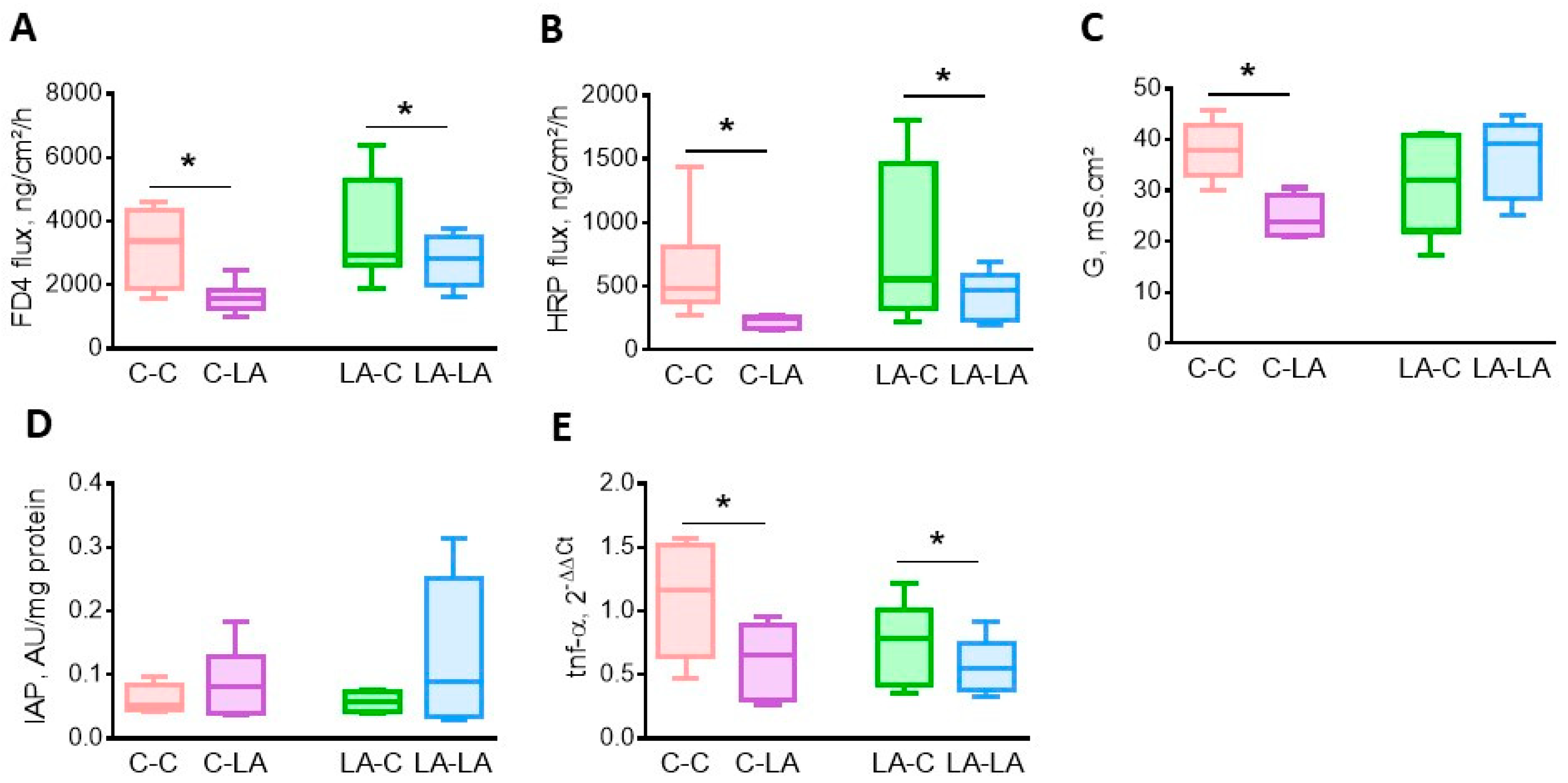
3.3. Maternal LA-Diet Had a Limited Impact on Gut Barrier Function and Adipose Tissue in Older Adults Compared to The Weaning Diet Itself
3.3.1. Cecal Barrier Function and Inflammation
3.3.2. Epididymal Adipose Tissue
3.3.3. Conjugated-Linoleic Acids
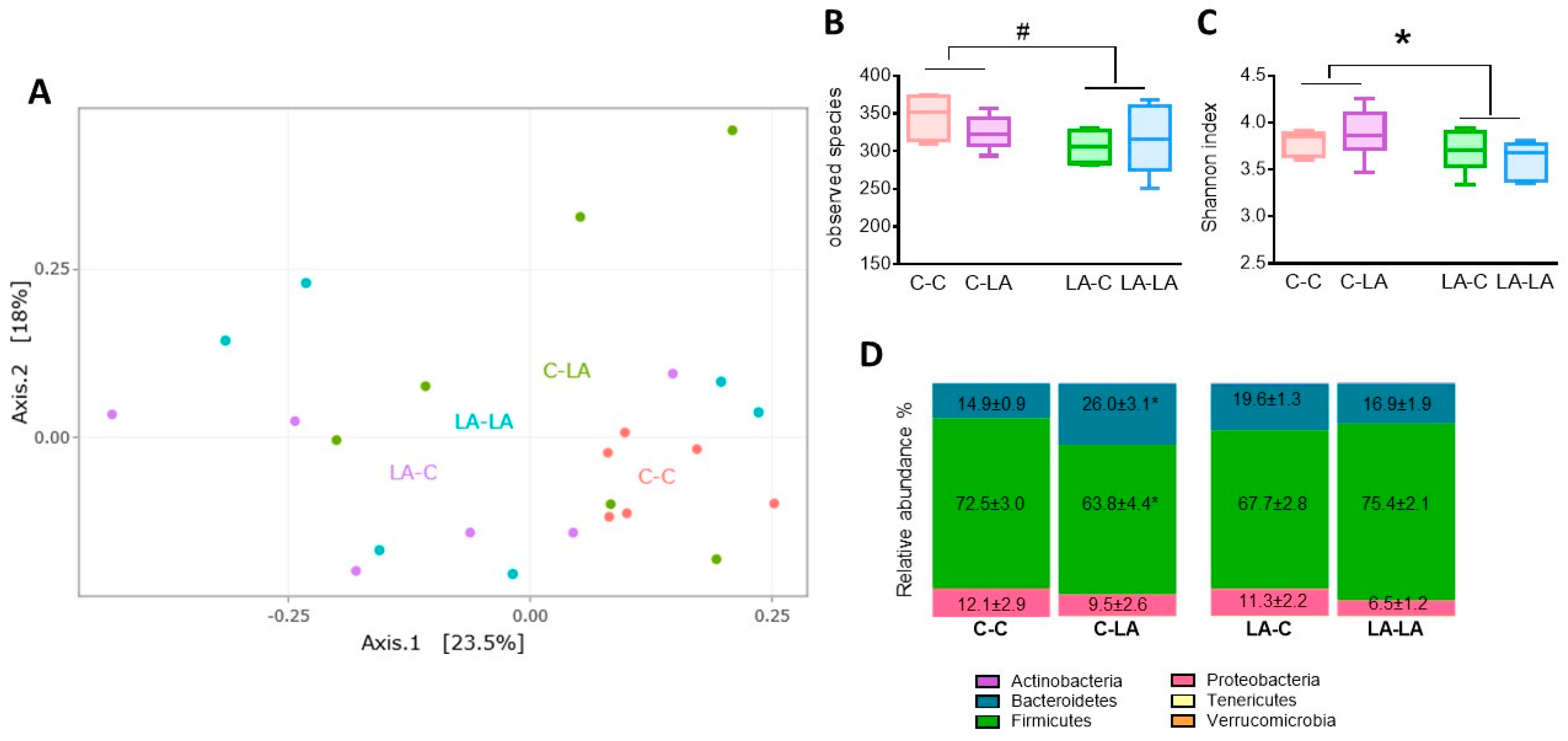
3.4. Cecal Microbiota
3.4.1. Cecal Microbiota at 3 Months of Age
3.4.2. Cecal Microbiota at 6 Months of Age
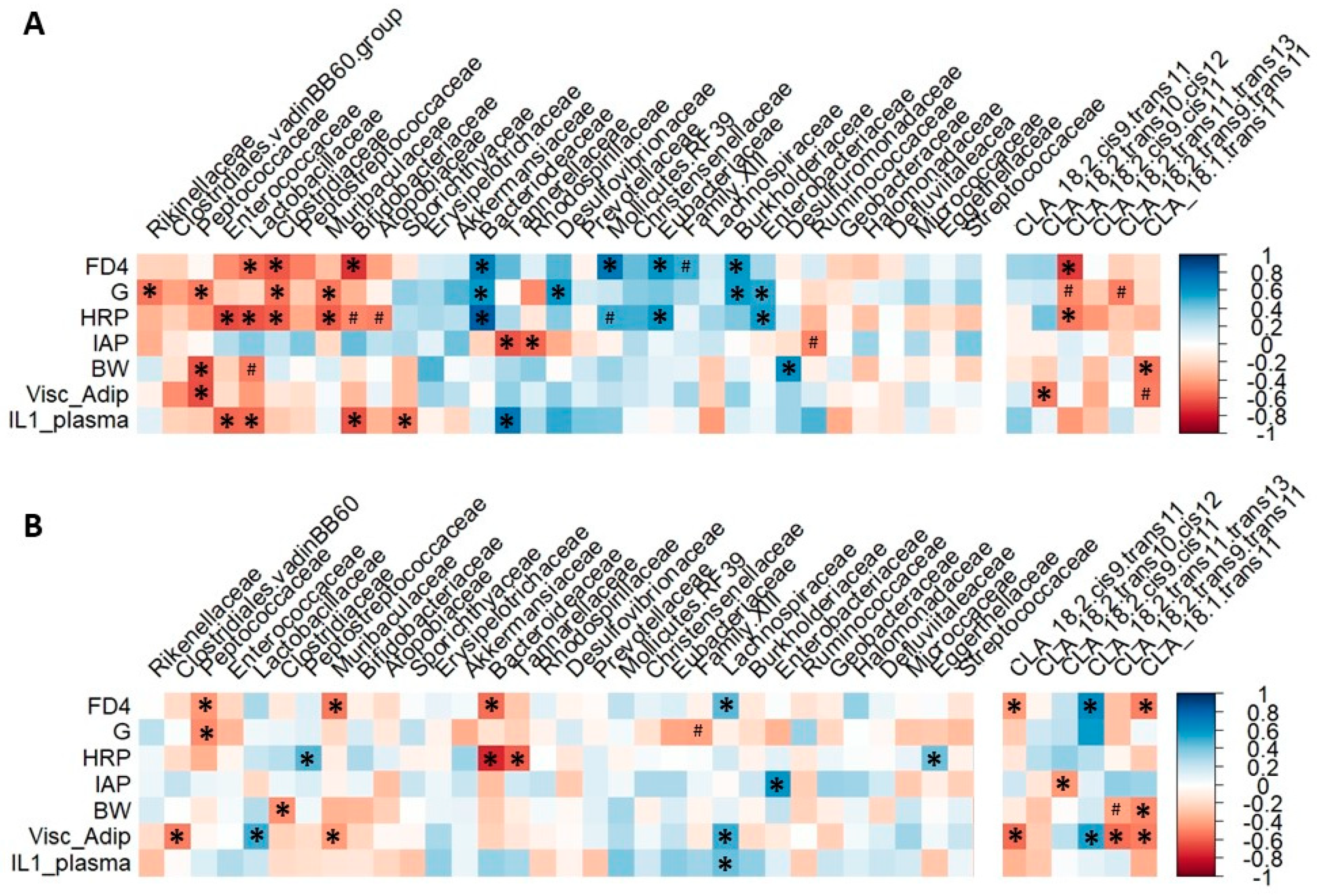
3.4.3. Microbiota Composition Correlates with Rat Gut Barrier and Obesity Phenotype at 3 but Not 6 Months of Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Ailhaud, G.; Massiera, F.; Weill, P.; Legrand, P.; Alessandri, J.-M.; Guesnet, P. Temporal changes in dietary fats: Role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog. Lipid Res. 2006, 45, 203–236. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Brands, B.; Grote, V.; Kirchberg, F.F.; Prell, C.; Rzehak, P.; Uhl, O.; Weber, M. Early Nutrition Programming Project Long-Term Health Impact of Early Nutrition: The Power of Programming. Ann. Nutr. Metab. 2017, 70, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Ailhaud, G.; Guesnet, P. Fatty acid composition of fats is an early determinant of childhood obesity: A short review and an opinion. Obes. Rev. 2004, 5, 21–26. [Google Scholar] [CrossRef]
- Mennitti, L.V.; Oliveira, J.L.; Morais, C.A.; Estadella, D.; Oyama, L.M.; Oller do Nascimento, C.M.; Pisani, L.P. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J. Nutr. Biochem. 2015, 26, 99–111. [Google Scholar] [CrossRef]
- Massiera, F.; Saint-Marc, P.; Seydoux, J.; Murata, T.; Kobayashi, T.; Narumiya, S.; Guesnet, P.; Amri, E.-Z.; Negrel, R.; Ailhaud, G. Arachidonic acid and prostacyclin signaling promote adipose tissue development: A human health concern? J. Lipid Res. 2003, 44, 271–279. [Google Scholar] [CrossRef]
- Massiera, F.; Barbry, P.; Guesnet, P.; Joly, A.; Luquet, S.; Moreilhon-Brest, C.; Mohsen-Kanson, T.; Amri, E.-Z.; Ailhaud, G. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J. Lipid Res. 2010, 51, 2352–2361. [Google Scholar] [CrossRef]
- Rudolph, M.C.; Young, B.E.; Lemas, D.J.; Palmer, C.E.; Hernandez, T.L.; Barbour, L.A.; Friedman, J.E.; Krebs, N.F.; MacLean, P.S. Early infant adipose deposition is positively associated with the n-6 to n-3 fatty acid ratio in human milk independent of maternal BMI. Int. J. Obes. (Lond.) 2017, 41, 510–517. [Google Scholar] [CrossRef]
- Bernard, J.Y.; Tint, M.-T.; Aris, I.M.; Chen, L.-W.; Quah, P.L.; Tan, K.H.; Yeo, G.S.-H.; Fortier, M.V.; Yap, F.; Shek, L.; et al. Maternal plasma phosphatidylcholine polyunsaturated fatty acids during pregnancy and offspring growth and adiposity. Prostaglandins Leukot. Essent. Fatty Acids 2017, 121, 21–29. [Google Scholar] [CrossRef]
- Vidakovic, A.J.; Gishti, O.; Voortman, T.; Felix, J.F.; Williams, M.A.; Hofman, A.; Demmelmair, H.; Koletzko, B.; Tiemeier, H.; Jaddoe, V.W.V.; et al. Maternal plasma PUFA concentrations during pregnancy and childhood adiposity: The Generation R Study. Am. J. Clin. Nutr. 2016, 103, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- de Vries, P.S.; Gielen, M.; Rizopoulos, D.; Rump, P.; Godschalk, R.; Hornstra, G.; Zeegers, M.P. Association between polyunsaturated fatty acid concentrations in maternal plasma phospholipids during pregnancy and offspring adiposity at age 7: The MEFAB cohort. Prostaglandins Leukot. Essent. Fatty Acids 2014, 91, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Harvey, N.C.; Robinson, S.M.; Ntani, G.; Davies, J.H.; Inskip, H.M.; Godfrey, K.M.; Dennison, E.M.; Calder, P.C.; Cooper, C.; et al. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J. Clin. Endocrinol. Metab. 2013, 98, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Donahue, S.M.A.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal fatty acid status and child adiposity at age 3 y: Results from a US pregnancy cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, N.; Gielen, M.; Margetaki, K.; Godschalk, R.W.; van der Wurff, I.; Rouschop, S.; Ibrahim, A.; Antoniou, E.; Chatzi, L.; de Groot, R.H.M.; et al. Polyunsaturated fatty acid levels at birth and child-to-adult growth: Results from the MEFAB cohort. Prostaglandins Leukot. Essent. Fatty Acids 2017, 126, 72–78. [Google Scholar] [CrossRef]
- Pedersen, L.; Lauritzen, L.; Brasholt, M.; Buhl, T.; Bisgaard, H. Polyunsaturated fatty acid content of mother’s milk is associated with childhood body composition. Pediatr. Res. 2012, 72, 631–636. [Google Scholar] [CrossRef]
- Hauner, H.; Much, D.; Vollhardt, C.; Brunner, S.; Schmid, D.; Sedlmeier, E.-M.; Heimberg, E.; Schuster, T.; Zimmermann, A.; Schneider, K.-T.M.; et al. Effect of reducing the n−6:n−3 long-chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: An open-label randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 383–394. [Google Scholar] [CrossRef]
- Rytter, D.; Bech, B.H.; Halldorsson, T.; Christensen, J.H.; Schmidt, E.B.; Danielsen, I.; Henriksen, T.B.; Olsen, S.F. No association between the intake of marine n-3 PUFA during the second trimester of pregnancy and factors associated with cardiometabolic risk in the 20-year-old offspring. Br. J. Nutr. 2013, 110, 2037–2046. [Google Scholar] [CrossRef]
- Martínez, J.A.; Cordero, P.; Campión, J.; Milagro, F.I. Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc. Nutr. Soc. 2012, 71, 276–283. [Google Scholar] [CrossRef]
- Kelly, J.R.; Minuto, C.; Cryan, J.F.; Clarke, G.; Dinan, T.G. Cross Talk: The Microbiota and Neurodevelopmental Disorders. Front. Neurosci. 2017, 11, 490. [Google Scholar] [CrossRef]
- Koh, A.; Bäckhed, F. From Association to Causality: The Role of the Gut Microbiota and Its Functional Products on Host Metabolism. Mol. Cell 2020, 78, 584–596. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Geurts, L.; Neyrinck, A.M.; Delzenne, N.M.; Knauf, C.; Cani, P.D. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: Novel insights into molecular targets and interventions using prebiotics. Benef. Microbes 2014, 5, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.J.; Frank, D.N.; Friedman, J.E. Early Microbes Modify Immune System Development and Metabolic Homeostasis-The “Restaurant” Hypothesis Revisited. Front. Endocrinol. (Lausanne) 2017, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef]
- Chong, C.Y.L.; Bloomfield, F.H.; O’Sullivan, J.M. Factors Affecting Gastrointestinal Microbiome Development in Neonates. Nutrients 2018, 10, 274. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef]
- Abulizi, N.; Quin, C.; Brown, K.; Chan, Y.K.; Gill, S.K.; Gibson, D.L. Gut Mucosal Proteins and Bacteriome Are Shaped by the Saturation Index of Dietary Lipids. Nutrients 2019, 11, 418. [Google Scholar] [CrossRef]
- Kaliannan, K.; Wang, B.; Li, X.-Y.; Kim, K.-J.; Kang, J.X. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Kaliannan, K.; Li, X.-Y.; Wang, B.; Pan, Q.; Chen, C.-Y.; Hao, L.; Xie, S.; Kang, J.X. Multi-omic analysis in transgenic mice implicates omega-6/omega-3 fatty acid imbalance as a risk factor for chronic disease. Commun. Biol. 2019, 2, 276. [Google Scholar] [CrossRef] [PubMed]
- Bidu, C.; Escoula, Q.; Bellenger, S.; Spor, A.; Galan, M.; Geissler, A.; Bouchot, A.; Dardevet, D.; Morio-Liondor, B.; Cani, P.D.; et al. The Transplantation of ω3 PUFA-Altered Gut Microbiota of Fat-1 Mice to Wild-Type Littermates Prevents Obesity and Associated Metabolic Disorders. Diabetes 2018, 67, 1512–1523. [Google Scholar] [CrossRef]
- Robertson, R.C.; Seira Oriach, C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Paul Ross, R.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Seira Oriach, C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Stanton, C. Deficiency of essential dietary n-3 PUFA disrupts the cecal microbiome and metabolome in mice. Br. J. Nutr. 2017, 118, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.J.; Hecht, P.M.; Jasarevic, E.; Beversdorf, D.Q.; Will, M.J.; Fritsche, K.; Gillespie, C.H. Sex-specific effects of docosahexaenoic acid (DHA) on the microbiome and behavior of socially-isolated mice. Brain Behav. Immun. 2017, 59, 38–48. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; El Aidy, S.; Crispie, F.; O’Sullivan, O.; Cotter, P.; Stanton, C.; Kelly, P.; Cryan, J.F.; Dinan, T.G. N-3 Polyunsaturated Fatty Acids (PUFAs) Reverse the Impact of Early-Life Stress on the Gut Microbiota. PLoS ONE 2015, 10, e0139721. [Google Scholar] [CrossRef]
- Shrestha, N.; Sleep, S.L.; Cuffe, J.S.M.; Holland, O.J.; McAinch, A.J.; Dekker Nitert, M.; Hryciw, D.H. Pregnancy and diet-related changes in the maternal gut microbiota following exposure to an elevated linoleic acid diet. Am. J. Physiol. Endocrinol. 2020, 318, E276–E285. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Escudié, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics 2018, 34, 1287–1294. [Google Scholar] [CrossRef]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Mahé, F.; Rognes, T.; Quince, C.; de Vargas, C.; Dunthorn, M. Swarm v2: Highly-scalable and high-resolution amplicon clustering. PeerJ 2015, 3, e1420. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing Large Minimum Evolution Trees with Profiles instead of a Distance Matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Chen, L.; Reeve, J.; Zhang, L.; Huang, S.; Wang, X.; Chen, J. GMPR: A robust normalization method for zero-inflated count data with application to microbiome sequencing data. PeerJ 2018, 6, e4600. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Guerville, M.; Leroy, A.; Sinquin, A.; Laugerette, F.; Michalski, M.-C.; Boudry, G. Western-diet consumption induces alteration of barrier function mechanisms in the ileum that correlates with metabolic endotoxemia in rats. Am. J. Physiol. Endocrinol. 2017, 313, E107–E120. [Google Scholar] [CrossRef] [PubMed]
- Marchix, J.; Catheline, D.; Duby, C.; Monthéan-Boulier, N.; Boissel, F.; Pédrono, F.; Boudry, G.; Legrand, P. Interactive effects of maternal and weaning high linoleic acid intake on hepatic lipid metabolism, oxylipins profile and hepatic steatosis in offspring. J. Nutr. Biochem. 2020, 75, 108241. [Google Scholar] [CrossRef]
- Druart, C.; Neyrinck, A.M.; Vlaeminck, B.; Fievez, V.; Cani, P.D.; Delzenne, N.M. Role of the lower and upper intestine in the production and absorption of gut microbiota-derived PUFA metabolites. PLoS ONE 2014, 9, e87560. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.L.; Gill, S.K.; Brown, K.; Tasnim, N.; Ghosh, S.; Innis, S.; Jacobson, K. Maternal exposure to fish oil primes offspring to harbor intestinal pathobionts associated with altered immune cell balance. Gut Microbes 2015, 6, 24–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, E.Y.; Leone, V.A.; Devkota, S.; Wang, Y.; Brady, M.J.; Chang, E.B. Composition of Dietary Fat Source Shapes Gut Microbiota Architecture and Alters Host Inflammatory Mediators in Mouse Adipose Tissue. JPEN J. Parenter. Enteral. Nutr. 2013, 37, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Cani, P.D. Interaction Between Obesity and the Gut Microbiota: Relevance in Nutrition. Annu. Rev. Nutr. 2011, 31, 15–31. [Google Scholar] [CrossRef]
- Nyangahu, D.D.; Lennard, K.S.; Brown, B.P.; Darby, M.G.; Wendoh, J.M.; Havyarimana, E.; Smith, P.; Butcher, J.; Stintzi, A.; Mulder, N.; et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 2018, 6, 124. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Debelius, J.; Song, S.J.; Vazquez-Baeza, Y.; Xu, Z.Z.; Gonzalez, A.; Knight, R. Tiny microbes, enormous impacts: What matters in gut microbiome studies? Genome Biol. 2016, 17, 217. [Google Scholar] [CrossRef]
- Kelly, B.J.; Gross, R.; Bittinger, K.; Sherrill-Mix, S.; Lewis, J.D.; Collman, R.G.; Bushman, F.D.; Li, H. Power and sample-size estimation for microbiome studies using pairwise distances and PERMANOVA. Bioinformatics 2015, 31, 2461–2468. [Google Scholar] [CrossRef]
- Lallès, J.-P. Intestinal alkaline phosphatase: Novel functions and protective effects. Nutr. Rev. 2014, 72, 82–94. [Google Scholar] [CrossRef]
- Malo, M.S.; Alam, S.N.; Mostafa, G.; Zeller, S.J.; Johnson, P.V.; Mohammad, N.; Chen, K.T.; Moss, A.K.; Ramasamy, S.; Faruqui, A.; et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut 2010, 59, 1476–1484. [Google Scholar] [CrossRef]
- Jiang, W.G.; Bryce, R.P.; Horrobin, D.F.; Mansel, R.E. Regulation of tight junction permeability and occludin expression by polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 1998, 244, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Kirpich, I.A.; Feng, W.; Wang, Y.; Liu, Y.; Barker, D.F.; Barve, S.S.; McClain, C.J. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol. Clin. Exp. Res. 2012, 36, 835–846. [Google Scholar] [CrossRef]
- Druart, C.; Neyrinck, A.M.; Dewulf, E.M.; De Backer, F.C.; Possemiers, S.; Van de Wiele, T.; Moens, F.; De Vuyst, L.; Cani, P.D.; Larondelle, Y.; et al. Implication of fermentable carbohydrates targeting the gut microbiota on conjugated linoleic acid production in high-fat-fed mice. Br. J. Nutr. 2013, 110, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Roche, H.M.; Terres, A.M.; Black, I.B.; Gibney, M.J.; Kelleher, D. Fatty acids and epithelial permeability: Effect of conjugated linoleic acid in Caco-2 cells. Gut 2001, 48, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.; Djokić, J.; Brdarić, E.; Dinić, M.; Terzić-Vidojević, A.; Golić, N.; Veljović, K. The Influence of Heat-Killed Enterococcus faecium BGPAS1-3 on the Tight Junction Protein Expression and Immune Function in Differentiated Caco-2 Cells Infected with Listeria monocytogenes ATCC 19111. Front. Microbiol. 2019, 10, 412. [Google Scholar] [CrossRef]
- Marques, T.M.; Wall, R.; O’Sullivan, O.; Fitzgerald, G.F.; Shanahan, F.; Quigley, E.M.; Cotter, P.D.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; et al. Dietary trans -10, cis -12-conjugated linoleic acid alters fatty acid metabolism and microbiota composition in mice. Br. J. Nutr. 2015, 113, 728–738. [Google Scholar] [CrossRef]
- Vyas, D.; Kadegowda, A.K.G.; Erdman, R.A. Dietary Conjugated Linoleic Acid and Hepatic Steatosis: Species-Specific Effects on Liver and Adipose Lipid Metabolism and Gene Expression. Nutr. Metab. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Park, Y.; Storkson, J.M.; Albright, K.J.; Liu, W.; Pariza, M.W. Evidence that the trans-10,cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids 1999, 34, 235–241. [Google Scholar] [CrossRef]
- Field, C.J.; Blewett, H.H.; Proctor, S.; Vine, D. Human health benefits of vaccenic acid. Appl. Physiol. Nutr. Metab. 2009, 34, 979–991. [Google Scholar] [CrossRef]
- Jacome-Sosa, M.M.; Borthwick, F.; Mangat, R.; Uwiera, R.; Reaney, M.J.; Shen, J.; Quiroga, A.D.; Jacobs, R.L.; Lehner, R.; Proctor, S.D.; et al. Diets enriched in trans-11 vaccenic acid alleviate ectopic lipid accumulation in a rat model of NAFLD and metabolic syndrome. J. Nutr. Biochem. 2014, 25, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jacome-Sosa, M.M.; Ruth, M.R.; Lu, Y.; Shen, J.; Reaney, M.J.; Scott, S.L.; Dugan, M.E.R.; Anderson, H.D.; Field, C.J.; et al. The intestinal bioavailability of vaccenic acid and activation of peroxisome proliferator-activated receptor-α and -γ in a rodent model of dyslipidemia and the metabolic syndrome. Mol. Nutr. Food Res. 2012, 56, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Hosokawa, M.; Tanaka, L.; Kohno, H.; Tanaka, T.; Miyashita, K. Potent inhibitory effect of trans9, trans11 isomer of conjugated linoleic acid on the growth of human colon cancer cells. J. Nutr. Biochem. 2006, 17, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Myles, I.A.; Fontecilla, N.M.; Janelsins, B.M.; Vithayathil, P.J.; Segre, J.A.; Datta, S.K. Parental dietary fat intake alters offspring microbiome and immunity. J. Immunol. 2013, 191, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Z.; Chen, L.; Tang, L.; Wen, S.; Liu, Y.; Yuan, J. Diet induced maternal obesity affects offspring gut microbiota and persists into young adulthood. Food Funct. 2018, 9, 4317–4327. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.M.; Antony, K.M.; Ma, J.; Prince, A.L.; Showalter, L.; Moller, M.; Aagaard, K.M. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016, 8, 77. [Google Scholar] [CrossRef]
- Robertson, R.C.; Kaliannan, K.; Strain, C.R.; Ross, R.P.; Stanton, C.; Kang, J.X. Maternal omega-3 fatty acids regulate offspring obesity through persistent modulation of gut microbiota. Microbiome 2018, 6, 95. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Hou, C.-Y.; Lee, C.-T.; Chan, J.Y.H.; Tain, Y.-L. The Interplay between Maternal and Post-Weaning High-Fat Diet and Gut Microbiota in the Developmental Programming of Hypertension. Nutrients 2019, 11, 1982. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Jiang, H. Combined maternal and post-weaning high fat diet inhibits male offspring’s prostate cancer tumorigenesis in transgenic adenocarcinoma of mouse prostate model. Prostate 2019, 79, 544–553. [Google Scholar] [CrossRef]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Piccolo, B.D.; Mercer, K.E.; Andres, A.; Thakali, K.M.; Shankar, K. Maternal High-Fat Diet Programs Offspring Liver Steatosis in a Sexually Dimorphic Manner in Association with Changes in Gut Microbial Ecology in Mice. Sci. Rep. 2018, 8, 16502. [Google Scholar] [CrossRef]
- Prince, A.L.; Pace, R.M.; Dean, T.; Takahashi, D.; Kievit, P.; Friedman, J.E.; Aagaard, K.M. The development and ecology of the Japanese macaque gut microbiome from weaning to early adolescence in association with diet. Am. J. Primatol. 2019, 81, e22980. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; de Vos, W.M. Early life colonization of the human gut: Microbes matter everywhere. Curr. Opin. Microbiol. 2018, 44, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.C.; Dinsmoor, A.M.; Wang, M.; Donovan, S.M. Microbiome Composition in Pediatric Populations from Birth to Adolescence: Impact of Diet and Prebiotic and Probiotic Interventions. Dig. Dis. Sci. 2020, 65, 706–722. [Google Scholar] [CrossRef]
- Caruso, R.; Ono, M.; Bunker, M.E.; Núñez, G.; Inohara, N. Dynamic and Asymmetric Changes of the Microbial Communities after Cohousing in Laboratory Mice. Cell Rep. 2019, 27, 3401–3412.e3. [Google Scholar] [CrossRef]
- Greiner, T.; Bäckhed, F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol. Metab. 2011, 22, 117–123. [Google Scholar] [CrossRef]


| Maternal Diet | C | LA | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Weaning Diet | C | LA | C | LA | Maternal Diet | Weaning Diet | x 1 |
| SFA | 24.5 (0.7) | 25.9 (1.3) | 25.5 (0.4) | 24.6 (1.3) | 0.89 | 0.76 | 0.27 |
| MUFA | 67.5 (0.5) | 31.2 (1.1) | 66.4 (0.2) | 30.7 (1.6) | 0.44 | <0.001 | 0.79 |
| n-6 PUFA | 6.4 (0.3) | 41.6 (1.8) | 6.7 (0.2) | 43.2 (1.4) | 0.39 | <0.001 | 0.54 |
| 18:2 n-6 | 6.2 (0.2) | 39.8 (2.0) | 6.5 (0.2) | 41.3 (1.4) | 0.44 | <0.001 | 0.59 |
| 20:4 n-6 | 0.1 (0.0) | 0.8 (0.1) | 0.1 (0.0) | 0.9 (0.1) | 0.33 | <0.001 | 0.50 |
| n-3 PUFA | 1.6 (0.0) ab | 1.6 (0.0) ab | 1.4 (0.1) a | 1.8 (0.0) b | 0.95 | 0.03 | 0.01 |
| n-6/n-3 | 4.0 (0.1) a | 26.7 (0.6) b | 4.9 (0.5) a | 24.2 (0.3) c | 0.07 | <0.001 | <0.01 |
| Maternal Diet | C | LA | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Weaning Diet | C | LA | C | LA | Maternal Diet | Weaning Diet | x 1 |
| 18:2 cis-9, trans-11 | 0.012 (0.003) a | 0.014 (0.002) a | 0.010 (0.002) a | 0.024 (0.002) b | 0.08 | 0.1 | 0.02 |
| 18:2 trans-10, cis-12 | 0.008 (0.002) | 0.057 (0.03) | 0.006 (0.001) | 0.013 (0.005) | 0.08 | 0.04 | 0.11 |
| 18:2 cis-9, cis-11 | 0.026 (0.009) | 0.012 (0.003) | 0.039 (0.006) | 0.013 (0.004) | 0.30 | 0.01 | 0.40 |
| 18:2 trans-11, trans-13 | 0.011 (0.003) | 0.009 (0.002) | 0.006 (0.001) | 0.007 (0.004) | 0.35 | 0.80 | 0.61 |
| 18:2 trans-9, trans-11 | 0.011 (0.003) | 0.004 (0.009) | 0.008 (0.003) | 0.015 (0.005) | 0.30 | 0.87 | 0.08 |
| 18:1 trans-11 | 0.042 (0.009) ab | 0.031 (0.005) ab | 0.027 (0.005) a | 0.050 (0.004) b | 0.77 | 0.34 | 0.02 |
| Maternal Diet | C | LA | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Weaning Diet | C | LA | C | LA | Maternal Diet | Weaning Diet | x 1 |
| SFA | 21.7 (0.3) | 23.1 (0.3) | 22.9 (0.4) | 24.2 (0.9) | 0.05 | 0.02 | 0.96 |
| MUFA | 70.8 (0.3) | 30.4 (0.7) | 69.2 (0.3) | 29.8 (0.7) | 0.08 | <0.001 | 0.36 |
| n-6 PUFA | 6.4 (0.1) | 44.9 (0.9) | 6.9 (0.1) | 44.5 (1.4) | 0.99 | <0.001 | 0.59 |
| 18:2 n-6 | 6.1 (0.1) | 43.4 (0.8) | 6.5 (0.1) | 42.8 (1.5) | 0.94 | <0.001 | 0.60 |
| 20:4 n-6 | 0.2 (0.0) | 0.7 (0.1) | 0.1 (0.0) | 0.8 (0.1) | 0.27 | <0.001 | 0.50 |
| n-3 PUFA | 1.0 (0.0) | 1.3 (0.1) | 1.0 (0.0) | 1.3 (0.0) | 0.08 | 0.02 | 0.66 |
| n-6/n-3 | 6.6 (0.1) | 34.9 (0.8) | 6.6 (0.0) | 33.2 (1.4) | 0.31 | <0.001 | 0.31 |
| Maternal Diet | C | LA | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Weaning Diet | C | LA | C | LA | Maternal Diet | Weaning Diet | x 1 |
| 18:2 cis-9, trans-11 | 0.015 (0.001) | 0.033 (0.004) | 0.011 (0.002) | 0.029 (0.002) | 0.21 | <0.001 | 0.98 |
| 18:2 trans-10, cis-12 | 0.025 (0.013) | 0.016 (0.003) | 0.020 (0.007) | 0.016 (0.004) | 0.75 | 0.37 | 0.78 |
| 18:2 cis-9, cis-11 | 0.033 (0.004) | 0.013 (0.002) | 0.024 (0.006) | 0.014 (0.004) | 0.34 | <0.01 | 0.25 |
| 18:2 trans-11, trans-13 | 0.013 (0.003) | 0.006 (0.001) | 0.012 (0.002) | 0.010 (0.003) | 0.64 | 0.04 | 0.35 |
| 18:2 trans-9, trans-11 | 0.022 (0.009) | 0.026 (0.004) | 0.008 (0.001) | 0.010 (0.003) | <0.01 | 0.54 | 0.89 |
| 18:1 trans-11 | 0.030 (0.005) | 0.045 (0.006) | 0.016 (0.004) | 0.037 (0.01) | 0.13 | 0.02 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchix, J.; Alain, C.; David-Le Gall, S.; Acuña-Amador, L.A.; Druart, C.; Delzenne, N.M.; Barloy-Hubler, F.; Legrand, P.; Boudry, G. Maternal Linoleic Acid Overconsumption Alters Offspring Gut and Adipose Tissue Homeostasis in Young but Not Older Adult Rats. Nutrients 2020, 12, 3451. https://doi.org/10.3390/nu12113451
Marchix J, Alain C, David-Le Gall S, Acuña-Amador LA, Druart C, Delzenne NM, Barloy-Hubler F, Legrand P, Boudry G. Maternal Linoleic Acid Overconsumption Alters Offspring Gut and Adipose Tissue Homeostasis in Young but Not Older Adult Rats. Nutrients. 2020; 12(11):3451. https://doi.org/10.3390/nu12113451
Chicago/Turabian StyleMarchix, Justine, Charlène Alain, Sandrine David-Le Gall, Luis Alberto Acuña-Amador, Céline Druart, Nathalie M. Delzenne, Frédérique Barloy-Hubler, Philippe Legrand, and Gaëlle Boudry. 2020. "Maternal Linoleic Acid Overconsumption Alters Offspring Gut and Adipose Tissue Homeostasis in Young but Not Older Adult Rats" Nutrients 12, no. 11: 3451. https://doi.org/10.3390/nu12113451
APA StyleMarchix, J., Alain, C., David-Le Gall, S., Acuña-Amador, L. A., Druart, C., Delzenne, N. M., Barloy-Hubler, F., Legrand, P., & Boudry, G. (2020). Maternal Linoleic Acid Overconsumption Alters Offspring Gut and Adipose Tissue Homeostasis in Young but Not Older Adult Rats. Nutrients, 12(11), 3451. https://doi.org/10.3390/nu12113451






