St. John’s Wort Suppresses Growth in Triple-Negative Breast Cancer Cell Line MDA-MB-231 by Inducing Prodeath Autophagy and Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture, Cell Viability, and Proliferation
2.3. Apoptosis Assay
2.4. Immunoblotting
2.5. Xenograft Mouse Study
2.6. H&E Staining and TUNEL Assay
2.7. Statistical Analysis
3. Results
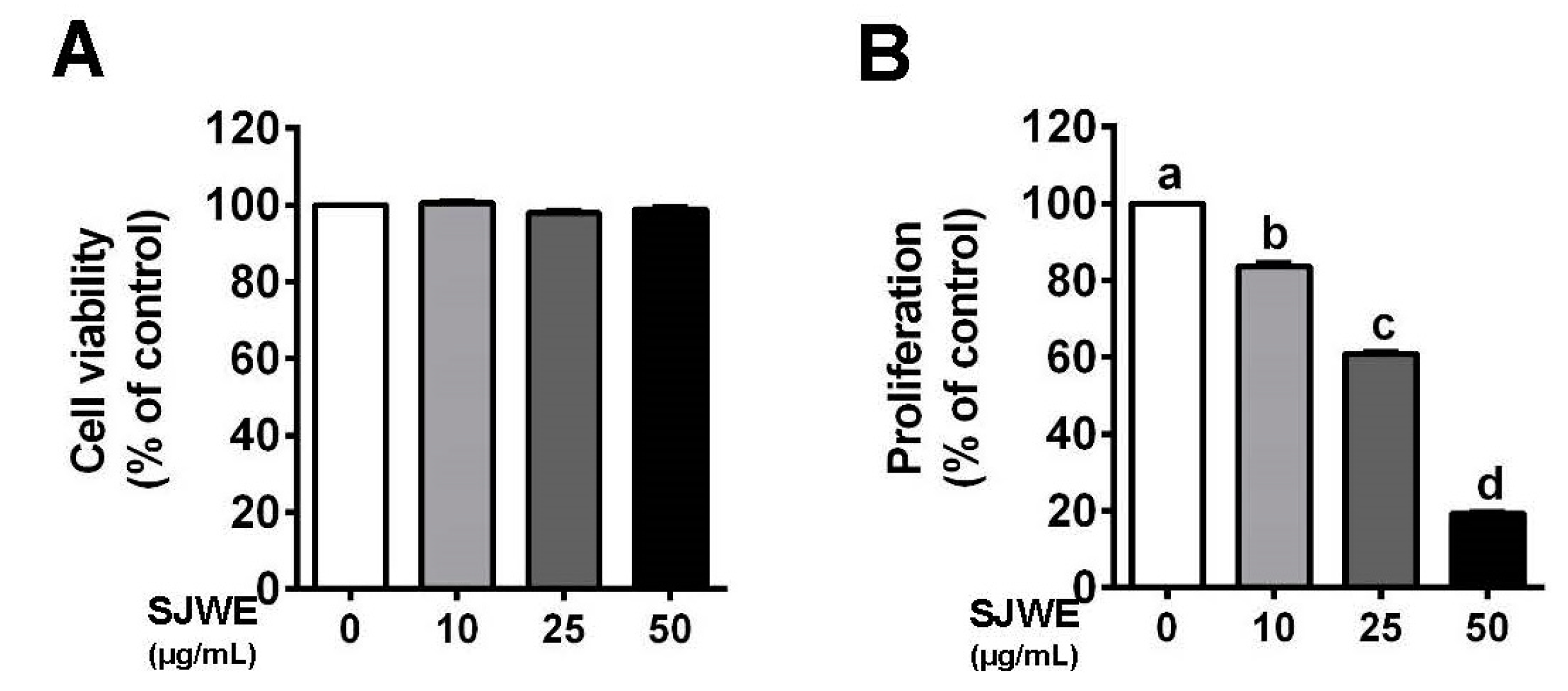
3.1. SJWE Inhibited the Proliferation in TNBC MDA-MB-231 Cells
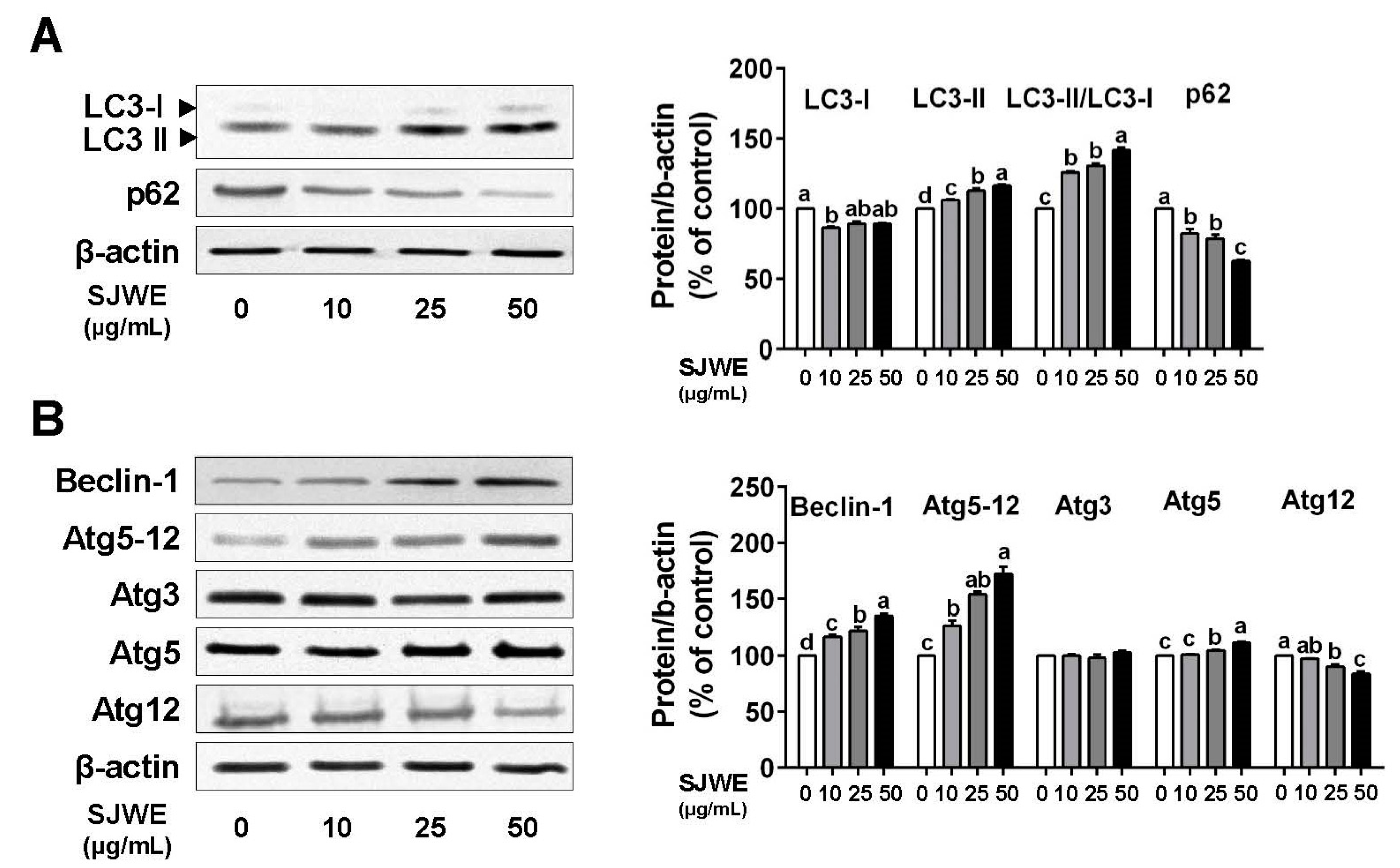
3.2. SJWE Induced Pro-Death Autophagy in TNBC Line MDA-MB-231 Cells
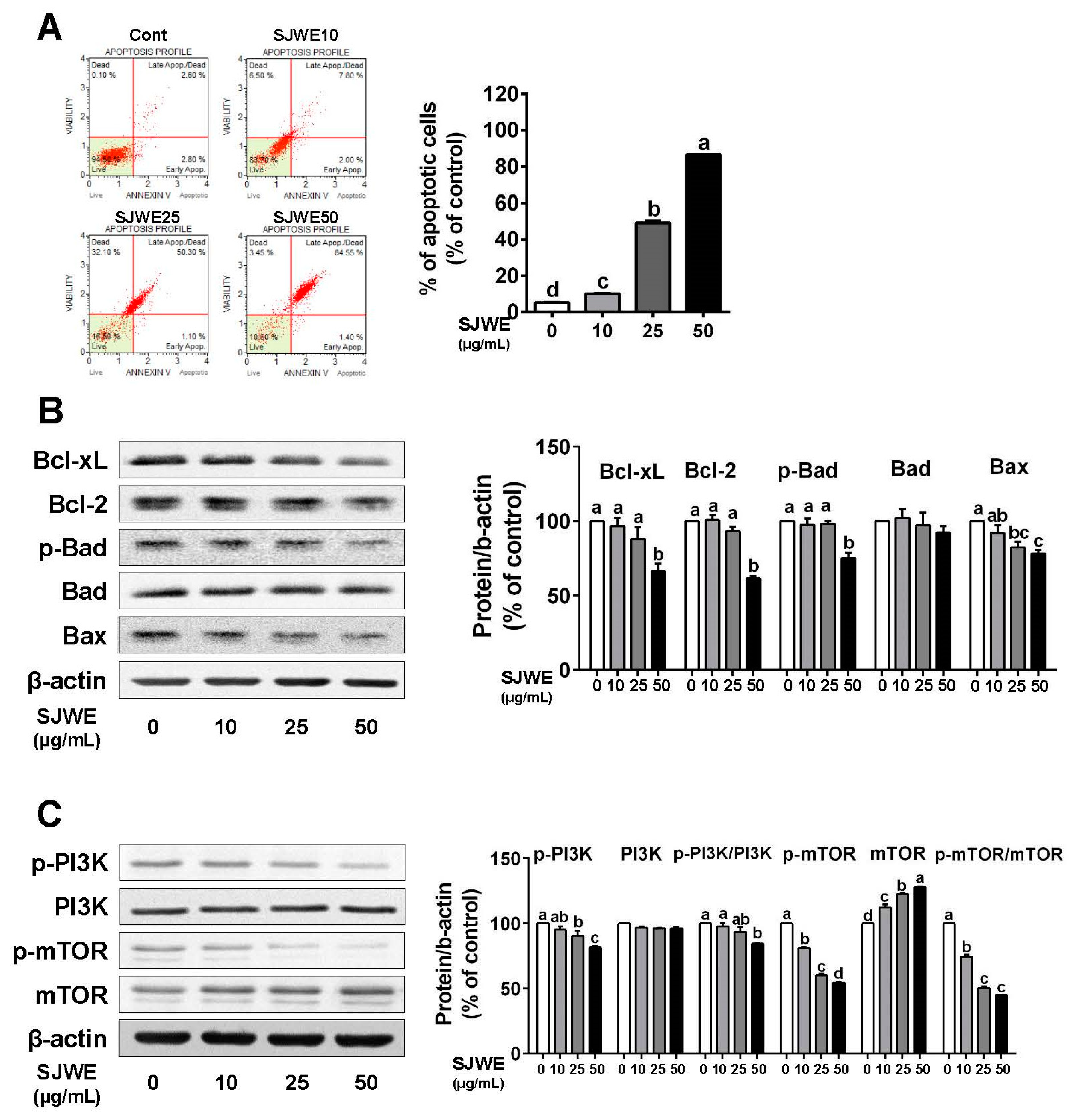
3.3. SJWE Induces Apoptosis via the Downregulation of Antiapoptic Proteins in TNBC MDA-MB-231 Cells
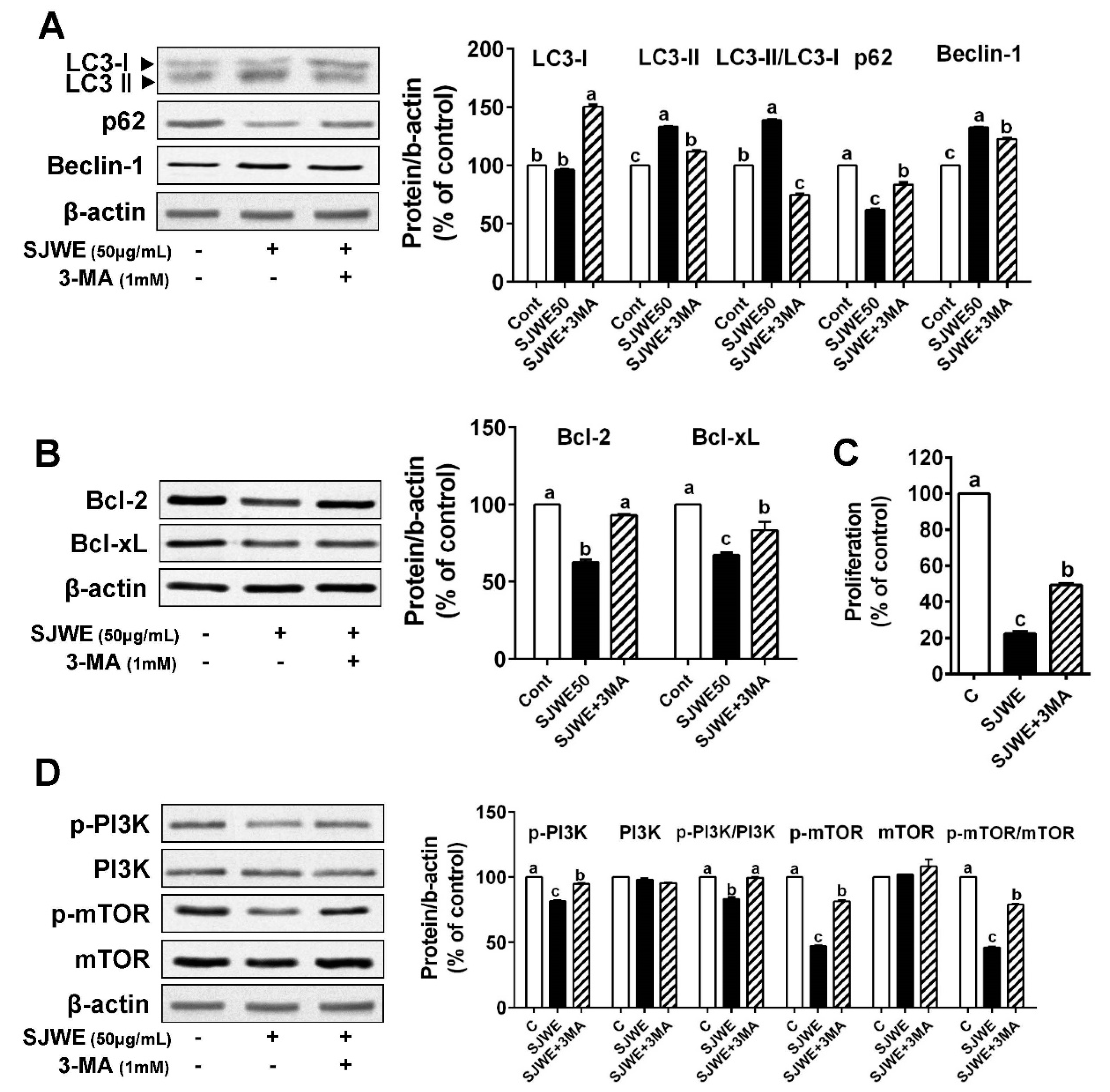
3.4. The Inhibition of Autophagy Reversed Apoptosis Induced by SJWE in TNBC Line MDA-MB-231 Cells
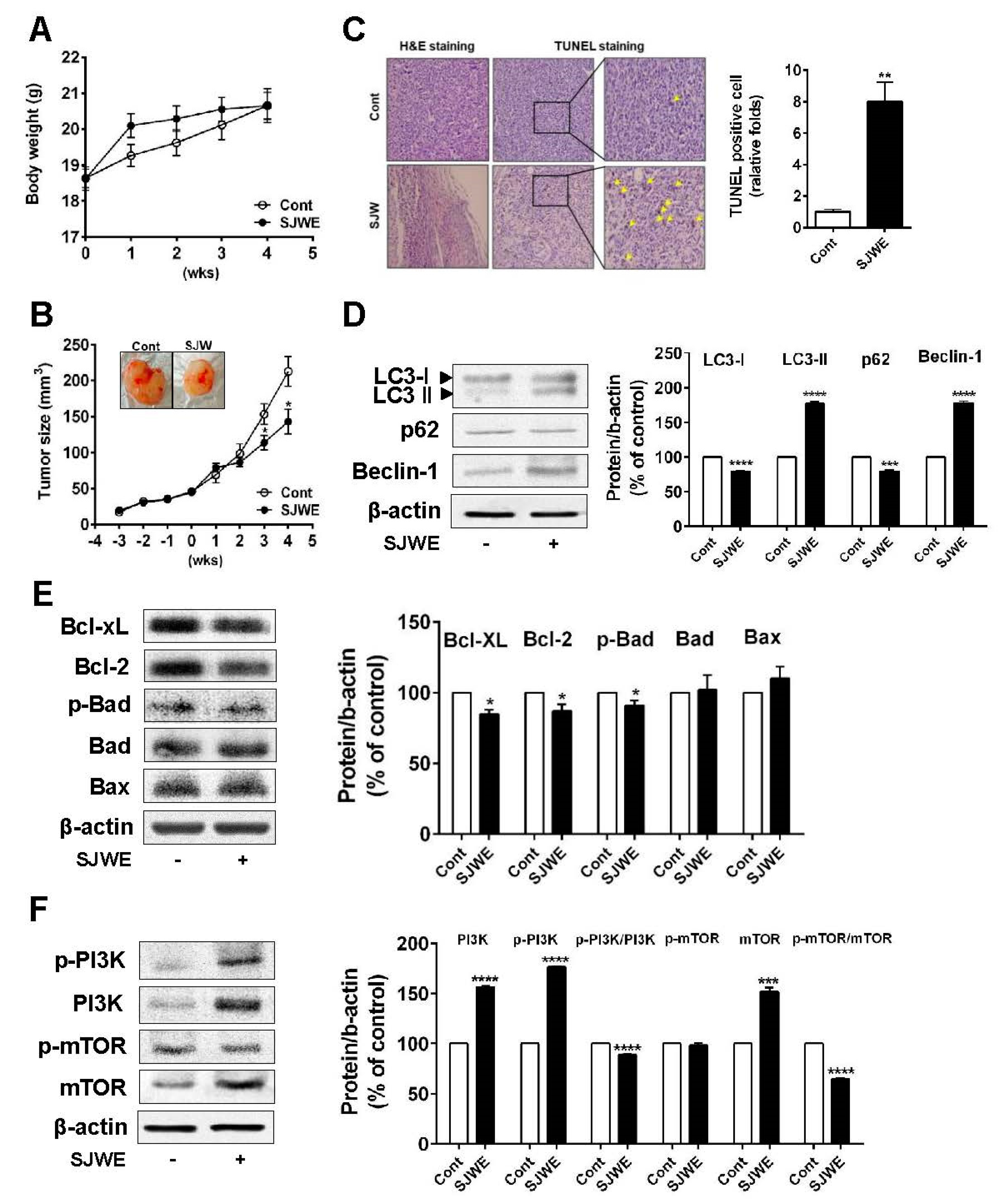
3.5. SJWE Inhibited Tumor Progression by Inducing Autophagic and Apoptotic Cell Death in MDA-MB-231 Cells Xenografted Athymic Nude Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LC3 | light chain 3 |
| Atg3, 5, 12, 5-12 | autophagy-related 3, 5, 12, 5-12, |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-xL | B-cell lymphoma-extra large |
| Bad | Bcl-2-associated death promoter |
| Bax | Bcl-2 like protein 4 |
| PI3K | phosphoinositide 3-kinases |
| mTOR | mammalian target of rapamycin |
| AMPK | AMP-activated protein kinase |
References
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Yorimitsu, T.; Klionsky, D.J. Autophagy: Molecular machinery for self-eating. Cell Death Differ. 2005, 12, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C. Autophagy induction rescues toxicity mediated by proteasome inhibition. Neuron 2007, 54, 854–856. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Denton, D.; Nicolson, S.; Kumar, S. Cell death by autophagy: Facts and apparent artefacts. Cell Death Differ. 2012, 19, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.A.; Salmani, J.M.M.; Jiang, Z.; Feng, L.; Song, J.; Jia, X.; Chen, B. Autophagy: An overview and its roles in cancer and obesity. Clin. Chim. Acta 2017, 468, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Karantza-Wadsworth, V.; Patel, S.; Kravchuk, O.; Chen, G.; Mathew, R.; Jin, S.; White, E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007, 21, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Karp, C.M.; Beaudoin, B.; Vuong, N.; Chen, G.; Chen, H.-Y.; Bray, K.; Reddy, A.; Bhanot, G.; Gelinas, C. Autophagy suppresses tumorigenesis through elimination of p62. Cell 2009, 137, 1062–1075. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342. [Google Scholar] [CrossRef]
- Hudis, C.A.; Gianni, L. Triple-negative breast cancer: An unmet medical need. Oncologist 2011, 16, 1–11. [Google Scholar] [CrossRef]
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef]
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer Registry. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.P.; Winer, E.P.; Foulkes, W.D.; Garber, J.; Perou, C.M.; Richardson, A.; Sledge, G.W.; Carey, L.A. Triple-negative breast cancer: Risk factors to potential targets. Clin. Cancer Res. 2008, 14, 8010–8018. [Google Scholar] [CrossRef]
- Liang, Z.; Bian, X.; Shim, H. Downregulation of microRNA-206 promotes invasion and angiogenesis of triple negative breast cancer. Biochem. Biophys. Res. Commun. 2016, 477, 461–466. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Mennini, T.; Gobbi, M. The antidepressant mechanism of Hypericum perforatum. Life Sci. 2004, 75, 1021–1027. [Google Scholar] [CrossRef]
- Brenner, R.; Azbel, V.; Madhusoodanan, S.; Pawlowska, M. Comparison of an extract of hypericum (LI 160) and sertraline in the treatment of depression: A double-blind, randomized pilot study. Clin. Ther. 2000, 22, 411–419. [Google Scholar] [CrossRef]
- Schrader, E. Equivalence of St John’s wort extract (Ze 117) and fluoxetine: A randomized, controlled study in mild-moderate depression. Int. Clin. Psychopharmacol. 2000, 15, 61–68. [Google Scholar] [CrossRef]
- Agostinis, P.; Vantieghem, A.; Merlevede, W.; de Witte, P.A. Hypericin in cancer treatment: More light on the way. Int. J. Biochem. Cell Biol. 2002, 34, 221–241. [Google Scholar] [CrossRef]
- Ferenc, P.; Solár, P.; Kleban, J.; Mikeš, J.; Fedoročko, P. Down-regulation of Bcl-2 and Akt induced by combination of photoactivated hypericin and genistein in human breast cancer cells. J. Photochem. Photobiol. B Biol. 2010, 98, 25–34. [Google Scholar] [CrossRef]
- Liu, C.D.; Kwan, D.; Saxton, R.E.; McFadden, D.W. Hypericin and photodynamic therapy decreases human pancreatic cancer in vitro and in vivo. J. Surg. Res. 2000, 93, 137–143. [Google Scholar] [CrossRef] [PubMed]
- VanderWerf, Q.M.; Saxton, R.E.; Chang, A.; Horton, D.; Paiva, M.B.; Anderson, J.; Foote, C.; Soudant, J.; Mathey, A.; Castro, D.J. Hypericin: A new laser phototargeting agent for human cancer cells. Laryngoscope 1996, 106, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Schempp, C.M.; Kirkin, V.; Simon-Haarhaus, B.; Kersten, A.; Kiss, J.; Termeer, C.C.; Gilb, B.; Kaufmann, T.; Borner, C.; Sleeman, J.P. Inhibition of tumour cell growth by hyperforin, a novel anticancer drug from St. John’s wort that acts by induction of apoptosis. Oncogene 2002, 21, 1242–1250. [Google Scholar] [CrossRef]
- Merhi, F.; Tang, R.; Piedfer, M.; Mathieu, J.; Bombarda, I.; Zaher, M.; Kolb, J.-P.; Billard, C.; Bauvois, B. Hyperforin inhibits Akt1 kinase activity and promotes caspase-mediated apoptosis involving Bad and Noxa activation in human myeloid tumor cells. PLoS ONE 2011, 6, e25963. [Google Scholar] [CrossRef] [PubMed]
- You, M.-K.; Kim, H.-J.; Kook, J.H.; Kim, H.-A. St. John’s wort regulates proliferation and apoptosis in MCF-7 human breast cancer cells by inhibiting AMPK/mTOR and activating the mitochondrial pathway. Int. J. Mol. Sci. 2018, 19, 966. [Google Scholar] [CrossRef]
- You, M.-K.; Rhuy, J.; Jeong, K.S.; Bang, M.; Kim, M.-S.; Kim, H.-A. Effect of St. John’s Wort (Hypericum perforatum) on obesity, lipid metabolism and uterine epithelial proliferation in ovariectomized rats. Nutr. Res. Pract. 2014, 8, 292–296. [Google Scholar] [CrossRef]
- Huang, R.; Liu, W. Identifying an essential role of nuclear LC3 for autophagy. Autophagy 2015, 11, 852–853. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef]
- Zhang, D.-M.; Liu, J.-S.; Deng, L.-J.; Chen, M.-F.; Yiu, A.; Cao, H.-H.; Tian, H.-Y.; Fung, K.-P.; Kurihara, H.; Pan, J.-X. Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis 2013, 34, 1331–1342. [Google Scholar] [CrossRef]
- Levine, B.; Yuan, J. Autophagy in cell death: An innocent convict? J. Clin. Investig. 2005, 115, 2679–2688. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Codogno, P.; Levine, B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 2012, 11, 709–730. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yu, J.; Chalmers, B.; Drisko, J.; Yang, J.; Li, B.; Chen, Q. Pharmacological ascorbate induces cytotoxicity in prostate cancer cells through ATP depletion and induction of autophagy. Anti-Cancer Drugs 2012, 23, 437–444. [Google Scholar] [CrossRef]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- Tai, W.; Shiau, C.; Chen, H.; Liu, C.; Lin, C.; Cheng, A.; Chen, P.; Chen, K. Mcl-1-dependent activation of Beclin 1 mediates autophagic cell death induced by sorafenib and SC-59 in hepatocellular carcinoma cells. Cell Death Dis. 2013, 4, e485. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.; Lotze, M.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Oh, S.; E, X.; Ni, D.; Pirooz, S.D.; Lee, J.-Y.; Lee, D.; Zhao, Z.; Lee, S.; Lee, H.; Ku, B. Downregulation of autophagy by Bcl-2 promotes MCF7 breast cancer cell growth independent of its inhibition of apoptosis. Cell Death Differ. 2011, 18, 452–464. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, W.; Guo, X.; Ren, J.; Gao, A. The crosstalk between autophagy and apoptosis was mediated by phosphorylation of Bcl-2 and beclin1 in benzene-induced hematotoxicity. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Carey, L.; Winer, E.; Viale, G.; Cameron, D.; Gianni, L. Triple-negative breast cancer: Disease entity or title of convenience? Nat. Rev. Clin. Oncol. 2010, 7, 683. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Campora, R.; Davalos-Casanova, G.; Beato-Moreno, A.; Garcia-Escudero, A.; Megia, M.J.P.; Montironi, R.; Lopez-Beltran, A. BCL-2, TP53 and BAX protein expression in superficial urothelial bladder carcinoma. Cancer Lett. 2007, 250, 292–299. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Qian, T.; He, L.; Kim, J.-S.; Elmore, S.P.; Cascio, W.E.; Brenner, D.A. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid. Redox Signal. 2002, 4, 769–781. [Google Scholar] [CrossRef]
- Luna-Dulcey, L.; Tomasin, R.; Naves, M.A.; da Silva, J.A.; Cominetti, M.R. Autophagy-dependent apoptosis is triggered by a semi-synthetic [6]-gingerol analogue in triple negative breast cancer cells. Oncotarget 2018, 9, 30787. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-F.; Wu, C.-L.; Huang, S.-C.; Wu, C.-M.; Hsiao, J.-R.; Yo, Y.-T.; Chen, Y.-H.; Shiau, A.-L.; Chou, C.-Y. Cathepsin L mediates resveratrol-induced autophagy and apoptotic cell death in cervical cancer cells. Autophagy 2009, 5, 451–460. [Google Scholar] [CrossRef]
- Tian, T.; Li, X.; Zhang, J. mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. Int. J. Mol. Sci. 2019, 20, 755. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Hasanzadeh, M.; Hassanian, S.M.; ShahidSales, S.; Ghayour-Mobarhan, M.; Ferns, G.A.; Avan, A. The potential value of the PI3K/Akt/mTOR signaling pathway for assessing prognosis in cervical cancer and as a target for therapy. J. Cell. Biochem. 2017, 118, 4163–4169. [Google Scholar] [CrossRef]
- Singh, B.N.; Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem. Pharmacol. 2012, 84, 1154–1163. [Google Scholar] [CrossRef]
- Din, F.V.; Valanciute, A.; Houde, V.P.; Zibrova, D.; Green, K.A.; Sakamoto, K.; Alessi, D.R.; Dunlop, M.G. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology 2012, 142, 1504–1515.e3. [Google Scholar] [CrossRef]
- Wang, J.; Qi, Q.; Feng, Z.; Zhang, X.; Huang, B.; Chen, A.; Prestegarden, L.; Li, X.; Wang, J. Berberine induces autophagy in glioblastoma by targeting the AMPK/mTOR/ULK1-pathway. Oncotarget 2016, 7, 66944. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, M.; Lee, Y.-H.; Kim, H.-J.; Kook, J.H.; Kim, H.-A. St. John’s Wort Suppresses Growth in Triple-Negative Breast Cancer Cell Line MDA-MB-231 by Inducing Prodeath Autophagy and Apoptosis. Nutrients 2020, 12, 3175. https://doi.org/10.3390/nu12103175
You M, Lee Y-H, Kim H-J, Kook JH, Kim H-A. St. John’s Wort Suppresses Growth in Triple-Negative Breast Cancer Cell Line MDA-MB-231 by Inducing Prodeath Autophagy and Apoptosis. Nutrients. 2020; 12(10):3175. https://doi.org/10.3390/nu12103175
Chicago/Turabian StyleYou, Mikyoung, Young-Hyun Lee, Hwa-Jin Kim, Ji Hyun Kook, and Hyeon-A Kim. 2020. "St. John’s Wort Suppresses Growth in Triple-Negative Breast Cancer Cell Line MDA-MB-231 by Inducing Prodeath Autophagy and Apoptosis" Nutrients 12, no. 10: 3175. https://doi.org/10.3390/nu12103175
APA StyleYou, M., Lee, Y.-H., Kim, H.-J., Kook, J. H., & Kim, H.-A. (2020). St. John’s Wort Suppresses Growth in Triple-Negative Breast Cancer Cell Line MDA-MB-231 by Inducing Prodeath Autophagy and Apoptosis. Nutrients, 12(10), 3175. https://doi.org/10.3390/nu12103175




