Bilberry Anthocyanins Ameliorate NAFLD by Improving Dyslipidemia and Gut Microbiome Dysbiosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Experiment Design
2.3. Measurement of Serum Biochemical Indexes
2.4. Measurement of 2-Thiobarbituric Acid Reactive Substances (TBARS)
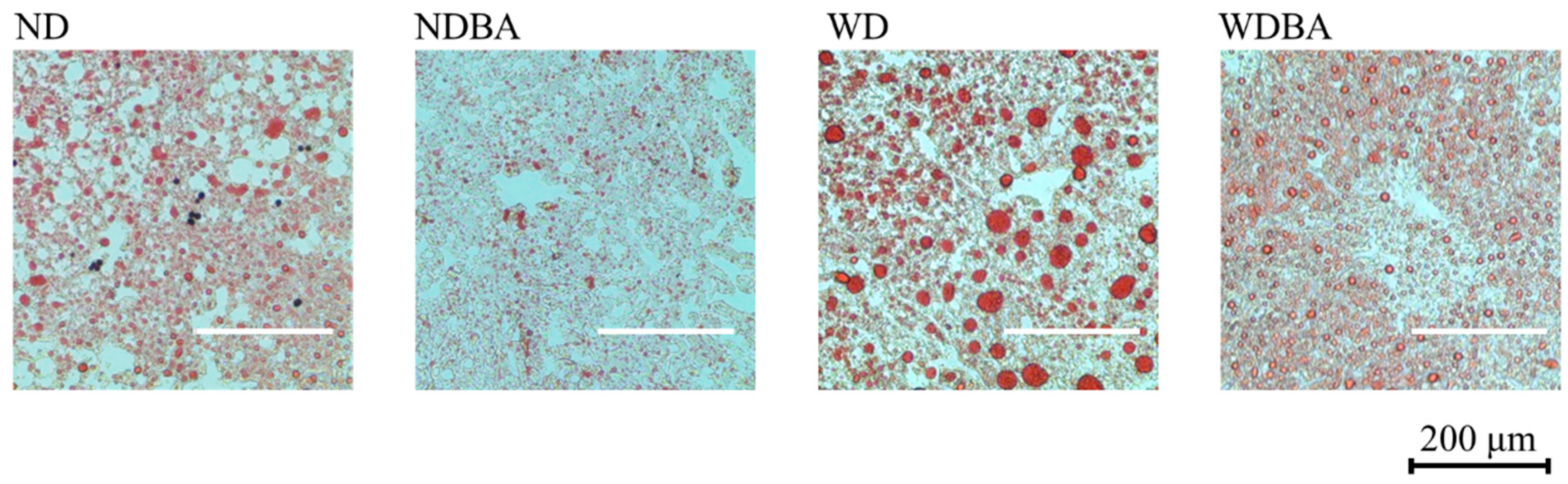
2.5. Histomorphological Analysis
2.6. Extraction of Hepatic and Fecal Lipid
2.7. Measurement of Short-Chain Fatty Acid (SCFA) Concentration in Caecum
2.8. Hepatic Protein Extraction and Western Blot Assay
2.9. Gut Microbiota Analysis by 16S rRNA Gene Sequencing
2.10. Statistical Analysis
3. Results
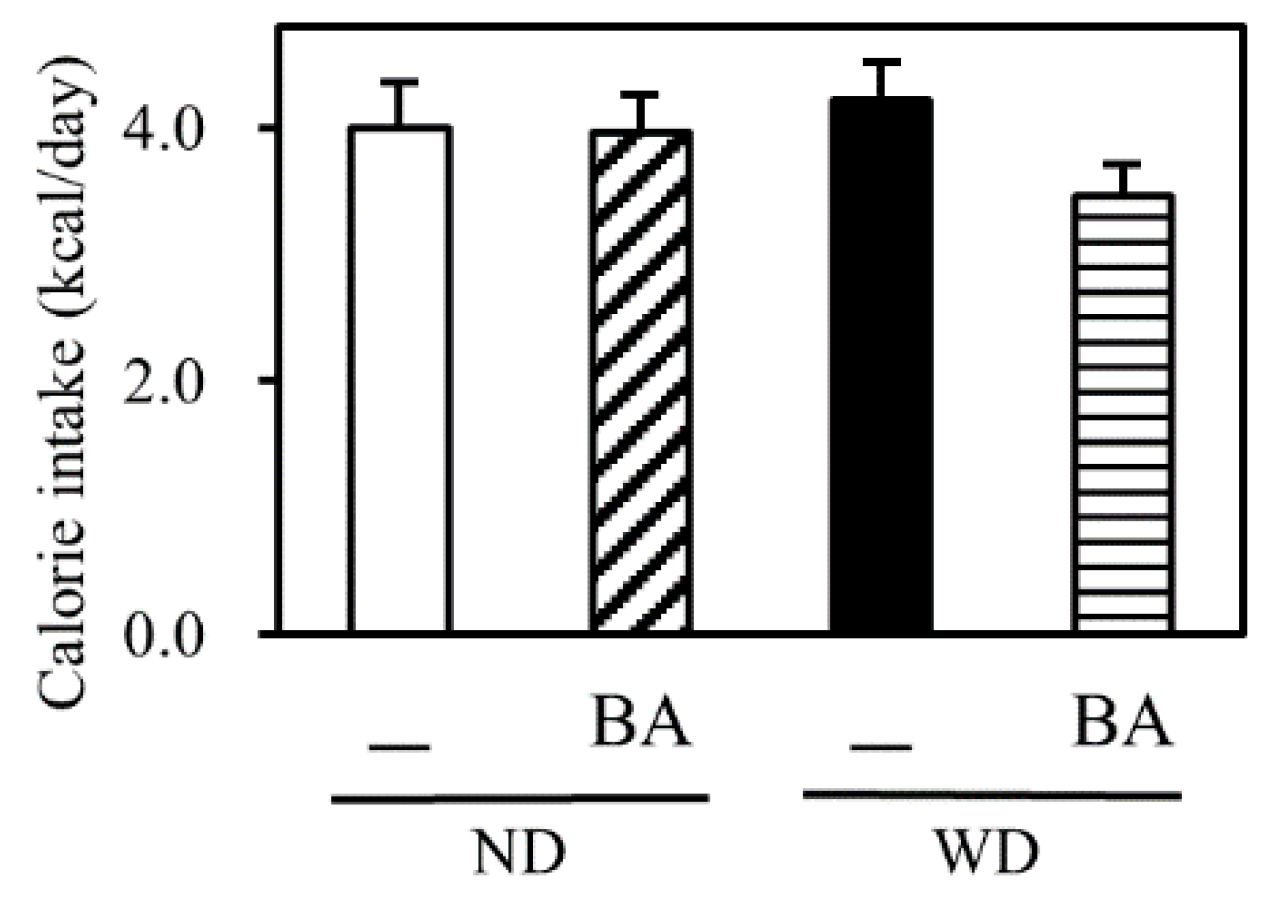
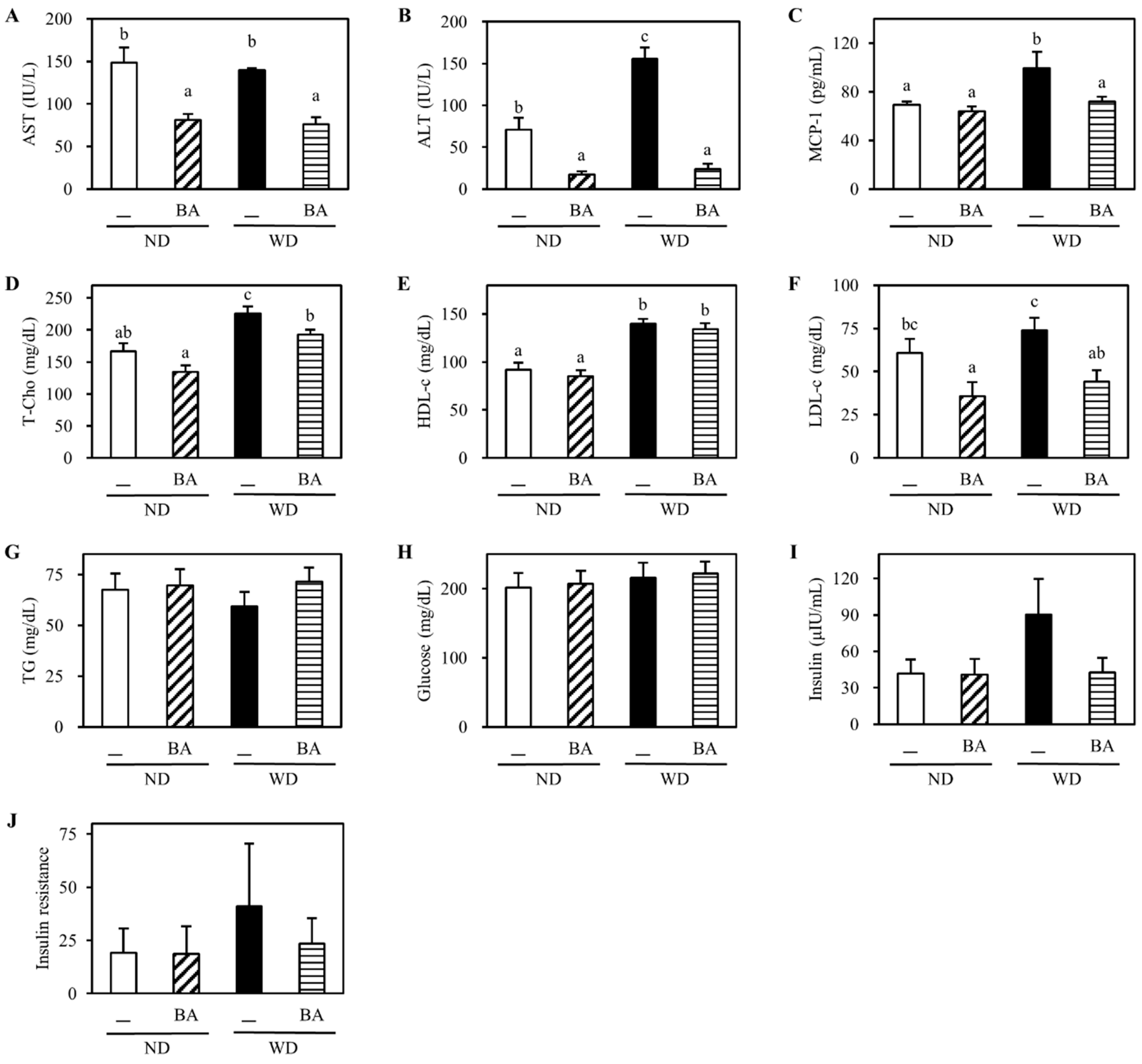
3.1. Body Weight and Indexes of Liver Injury
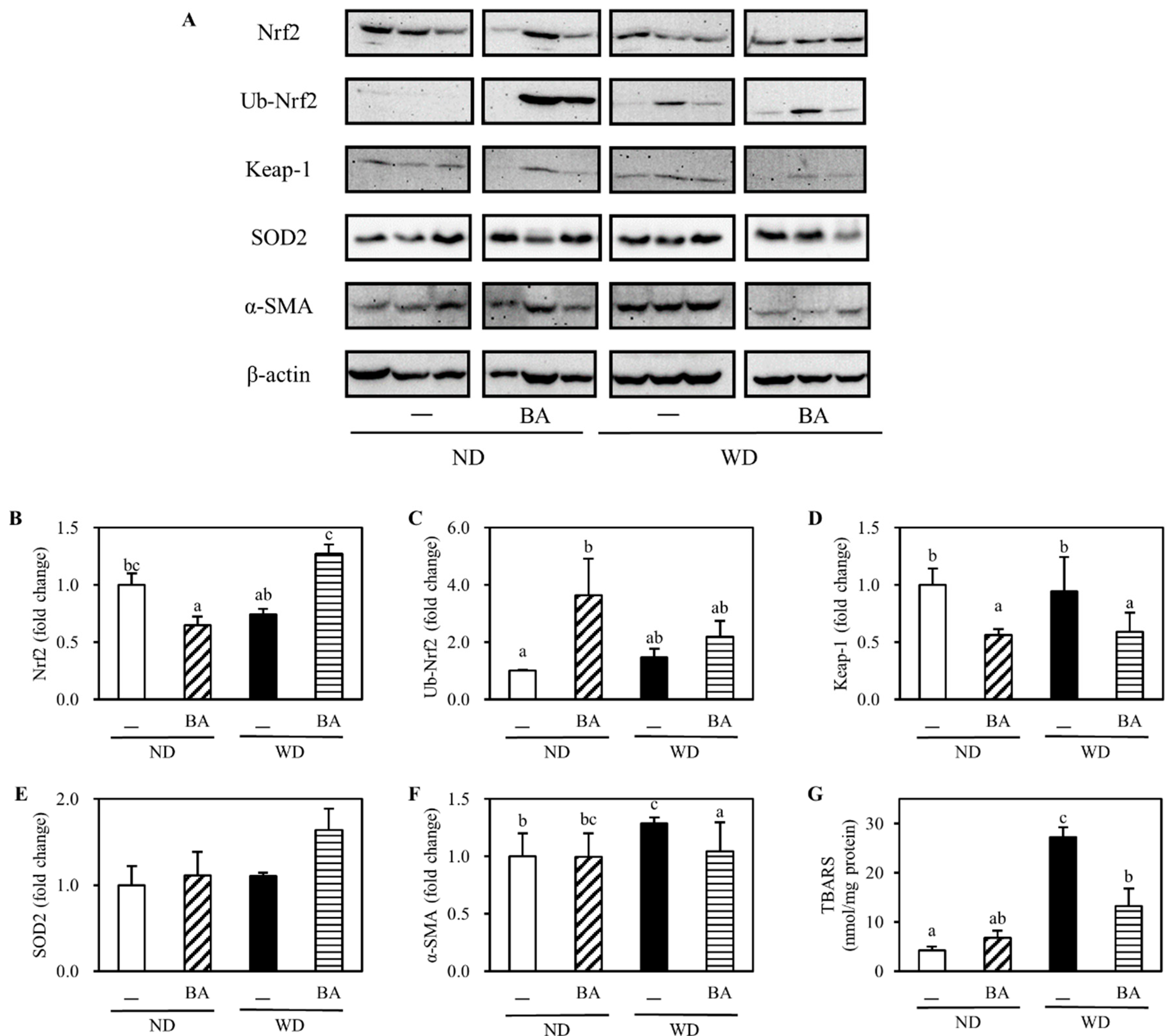
3.2. Activation of Nrf2-Antioxidant Pathway and Amelioration of Non-Alcoholic Fatty Liver Disease (NAFLD)-Associated Markers
3.3. The Concentration of Caecal Organic Acids
3.4. Modulation of Mouse Gut Microbiome
3.5. A Correlation Between Gut Microbiota and NAFLD-Associated Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Components (%) | ND | WD | ||
|---|---|---|---|---|
| — | BA | — | BA | |
| Lard | 3 | 3 | 30 | 30 |
| Soybean oil | 3 | 3 | 3 | 3 |
| Corn Starch | 45 | 43 | 16.5 | 14.5 |
| Casein | 20 | 20 | 20 | 20 |
| Sucrose | 20 | 20 | 20 | 20 |
| Cellulose | 4 | 4 | 4 | 4 |
| Mineral Mix | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin Mix | 1 | 1 | 1 | 1 |
| Cholesterol | 0 | 0 | 1.5 | 1.5 |
| Choline Bitartrate | 0.2 | 0.2 | 0.2 | 0.2 |
| Methionine | 0.3 | 0.3 | 0.3 | 0.3 |
| Bilberry anthocyanins (BA) | 2 | 2 | ||
| Total calories (kcal/100 g) | 374 | 367 | 517 | 510 |
Appendix B
References
- ICD-11 for Mortality and Morbidity Statistics (Version: 09/2020). Available online: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/1912806631 (accessed on 25 September 2020).
- Definition & Facts of NAFLD & NASH. Available online: https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/definition-facts (accessed on 26 September 2020).
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Moolla, A.; Cobbold, J.F.; Hodson, L.; Tomlinson, J.W. Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management. Endocr. Rev. 2020, 41, 1. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, B.; Brenner, D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Huber, Y.; Galle, P.R.; Schattenberg, J.M. What is the (right) target for non-alcoholic fatty liver disease (NAFLD). Z. Gastroenterol. 2020. [Google Scholar] [CrossRef]
- Tutin, T.G. Flora Europaea; Cambridge University Press: Cambridge, UK, 1972; Volume 3, pp. 12–13. [Google Scholar]
- Cassinese, C.; De Combarieu, E.; Falzoni, M.; Fuzzati, N.; Pace, R.; Sardone, N. New liquid chromatography method with ultraviolet detection for analysis of anthocyanins and anthocyanidins in Vaccinium myrtillus fruit dry extracts and commercial preparations. J. AOAC Int. 2007, 90, 911–919. [Google Scholar] [CrossRef]
- Popović, D.; Đukić, D.; Katić, V.; Jović, Z.; Jović, M.; Lalić, J.; Golubović, I.; Stojanović, S.; Ulrih, N.P.; Stanković, M.; et al. Antioxidant and proapoptotic effects of anthocyanins from bilberry extract in rats exposed to hepatotoxic effects of carbon tetrachloride. Life Sci. 2016, 157, 168–177. [Google Scholar] [CrossRef]
- Hoggard, N.; Cruickshank, M.; Moar, K.M.; Bestwick, C.; Holst, J.J.; Russell, W.; Horgan, G. A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36 % wet weight anthocyanins) modifies glycaemic response in individuals with type 2 diabetes controlled by diet and lifestyle. J. Nutr. Sci. 2013, 2, 1–9. [Google Scholar] [CrossRef]
- Morrison, M.C.; Liang, W.; Mulder, P.; Verschuren, L.; Pieterman, E.; Toet, K.; Heeringa, P.; Wielinga, P.Y.; Kooistra, T.; Kleemann, R. Mirtoselect, an anthocyanin-rich bilberry extract, attenuates non-alcoholic steatohepatitis and associated fibrosis in ApoE∗3Leiden mice. J. Hepatol. 2015. [Google Scholar] [CrossRef]
- Xie, K.; He, X.; Chen, K.; Sakao, K.; Hou, D.X. Ameliorative effects and molecular mechanisms of vine tea on western diet-induced NAFLD. Food Funct. 2020. [Google Scholar] [CrossRef] [PubMed]
- Warnick, G.R.; Knopp, R.H.; Fitzpatrick, V.; Branson, L. Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin. Chem. 1990, 36, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.C.; Holman, R.R.; Matthews, D.; Hockaday, T.D.R.; Peto, J. Insulin deficiency and insulin resistance interaction in diabetes: Estimation of their relative contribution by feedback analysis from basal plasma insulin and glucose concentrations. Metabolism 1979, 28, 1086–1096. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Wu, S.; He, X.; Wu, X.; Qin, S.; He, J.; Zhang, S.; Hou, D.-X. Inhibitory effects of blue honeysuckle (Lonicera caerulea L.) on adjuvant-induced arthritis in rats: Crosstalk of anti-inflammatory and antioxidant effects. J. Funct. Foods 2015, 17, 514–523. [Google Scholar] [CrossRef]
- Wu, S.; Hu, R.; Nakano, H.; Chen, K.; Liu, M.; He, X.; Zhang, H.; He, J.; Hou, D.X. Modulation of gut microbiota by Lonicera caerulea l. Berry polyphenols in a mouse model of fatty liver induced by high fat diet. Molecules 2018, 23, 3213. [Google Scholar] [CrossRef]
- Ogawa, K.; Sakakibara, H.; Iwata, R.; Ishii, T.; Sato, T.; Goda, T.; Shimoi, K.; Kumazawa, S. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J. Agric. Food Chem. 2008. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Sanches, S.C.L.; Ramalho, L.N.Z.; Augusto, M.J.; Da Silva, D.M.; Ramalho, F.S. Nonalcoholic steatohepatitis: A search for factual animal models. Biomed Res. Int. 2014, 2015, 1–13. [Google Scholar] [CrossRef]
- Xu, D.; Xu, M.; Jeong, S.; Qian, Y.; Wu, H.; Xia, Q.; Kong, X. The role of Nrf2 in liver disease: Novel molecular mechanisms and therapeutic approaches. Front. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Tanaka, Y.; Aleksunes, L.M.; Yeager, R.L.; Gyamfi, M.A.; Esterly, N.; Guo, G.L.; Klaassen, C.D. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 2008. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tabbi-Anneni, I.; Gunda, V.; Wang, L. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Baker, S.S.; Nugent, C.A.; Tsompana, M.; Cai, L.; Wang, Y.; Buck, M.J.; Genco, R.J.; Baker, R.D.; Zhu, R.; et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: A meta-analysis. Physiol. Genomics 2018, 50, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Eifert, J.D.; Hackney, C.R.; Pierson, M.D.; Duncan, S.E.; Eigel, W.N. Acetic, lactic, and hydrochloric acid effects on Staphylococcus aureus 196E growth based on a predictive model. J. Food Sci. 1997. [Google Scholar] [CrossRef]
- Paturi, G.; Butts, C.A.; Monro, J.A.; Hedderley, D. Effects of blackcurrant and dietary fibers on large intestinal health biomarkers in rats. Plant Foods Hum. Nutr. 2018. [Google Scholar] [CrossRef]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Kołodziejczyk, K.; Milala, J.; Kosmala, M.; Zduńczyk, Z. Anthocyanins in strawberry polyphenolic extract enhance the beneficial effects of diets with fructooligosaccharides in the rat cecal environment. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Keilbaugh, S.U.E.A.; Hamady, M.; Knight, R.O.B.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity MARIE. Gastroenterology 2009, 137, 1716–1724. [Google Scholar] [CrossRef]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef]
- Koh, G.Y.; Kane, A.; Lee, K.; Xu, Q.; Wu, X.; Roper, J.; Mason, J.B.; Crott, J.W. Parabacteroides distasonis attenuates toll-like receptor 4 signaling and Akt activation and blocks colon tumor formation in high-fat diet-fed azoxymethane-treated mice. Int. J. Cancer 2018, 143, 1797–1805. [Google Scholar] [CrossRef]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; De Vos, W.M. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
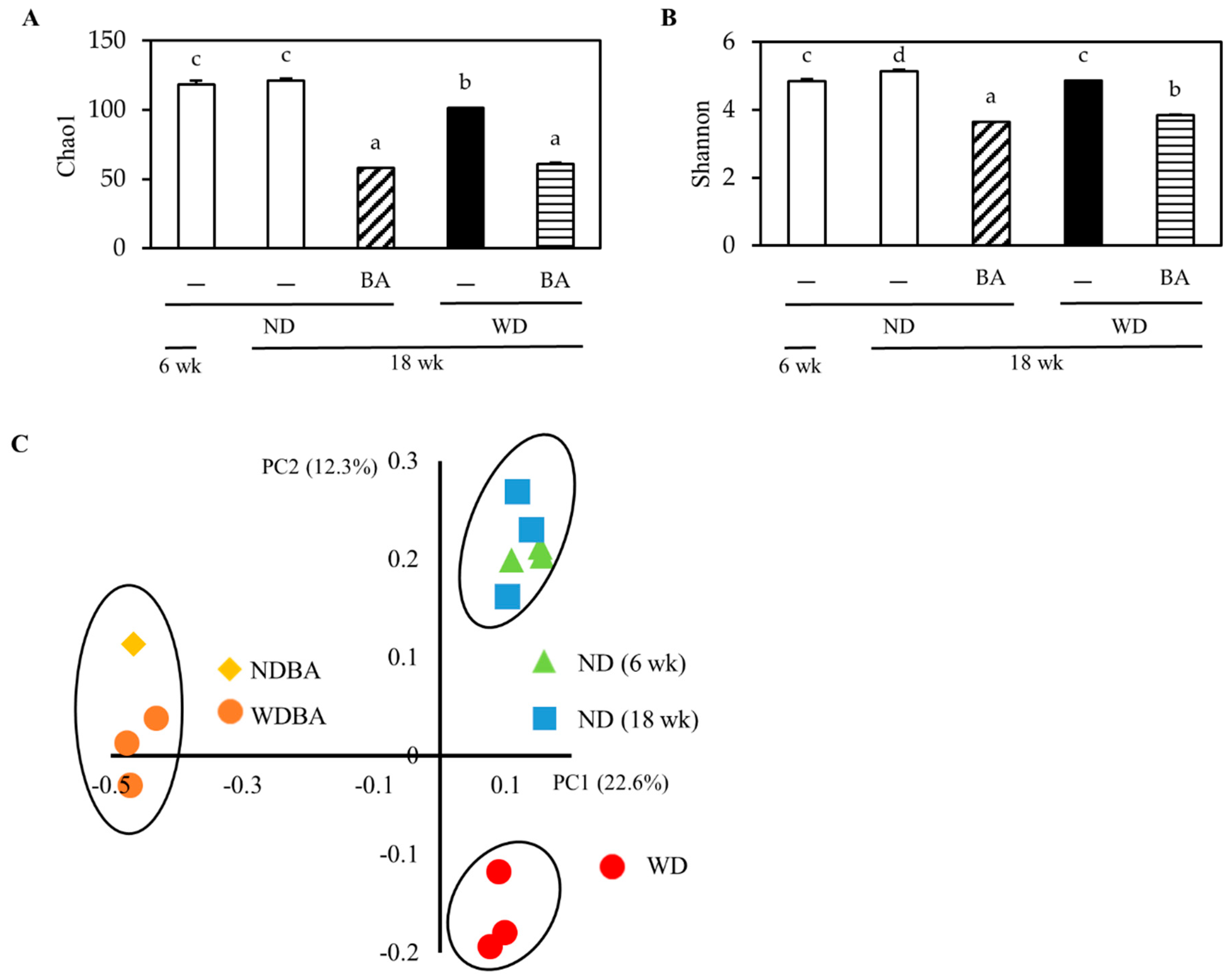
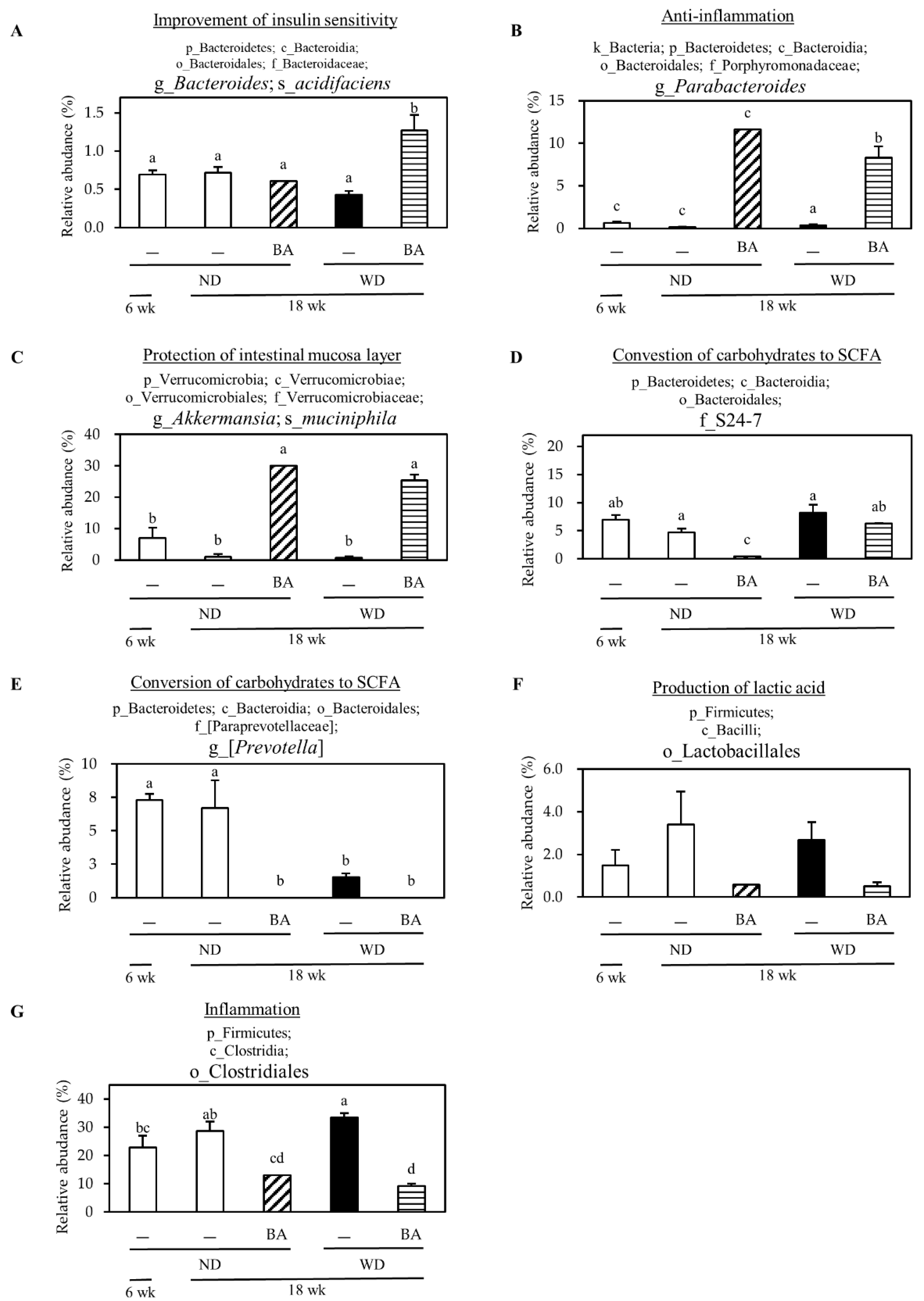
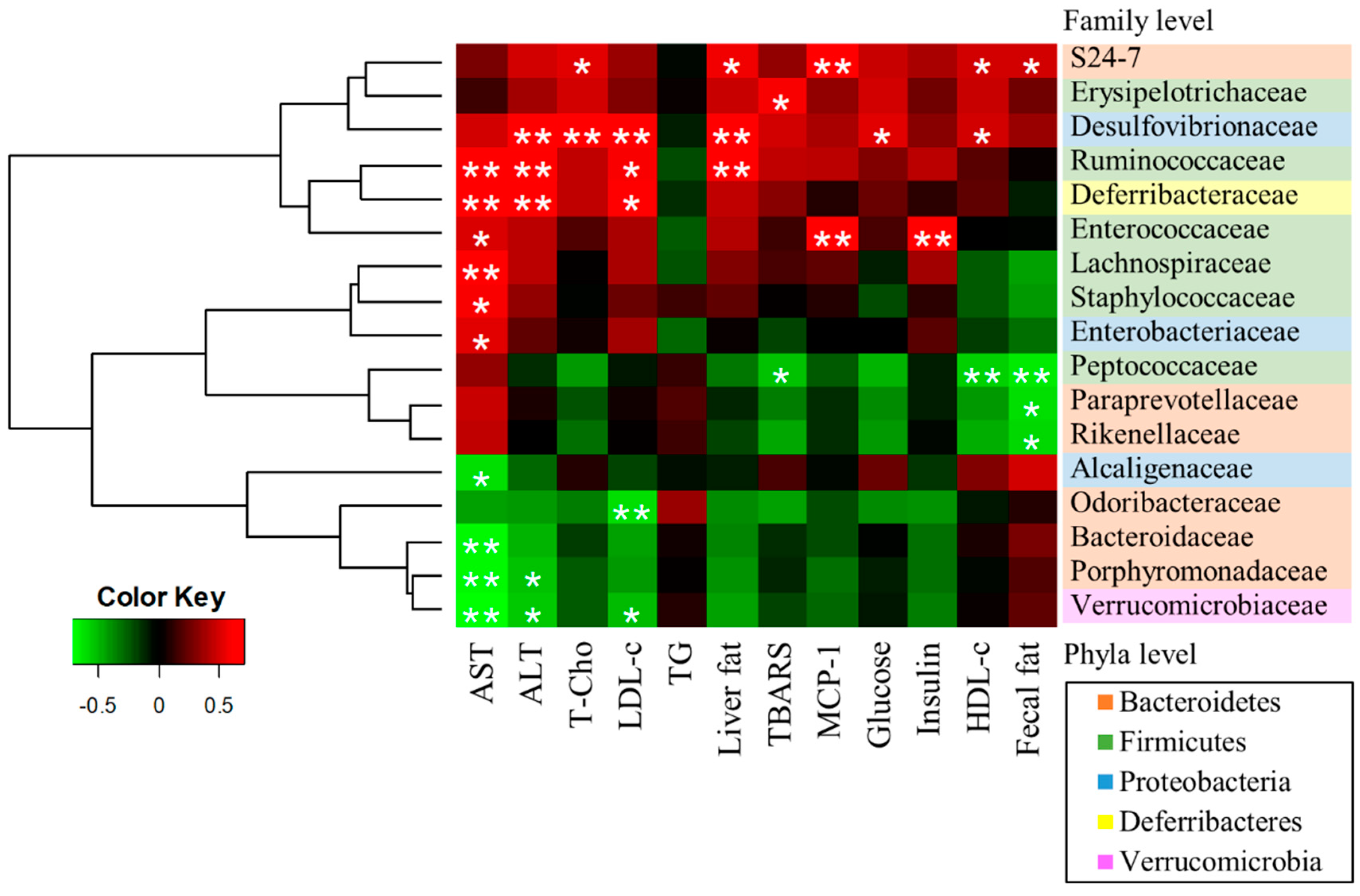
| Index | ND | WD | ||
|---|---|---|---|---|
| — | BA | — | BA | |
| Body weight (g) | 35.3 ± 0.9 a | 33.3 ± 1.0 a | 40.7 ± 0.4 b | 35.8 ± 1.6 a |
| Liver weight (g) | 1.3 ± 0.1 a,b | 1.1 ± 0.0 a | 2.0 ± 0.1 c | 1.4 ± 0.1 b |
| Epididymis fat weight (g) | 1.7 ± 0.1 a,b | 1.3 ± 0.2 a | 2.2 ± 0.1 c | 1.9 ± 0.2 b,c |
| Liver weight/body weight (%) | 3.6 ± 0.2 a,b | 3.2 ± 0.1 a | 4.8 ± 0.2 c | 3.8 ± 0.1 b |
| Epididymis fat weight/body weight (%) | 4.8 ± 0.3 a,b | 3.9 ± 0.4 a | 5.5 ± 0.2 b | 5.3 ± 0.4 b |
| Liver fat weight (%) | 7.6 ± 1.2 a | 4.0 ± 0.4 a | 27.4 ± 2.9 b | 9.5 ± 1.8 a |
| Total feces fat at 18 week (mg) | 52.8 ± 7.2 a | 45.9 ± 2.4 a | 87.1 ± 4.9 b | 100.1 ± 4.4 b |
| Index | ND | WD | ||
|---|---|---|---|---|
| — | BA | — | BA | |
| Caecum (g/body weight, %) | 0.64 ± 0.08 b | 1.04 ± 0.09 a | 0.69 ± 0.08 b | 0.96 ± 0.06 a |
| Lactic acid (μmol/total cecum) | 0.59 ± 0.08 a | 2.19 ± 0.49 b | 1.17 ± 0.20 a,b | 2.07 ± 0.49 b |
| Acetic acid (μmol/total cecum) | 3.92 ± 0.53 | 4.69 ± 0.71 | 2.84 ± 0.82 | 2.79 ± 0.16 |
| Propionic acid (μmol/total cecum) | 1.72 ± 0.49 | 1.95 ± 0.61 | 1.10 ± 0.16 | 1.17 ± 0.05 |
| Butyric acid (μmol/total cecum) | 0.66 ± 0.14 b | 0.21 ± 0.05 a | 0.77 ± 0.21 b | 0.55 ± 0.09 a,b |
| Total SCFAs (μmol/total cecum) | 6.88 ± 1.21 | 9.04 ± 1.66 | 5.89 ± 1.48 | 6.58 ± 0.46 |
| Phylum Level of Gut Microbiome (%) | 6 week | 18 week | |||
|---|---|---|---|---|---|
| ND | WD | ||||
| — | — | BA | — | BA | |
| Firmicutes | 37.0 ± 5.7 a | 42.6 ± 3.7 a | 17.6 ± 0.0 b | 48.3 ± 0.9 a | 12.5 ± 2.1 b |
| Bacteroidetes | 36.0 ± 3.6 b,c | 37.0 ± 4.3 b,c | 43.0 ± 0.0 a,b | 26.2 ± 2.7 c | 48.6 ± 2.3 a |
| Verrucomicrobia | 7.0 ± 3.3 b | 1.0 ± 0.9 b | 30.0 ± 0.0 a | 0.7 ± 0.4 b | 25.3 ± 1.9 a |
| Actinobacteria | 0.1 ± 0.0 | 0.9 ± 0.5 | 0.3 ± 0.0 | 0.9 ± 0.5 | 0.2 ± 0.1 |
| Proteobacteria | 16.4 ± 3.0 | 16.5 ± 4.2 | 9.1 ± 0.0 | 20.9 ± 1.2 | 13.4 ± 1.3 |
| Deferribacteres | 3.5 ± 0.7 a | 2.0 ± 0.9 a,b | 0.0 ± 0.0 a,b | 3.0 ± 1.1 a | 0.0 ± 0.0 b |
| F/B | 1.1 ± 0.3 b | 1.2 ± 0.2 b | 0.4 ± 0.0 b,c | 1.9 ± 0.2 a | 0.3 ± 0.1 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, H.; Wu, S.; Sakao, K.; Hara, T.; He, J.; Garcia, S.; Shetty, K.; Hou, D.-X. Bilberry Anthocyanins Ameliorate NAFLD by Improving Dyslipidemia and Gut Microbiome Dysbiosis. Nutrients 2020, 12, 3252. https://doi.org/10.3390/nu12113252
Nakano H, Wu S, Sakao K, Hara T, He J, Garcia S, Shetty K, Hou D-X. Bilberry Anthocyanins Ameliorate NAFLD by Improving Dyslipidemia and Gut Microbiome Dysbiosis. Nutrients. 2020; 12(11):3252. https://doi.org/10.3390/nu12113252
Chicago/Turabian StyleNakano, Hironobu, Shusong Wu, Kozue Sakao, Taichi Hara, Jianhua He, Santos Garcia, Kalidas Shetty, and De-Xing Hou. 2020. "Bilberry Anthocyanins Ameliorate NAFLD by Improving Dyslipidemia and Gut Microbiome Dysbiosis" Nutrients 12, no. 11: 3252. https://doi.org/10.3390/nu12113252
APA StyleNakano, H., Wu, S., Sakao, K., Hara, T., He, J., Garcia, S., Shetty, K., & Hou, D.-X. (2020). Bilberry Anthocyanins Ameliorate NAFLD by Improving Dyslipidemia and Gut Microbiome Dysbiosis. Nutrients, 12(11), 3252. https://doi.org/10.3390/nu12113252






