Prospective Evaluation of Mango Fruit Intake on Facial Wrinkles and Erythema in Postmenopausal Women: A Randomized Clinical Pilot Study
Abstract
1. Introduction
2. Materials and Methods
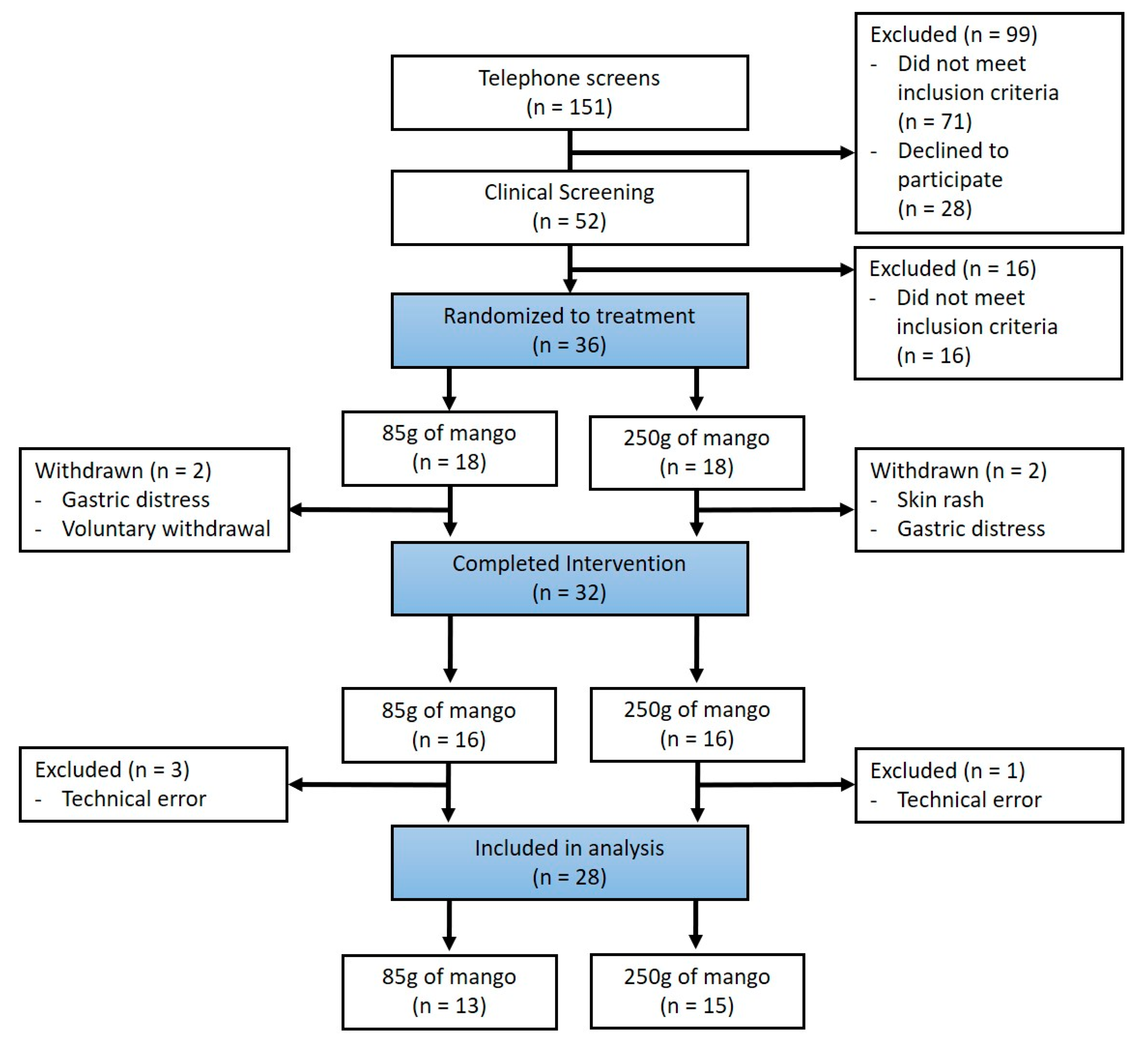
2.1. Participants
2.2. Study Design
2.3. Ataulfo Mangos
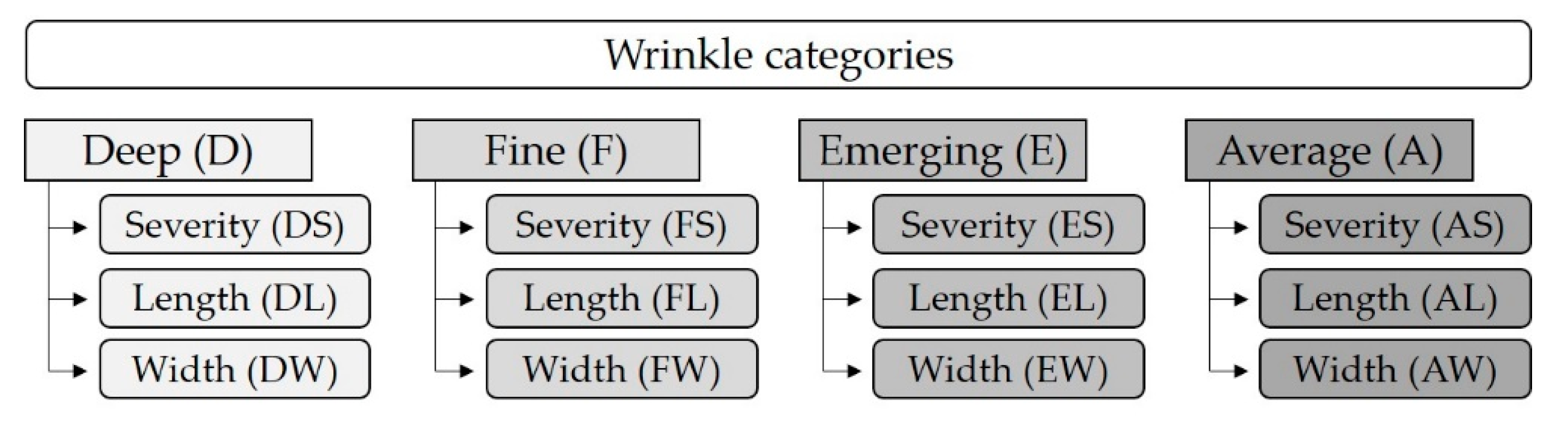
2.4. Wrinkles and Erythema
2.5. Skin Carotenoids
2.6. Blood Pressure and Lipids
2.7. Dietary Intake
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Dietary Intake
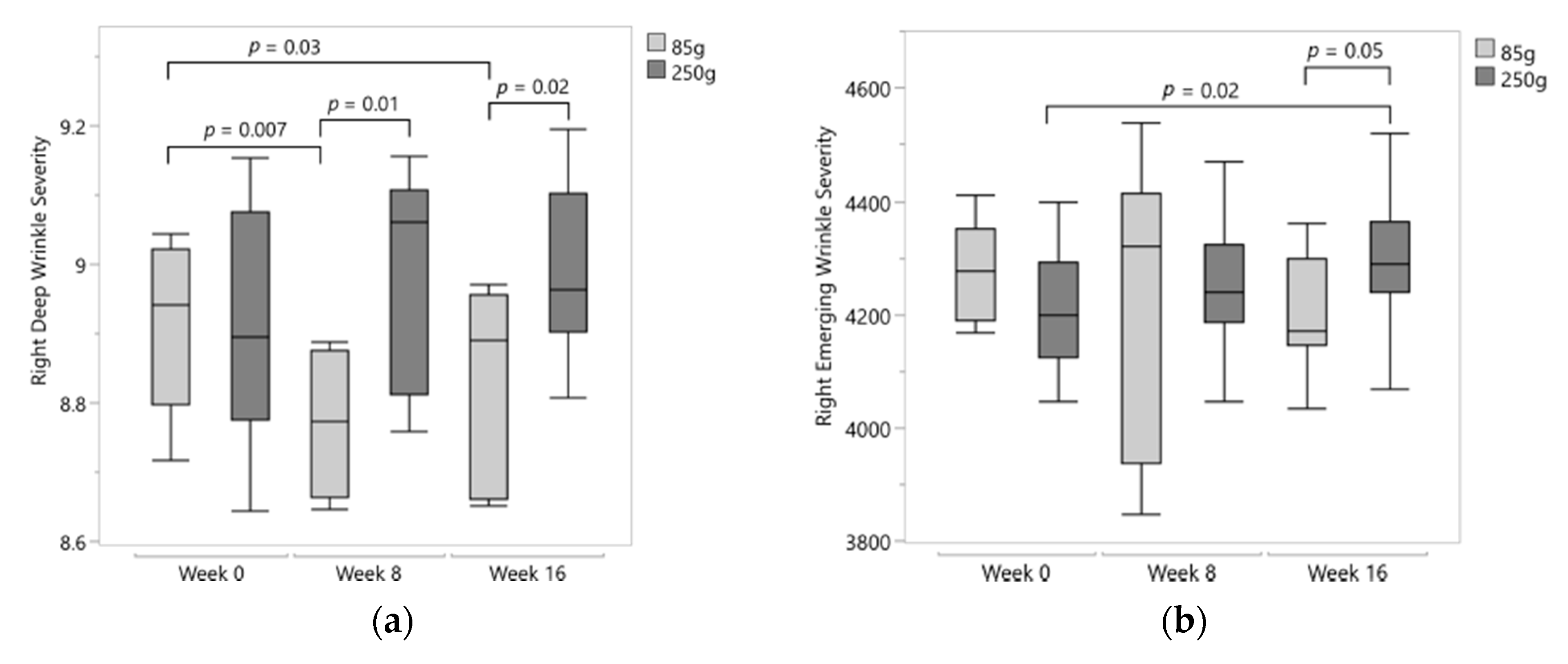
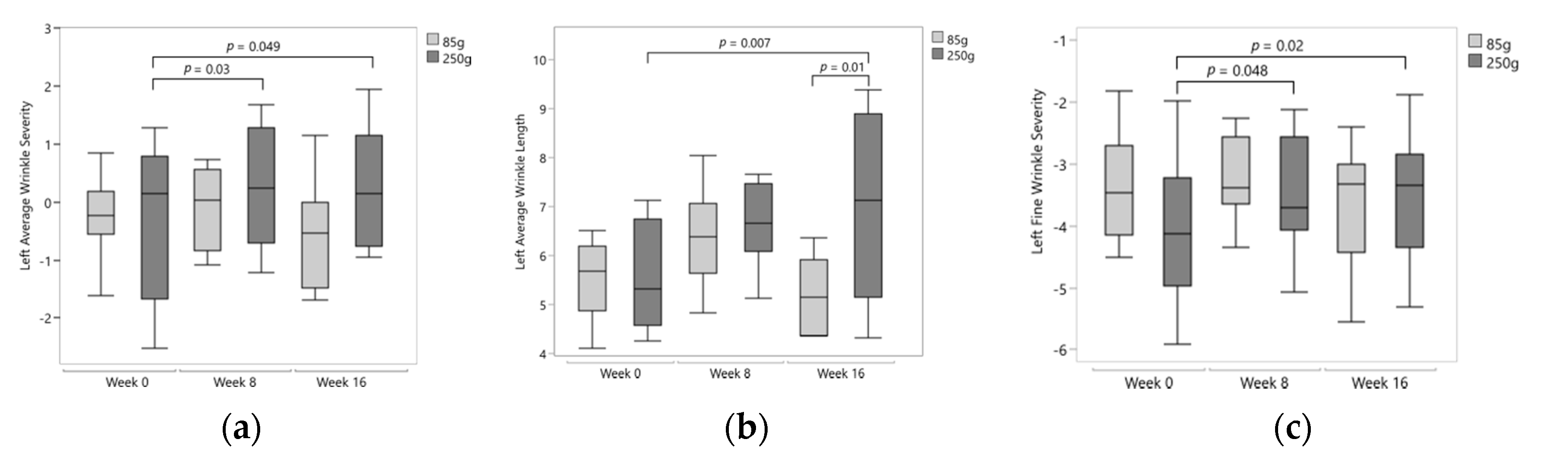
3.3. Facial Wrinkles and Erythema
3.4. Skin Carotenoids
3.5. Blood Pressure and Plasma Lipids
3.6. Correlations
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and Extrinsic Factors in Skin Ageing: A Review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Chien, A.L.; Kang, S. Photoaging. Dermatol. Clin. 2014, 32, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, Prevention and Therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Friedrich, A.; Tscherch, K.; Haag, S.F.; Darvin, M.E.; Vollert, H.; Groth, N.; Lademann, J.; Rohn, S. Influence of Dietary Carotenoids on Radical Scavenging Capacity of the Skin and Skin Lipids. Eur. J. Pharm. Biopharm. 2013, 84, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Nowbary, C.K.; Schanzer, S.; Vollert, H.; Lademann, J.; Darvin, M.E. Influences of Orally Taken Carotenoid-Rich Curly Kale Extract on Collagen I/Elastin Index of the Skin. Nutrients 2017, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.; Wainwright, L.J.; Holland, R.; Barrett, K.E.; Casey, J. Wrinkle Reduction in Post-Menopausal Women Consuming a Novel Oral Supplement: A Double-Blind Placebo-Controlled Randomized Study. Int. J. Cosmet. Sci. 2014, 36, 22–31. [Google Scholar] [CrossRef]
- Costa, A.; Pegas Pereira, E.S.; Assumpção, E.C.; Calixto dos Santos, F.B.; Ota, F.S.; de Oliveira Pereira, M.; Fidelis, M.C.; Fávaro, R.; Barros Langen, S.S.; Favaro de Arruda, L.H.; et al. Assessment of Clinical Effects and Safety of an Oral Supplement Based on Marine Protein, Vitamin C, Grape Seed Extract, Zinc, and Tomato Extract in the Improvement of Visible Signs of Skin Aging in Men. Clin. Cosmet. Investig. Dermatol. 2015, 8, 319–328. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular Evidence That Oral Supplementation with Lycopene or Lutein Protects Human Skin against Ultraviolet Radiation: Results from a Double-Blinded, Placebo-Controlled, Crossover Study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. β-Carotene and Other Carotenoids in Protection from Sunlight. Am. J. Clin. Nutr. 2012, 96, 1179S–1184S. [Google Scholar] [CrossRef]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef]
- Manthey, J.A.; Perkins-Veazie, P. Influences of Harvest Date and Location on the Levels of β-Carotene, Ascorbic Acid, Total Phenols, the in Vitro Antioxidant Capacity, and Phenolic Profiles of Five Commercial Varieties of Mango (Mangifera Indica L.). J. Agric. Food Chem. 2009, 57, 10825–10830. [Google Scholar] [CrossRef] [PubMed]
- Ornelas-Paz, J.D.J.; Failla, M.L.; Yahia, E.M.; Gardea-Bejar, A. Impact of the Stage of Ripening and Dietary Fat on in Vitro Bioaccessibility of β-Carotene in ‘Ataulfo’ Mango. J. Agric. Food Chem. 2008, 56, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Gil-Chávez, J.; Sotelo-Mundo, R.R.; Namiesnik, J.; Gorinstein, S.; González-Aguilar, G.A. Antioxidant Interactions between Major Phenolic Compounds Found in ‘Ataulfo’ Mango Pulp: Chlorogenic, Gallic, Protocatechuic and Vanillic Acids. Molecules 2012, 17, 12657–12664. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.-Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.; Yi, T.H. Gallic Acid Regulates Skin Photoaging in UVB-Exposed Fibroblast and Hairless Mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef]
- Fukushima, Y.; Takahashi, Y.; Hori, Y.; Kishimoto, Y.; Shiga, K.; Tanaka, Y.; Masunaga, E.; Tani, M.; Yokoyama, M.; Kondo, K. Skin Photoprotection and Consumption of Coffee and Polyphenols in Healthy Middle-Aged Japanese Females. Int. J. Dermatol. 2015, 54, 410–418. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.-H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A Natural Miracle Bioactive Compound against Lifestyle Related Disorders. Lipids Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef]
- Ochocka, R.; Hering, A.; Stefanowicz–Hajduk, J.; Cal, K.; Barańska, H. The Effect of Mangiferin on Skin: Penetration, Permeation and Inhibition of ECM Enzymes. PLoS ONE 2017, 12, e0181542. [Google Scholar] [CrossRef]
- Song, J.H.; Bae, E.Y.; Choi, G.; Hyun, J.W.; Lee, M.Y.; Lee, H.W.; Chae, S. Protective Effect of Mango (Mangifera Indica L.) against UVB-Induced Skin Aging in Hairless Mice. Photodermatol. Photoimmunol. Photomed. 2013, 29, 84–89. [Google Scholar] [CrossRef]
- Dietary Guidelines—Health.gov. 2015–2020. Available online: https://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 25 October 2019).
- Lee, S.; Choi, Y.; Jeong, H.S.; Lee, J.; Sung, J. Effect of Different Cooking Methods on the Content of Vitamins and True Retention in Selected Vegetables. Food Sci. Biotechnol. 2017, 27, 333–342. [Google Scholar] [CrossRef]
- Jahns, L.; Johnson, L.K.; Conrad, Z.; Bukowski, M.; Raatz, S.K.; Jilcott Pitts, S.; Wang, Y.; Ermakov, I.V.; Gellermann, W. Concurrent Validity of Skin Carotenoid Status as a Concentration Biomarker of Vegetable and Fruit Intake Compared to Multiple 24-h Recalls and Plasma Carotenoid Concentrations across One Year: A Cohort Study. Nutr. J. 2019, 18, 78. [Google Scholar] [CrossRef]
- Danby, F.W. Nutrition and Aging Skin: Sugar and Glycation. Clin. Dermatol. 2010, 28, 409–411. [Google Scholar] [CrossRef]
- Foolad, N.; Vaughn, A.R.; Rybak, I.; Burney, W.A.; Chodur, G.M.; Newman, J.W.; Steinberg, F.M.; Sivamani, R.K. Prospective Randomized Controlled Pilot Study on the Effects of Almond Consumption on Skin Lipids and Wrinkles. Phytother. Res. 2019, 33, 3212–3217. [Google Scholar] [CrossRef]
- Cho, S.; Lee, D.H.; Won, C.-H.; Kim, S.M.; Lee, S.; Lee, M.-J.; Chung, J.H. Differential Effects of Low-Dose and High-Dose Beta-Carotene Supplementation on the Signs of Photoaging and Type I Procollagen Gene Expression in Human Skin in Vivo. Dermatology 2010, 221, 160–171. [Google Scholar] [CrossRef]
- Han, J.-H.; Yang, Y.-X.; Feng, M.-Y. Contents of Phytosterols in Vegetables and Fruits Commonly Consumed in China. Biomed. Environ. Sci. 2008, 21, 449–453. [Google Scholar] [CrossRef]
- Cabral, C.E.; Klein, M.R.S.T. Phytosterols in the Treatment of Hypercholesterolemia and Prevention of Cardiovascular Diseases. Arq. Bras. Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Vermeer, M.A.; Hiemstra, H.; Ras, R.T. LDL-Cholesterol Lowering of Plant Sterols and Stanols—Which Factors Influence Their Efficacy? Nutrients 2018, 10, 1262. [Google Scholar] [CrossRef]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-Lowering Effects of Dietary Fiber: A Meta-Analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, Technological and in Vitro Antioxidant Properties of Mango, Guava, Pineapple and Passion Fruit Dietary Fibre Concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Astiazaran-Garcia, H.; Wall-Medrano, A.; de la Rosa, L.A.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Mango Phenolics Increase the Serum Apolipoprotein A1/B Ratio in Rats Fed High Cholesterol and Sodium Cholate Diets. J. Sci. Food Agric. 2019, 99, 1604–1612. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Hamm, L.; Batuman, V.; Whelton, P.K. Serum Antioxidant Vitamins and Blood Pressure in the United States Population. Hypertension 2002, 40, 810–816. [Google Scholar] [CrossRef]
- Buijsse, B.; Feskens, E.J.M.; Kwape, L.; Kok, F.J.; Kromhout, D. Both α- and β-Carotene, but Not Tocopherols and Vitamin C, Are Inversely Related to 15-Year Cardiovascular Mortality in Dutch Elderly Men. J. Nutr. 2008, 138, 344–350. [Google Scholar] [CrossRef] [PubMed]
| 85 g Group (n = 13) | 250 g Group (n = 15) | p | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 61 ± 5.1 | 60 ± 5.3 | 0.58 |
| BMI (kg/m2) | 26.4 ± 4.0 | 22.9 ± 2.6 | 0.01 * |
| WC (cm) | 89 ± 12.0 | 83 ± 9.4 | 0.13 |
| SBP (mmHg) | 115 ± 10.7 | 113 ± 6.9 | 0.96 † |
| DBP (mmHg) | 75 ± 6.5 | 74 ± 4.0 | 0.61 |
| HR (BPM) | 63 ± 6.2 | 61 ± 7.2 | 0.53 |
| RS Carotenoid (nm wavelength) | 363 ± 78 | 432 ± 105 | 0.06 |
| Glucose (mg/dL) | 97.5 ± 6.5 | 95.1 ± 7.5 | 0.46 ‡ |
| Cholesterol (mg/dL) | 220 ± 31.9 | 225 ± 44.2 | 0.83 ‡ |
| LDL (mg/dL) | 138 ± 25.6 | 127 ± 27.7 | 0.42 ‡ |
| Non-HDL (mg/dL) | 152 ± 24.7 | 143 ± 27.1 | 0.49 ‡ |
| Triglycerides (mg/dL) | 71 ± 21.6 | 83 ± 69.3 | 0.16 ‡,† |
| Left lateral canthus | |||
| Deep Wrinkle Severity | 7575 ± 1063.4 | 8037 ± 1403.2 | 0.46 Ŧ |
| Deep Wrinkle Length (mm) | 17.61 ± 5.05 | 17.68 ± 3.77 | 0.98 Ŧ |
| Deep Wrinkle Width (mm) | 1.63 ± 0.25 | 1.84 ± 0.24 | 0.09 Ŧ |
| Fine Wrinkle Severity | 5866 ± 680.4 | 5148 ± 1510.4 | 0.08 † |
| Fine Wrinkle Length (mm) | 5.49 ± 1.05 | 4.01 ± 1.40 | 0.004 * |
| Fine Wrinkle Width (mm) | 1.46 ± 0.18 | 1.29 ± 0.40 | 0.24 |
| Emerging Wrinkle Severity | 4279 ± 123.8 | 4249 ± 110.2 | 0.51 |
| Emerging Wrinkle Length (mm) | 5.06 ± 2.92 | 3.64 ± 0.61 | 0.02 *,† |
| Emerging Wrinkle Width (mm) | 1.20 ± 0.13 | 1.21 ± 0.13 | 0.90 |
| Average Wrinkle Severity | 5166 ± 379.1 | 5329 ± 639.3 | 0.54 Ŧ |
| Average Wrinkle Length (mm) | 5.71 ± 1.18 | 5.57 ± 1.08 | 0.80 Ŧ |
| Average Wrinkle Width (mm) | 1.28 ± 0.09 | 1.35 ± 0.18 | 0.47 Ŧ,† |
| Right lateral canthus | |||
| Deep Wrinkle Severity | 7487 ± 922.1 | 7537 ± 1338.0 | 0.93 Ŧ |
| Deep Wrinkle Length (mm) | 16.62 ± 5.53 | 17.41 ± 6.27 | 0.78 Ŧ |
| Deep Wrinkle Width (mm) | 1.70 ± 0.20 | 1.71 ± 0.27 | 0.93 Ŧ |
| Fine Wrinkle Severity | 5730 ± 330.4 | 5778 ± 485.1 | 0.76 |
| Fine Wrinkle Length (mm) | 5.75 ± 3.07 | 4.91 ± 0.85 | 0.85 † |
| Fine Wrinkle Width (mm) | 1.52 ± 0.23 | 1.38 ± 0.15 | 0.08 |
| Emerging Wrinkle Severity | 4240 ± 160.6 | 4178 ± 186.9 | 0.18 † |
| Emerging Wrinkle Length (mm) | 4.00 ± 0.61 | 3.62 ± 0.52 | 0.09 |
| Emerging Wrinkle Width (mm) | 1.22 ± 0.12 | 1.26 ± 0.16 | 0.47 |
| Average Wrinkle Severity | 5169 ± 256.9 | 5336 ± 541.7 | 0.40 Ŧ |
| Average Wrinkle Length (mm) | 6.10 ± 1.84 | 6.12 ± 1.44 | 0.82 Ŧ,† |
| Average Wrinkle Width (mm) | 1.36 ± 0.13 | 1.38 ± 0.16 | 0.81 Ŧ |
| Erythema | |||
| Left Cheek | 14.73 ± 6.90 | 16.18 ± 4.96 | 0.55 |
| Right Cheek | 18.02 ± 13.08 | 14.00 ± 8.85 | 0.43 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fam, V.W.; Holt, R.R.; Keen, C.L.; Sivamani, R.K.; Hackman, R.M. Prospective Evaluation of Mango Fruit Intake on Facial Wrinkles and Erythema in Postmenopausal Women: A Randomized Clinical Pilot Study. Nutrients 2020, 12, 3381. https://doi.org/10.3390/nu12113381
Fam VW, Holt RR, Keen CL, Sivamani RK, Hackman RM. Prospective Evaluation of Mango Fruit Intake on Facial Wrinkles and Erythema in Postmenopausal Women: A Randomized Clinical Pilot Study. Nutrients. 2020; 12(11):3381. https://doi.org/10.3390/nu12113381
Chicago/Turabian StyleFam, Vivien W., Roberta R. Holt, Carl L. Keen, Raja K. Sivamani, and Robert M. Hackman. 2020. "Prospective Evaluation of Mango Fruit Intake on Facial Wrinkles and Erythema in Postmenopausal Women: A Randomized Clinical Pilot Study" Nutrients 12, no. 11: 3381. https://doi.org/10.3390/nu12113381
APA StyleFam, V. W., Holt, R. R., Keen, C. L., Sivamani, R. K., & Hackman, R. M. (2020). Prospective Evaluation of Mango Fruit Intake on Facial Wrinkles and Erythema in Postmenopausal Women: A Randomized Clinical Pilot Study. Nutrients, 12(11), 3381. https://doi.org/10.3390/nu12113381






