How Does Our Brain Process Sugars and Non-Nutritive Sweeteners Differently: A Systematic Review on Functional Magnetic Resonance Imaging Studies
Abstract
1. Introduction
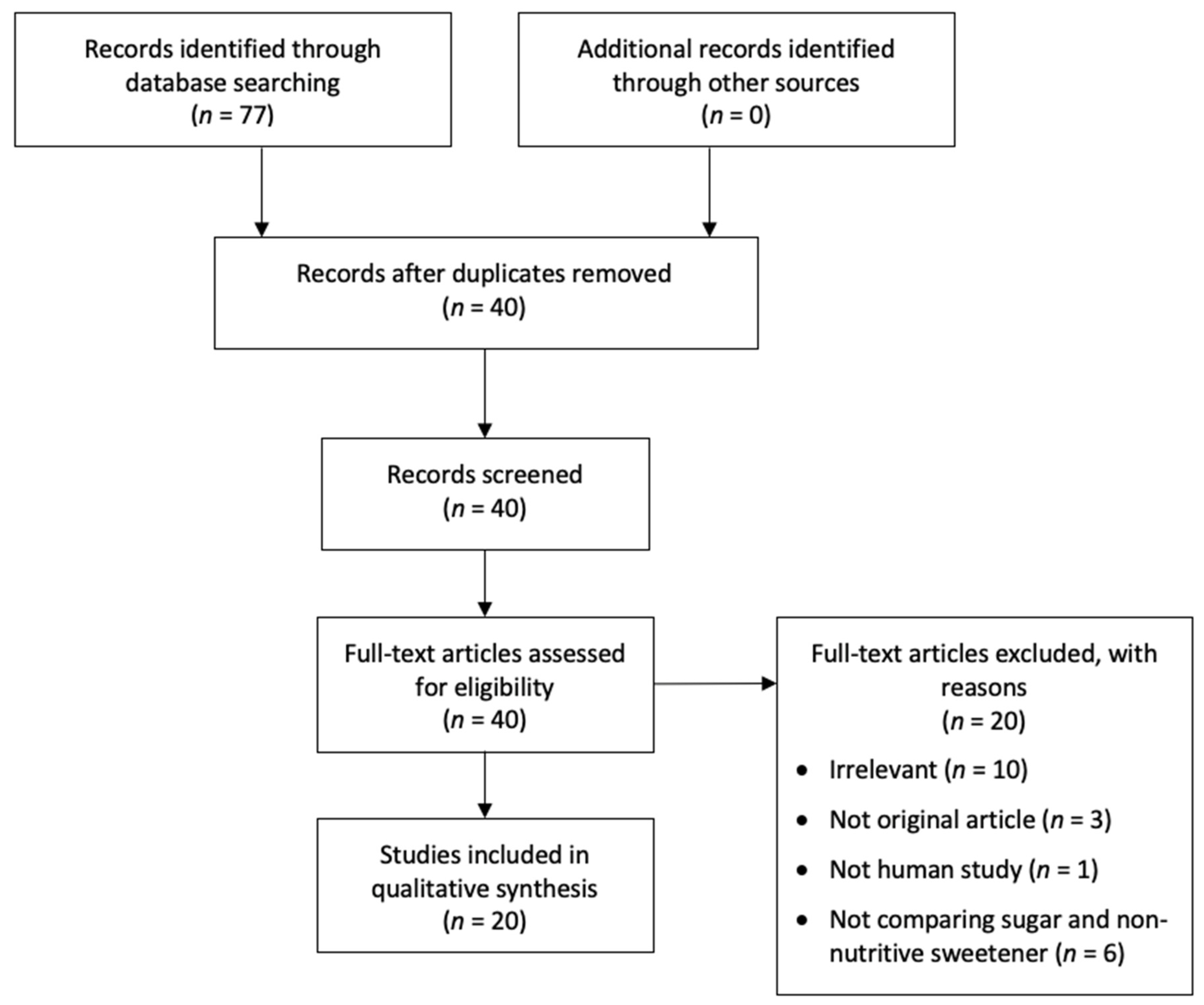
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Data Extracted from the Analyzed Studies
2.3. Study Quality Assessment
3. Results
Study Characteristics
4. Discussion
4.1. Differential Brain Responses during Tasting
4.2. Differential Brain Responses during Tasks after Pre-Loaded with Sweet Beverages
4.3. Differential Brain Activity Levels at Resting State after Pre-Loaded with Sweet Beverages
4.4. Limitations of This Study and Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, P.E.; Perez, V. Low-calorie sweeteners and body weight and composition: A meta-analysis of randomized controlled trials and prospective cohort studies. Am. J. Clin. Nutr. 2014, 100, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.; Hogenkamp, P.; De Graaf, C.; Higgs, S.; Lluch, A.; Ness, A.; Penfold, C.; Perry, R.; Putz, P.; Yeomans, M. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int. J. Obes. 2016, 40, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; De Banate, M.A.; Rother, K.I. Artificial sweeteners: A systematic review of metabolic effects in youth. Int. J. Pediatr. Obes. 2010, 5, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ruanpeng, D.; Thongprayoon, C.; Cheungpasitporn, W.; Harindhanavudhi, T. Sugar and artificially sweetened beverages linked to obesity: A systematic review and meta-analysis. QJM Int. J. Med. 2017, 110, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Reid, A.E.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Abou-Setta, A.M.; Fiander, M.; MacKay, D.S.; McGavock, J. Early exposure to nonnutritive sweeteners and long-term metabolic health: A systematic review. Pediatrics 2016, 137, e20153603. [Google Scholar] [CrossRef]
- Romo-Romo, A.; Aguilar-Salinas, C.A.; Brito-Córdova, G.X.; Díaz, R.A.G.; Valentín, D.V.; Almeda-Valdes, P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: Systematic review of observational prospective studies and clinical trials. PLoS ONE 2016, 11, e0161264. [Google Scholar] [CrossRef]
- Shankar, P.; Ahuja, S.; Sriram, K. Non-nutritive sweeteners: Review and update. Nutrition 2013, 29, 1293–1299. [Google Scholar] [CrossRef]
- Lohner, S.; Toews, I.; Meerpohl, J.J. Health outcomes of non-nutritive sweeteners: Analysis of the research landscape. Nutr. J. 2017, 16, 55. [Google Scholar] [CrossRef]
- Rogers, P.J.; Blundell, J.E. Separating the actions of sweetness and calories: Effects of saccharin and carbohydrates on hunger and food intake in human subjects. Physiol. Behav. 1989, 45, 1093–1099. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. Calorie restriction: Is AMPK a key sensor and effector? Physiology 2011, 26, 214–224. [Google Scholar] [CrossRef]
- Nichol, A.D.; Holle, M.J.; An, R. Glycemic impact of non-nutritive sweeteners: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2018, 72, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.; Bridge, M.; Jones, D. Carbohydrate sensing in the human mouth: Effects on exercise performance and brain activity. J. Physiol. 2009, 587, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Connolly, L.; Coveleskie, K.; Kilpatrick, L.; Labus, J.; Ebrat, B.; Stains, J.; Jiang, Z.; Tillisch, K.; Raybould, H.E.; Mayer, E. Differences in brain responses between lean and obese women to a sweetened drink. Neurogastroenterol. Motil. 2013, 25, 579-e460. [Google Scholar] [CrossRef] [PubMed]
- Di Salle, F.; Cantone, E.; Savarese, M.F.; Aragri, A.; Prinster, A.; Nicolai, E.; Sarnelli, G.; Iengo, M.; Buyckx, M.; Cuomo, R. Effect of carbonation on brain processing of sweet stimuli in humans. Gastroenterology 2013, 145, 537–539 e533. [Google Scholar] [CrossRef]
- Frank, G.K.; Oberndorfer, T.A.; Simmons, A.N.; Paulus, M.P.; Fudge, J.L.; Yang, T.T.; Kaye, W.H. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage 2008, 39, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Gramling, L.; Kapoulea, E.; Murphy, C. Taste perception and caffeine consumption: An fMRI study. Nutrients 2019, 11, 34. [Google Scholar] [CrossRef]
- Green, E.; Murphy, C. Altered processing of sweet taste in the brain of diet soda drinkers. Physiol. Behav. 2012, 107, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Griffioen-Roose, S.; Smeets, P.A.; Weijzen, P.L.; van Rijn, I.; van den Bosch, I.; de Graaf, C. Effect of replacing sugar with non-caloric sweeteners in beverages on the reward value after repeated exposure. PLoS ONE 2013, 8, e81924. [Google Scholar] [CrossRef] [PubMed]
- Haase, L.; Cerf-Ducastel, B.; Murphy, C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage 2009, 44, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Li, X.; DuBois, G.E.; Zhou, L.; Hu, X.P. Prolonged insula activation during perception of aftertaste. Neuroreport 2009, 20, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, L.A.; Coveleskie, K.; Connolly, L.; Labus, J.S.; Ebrat, B.; Stains, J.; Jiang, Z.; Suyenobu, B.Y.; Raybould, H.E.; Tillisch, K. Influence of sucrose ingestion on brainstem and hypothalamic intrinsic oscillations in lean and obese women. Gastroenterology 2014, 146, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Oberndorfer, T.A.; Frank, G.K.; Simmons, A.N.; Wagner, A.; McCurdy, D.; Fudge, J.L.; Yang, T.T.; Paulus, M.P.; Kaye, W.H. Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. Am. J. Psychiatry 2013, 170, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.B.; Krebs-Kraft, D.L.; Ryan, J.P.; Wilson, J.S.; Harenski, C.; Hamann, S. Glucose administration enhances fMRI brain activation and connectivity related to episodic memory encoding for neutral and emotional stimuli. Neuropsychologia 2011, 49, 1052–1066. [Google Scholar] [CrossRef]
- Smeets, P.A.; de Graaf, C.; Stafleu, A.; van Osch, M.J.; van der Grond, J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am. J. Clin. Nutr. 2005, 82, 1011–1016. [Google Scholar] [CrossRef]
- Smeets, P.A.; Weijzen, P.; de Graaf, C.; Viergever, M.A. Consumption of caloric and non-caloric versions of a soft drink differentially affects brain activation during tasting. Neuroimage 2011, 54, 1367–1374. [Google Scholar] [CrossRef]
- Stone, W.S.; Thermenos, H.W.; Tarbox, S.I.; Poldrack, R.A.; Seidman, L.J. Medial temporal and prefrontal lobe activation during verbal encoding following glucose ingestion in schizophrenia: A pilot fMRI study. Neurobiol. Learn. Mem. 2005, 83, 54–64. [Google Scholar] [CrossRef]
- Tryon, M.S.; Stanhope, K.L.; Epel, E.S.; Mason, A.E.; Brown, R.; Medici, V.; Havel, P.J.; Laugero, K.D. Excessive sugar consumption may be a difficult habit to break: A view from the brain and body. J. Clin. Endocrinol. Metab. 2015, 100, 2239–2247. [Google Scholar] [CrossRef]
- van Opstal, A.M.; Hafkemeijer, A.; van den Berg-Huysmans, A.A.; Hoeksma, M.; Mulder, T.P.; Pijl, H.; Rombouts, S.A.; van der Grond, J. Brain activity and connectivity changes in response to nutritive natural sugars, non-nutritive natural sugar replacements and artificial sweeteners. Nutr. Neurosci. 2019. [Google Scholar] [CrossRef]
- van Opstal, A.M.; Kaal, I.; van den Berg-Huysmans, A.; Hoeksma, M.; Blonk, C.; Pijl, H.; Rombouts, S.; van der Grond, J. Dietary sugars and non-caloric sweeteners elicit different homeostatic and hedonic responses in the brain. Nutrition 2019, 60, 80–86. [Google Scholar] [CrossRef]
- van Rijn, I.; de Graaf, C.; Smeets, P.A. Tasting calories differentially affects brain activation during hunger and satiety. Behav. Brain Res. 2015, 279, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Simmons, A.N.; Oberndorfer, T.A.; Frank, G.K.; McCurdy-McKinnon, D.; Fudge, J.L.; Yang, T.T.; Paulus, M.P.; Kaye, W.H. Altered sensitization patterns to sweet food stimuli in patients recovered from anorexia and bulimia nervosa. Psychiatry Res. Neuroimaging 2015, 234, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Goto, T.K.; Leung, W.K. Basic taste processing recruits bilateral anteroventral and middle dorsal insulae: An activation likelihood estimation meta-analysis of fMRI studies. Brain Behav. 2017, 7, e00655. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Goto, T.K.; Leung, W.K. Affective value, intensity and quality of liquid tastants/food discernment in the human brain: An activation likelihood estimation meta-analysis. Neuroimage 2018, 169, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, Y.; Preuschhof, C.; Bohner, G.; Bauknecht, H.-C.; Klingebiel, R.; Flor, H.; Klapp, B.F. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007, 37, 410–421. [Google Scholar] [CrossRef]
- Dimitropoulos, A.; Tkach, J.; Ho, A.; Kennedy, J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite 2012, 58, 303–312. [Google Scholar] [CrossRef]
- Ochner, C.N.; Kwok, Y.; Conceição, E.; Pantazatos, S.P.; Puma, L.M.; Carnell, S.; Teixeira, J.; Hirsch, J.; Geliebter, A. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann. Surg. 2011, 253, 502–507. [Google Scholar] [CrossRef]
- Schneeberger, M.; Gomis, R.; Claret, M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J. Endocrinol. 2014, 220, T25–T46. [Google Scholar] [CrossRef]
- Waterson, M.J.; Horvath, T.L. Neuronal regulation of energy homeostasis: Beyond the hypothalamus and feeding. Cell Metab. 2015, 22, 962–970. [Google Scholar] [CrossRef]
- Chumbley, J.; Worsley, K.; Flandin, G.; Friston, K. Topological FDR for neuroimaging. Neuroimage 2010, 49, 3057–3064. [Google Scholar] [CrossRef]
- Crézé, C.; Candal, L.; Cros, J.; Knebel, J.-F.; Seyssel, K.; Stefanoni, N.; Schneiter, P.; Murray, M.M.; Tappy, L.; Toepel, U. The impact of caloric and non-caloric sweeteners on food intake and brain responses to food: A randomized crossover controlled trial in healthy humans. Nutrients 2018, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Zeffiro, T.A. Hunger and BMI modulate neural responses to sweet stimuli: fMRI meta-analysis. Int. J. Obes. 2020, 44, 1636–1652. [Google Scholar] [CrossRef] [PubMed]
- Yunker, A.G.; Patel, R.; Page, K.A. Effects of Non-nutritive Sweeteners on Sweet Taste Processing and Neuroendocrine Regulation of Eating Behavior. Curr. Nutr. Rep. 2020, 9, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. Ten ironic rules for non-statistical reviewers. Neuroimage 2012, 61, 1300–1310. [Google Scholar] [CrossRef]
| Study | Journal (2018 Impact Factor) | Sample Size | Age, Mean ± SD | BMI ± SD | Medical Condition Involved | Sugar Used | Non-Nutritive Sweetener Used | Fasting before Experiment | Task of fMRI | Any Statistical Tests to Directly Compare Brain Responses to Sugar and Sweetener | Statistical Threshold a,b | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chambers et al. 2009 [13] | J Physiol-London (4.984) | 8 (8M) | 29 ± 9 | 23.8 ± 2.5 | Healthy | Glucose | Saccharin | Overnight | Passive tasting of the sweet solutions | No (separate tests against baseline) | p < 0.05, FWE corrected | Sugar caused larger brain responses in anterior cingulate and striatum |
| Connolly et al. 2013 [14] | Neurogastroenterol Motil (3.803) | 20 (20F) | 25.6, range = 18–40 | 27.7, range = 19–37 | Obesity | Sucrose | Truvia (stevia-based) | 6 h | Reported brain responses to viewing food images after drinking sweet beverages | Yes | Clusters with a peak z >3.30 and >60 voxels | Sugar and sweetener engaged similar brain regions. Females with obesity had larger brain responses than lean females for sugar but not sweetener condition in anterior cingulate, anterior insula, amygdala and hippocampus |
| Di Salle et al. 2013 [15] | Gastroenterology (19.809) | 9 (5M, 4F) | 23 ± NA | NA | Healthy | Sucrose | Aspartame + acesulfame | Unclear | Passive tasting of the sweet solutions | Yes | p < 0.05, corrected with unknown method | Sugar and sweetener caused increased responses in different brain regions. With carbonation, the differential responses largely diminished |
| Frank et al. 2008 [16] | NeuroImage (5.812) | 12 (12F) | 27 ± 6 | 22 ± 2 | Healthy | Sucrose | Sucralose | Overnight | Passive tasting of the sweet solutions | Yes | p < 0.05 with clusters >8 voxels, uncorrected | Sugar caused larger brain responses in anterior insula, anterior cingulate, caudate, and superior frontal gyrus. Sugar engaged dopaminergic midbrain regions but not sweetener |
| Gramling et al. 2019 [17] | Nutrients (4.171) | 28 (12M, 16F) | 50.9 ± 17.4 | 29.6 ± 6.5 | Obesity | Sucrose | Saccharin | 12 h | Tasting of sweet solutions and evaluated the pleasantness | No (separate tests against baseline) | p < 0.015, FWE corrected | Sugar caused greater responses in memory and reward regions. Sweetener caused greater responses in memory and information processing regions |
| Green and Murphy 2012 [18] | Physiol Behav (2.635) | 24 (10M, 14F) | 23.5 ± 2.8 | 26.1 ± 5.9 | Obesity | Sucrose | Saccharin | 12 h | Tasting of sweet solutions and evaluated the pleasantness | Yes | p < 0.01, FWE corrected | Sweetener caused greater responses than sugar in non-diet soda drinkers in orbitofrontal cortex. For diet soda drinkers, there was no difference |
| Griffioen-Roose et al. 2013 [19] | PLOS One (2.776) | 40 (15M, 25F) | 21 ± 2 | 21.5 ± 1.7 | Healthy | Sucrose | Sucralose + acesulfame | 3 h | Tasting of sweet solutions and evaluated the pleasantness | Yes | p < 0.05 with clusters >8 voxels, uncorrected | Sugar caused larger brain responses in Rolandic operculum, precentral gyrus and middle cingulate |
| Haase et al. 2009 [20] | NeuroImage (5.812) | 18 (9M, 9F) | 20.7 ± 1.0 | 23.7 ± NA | Healthy | Sucrose | Saccharin | 12 h | Passive tasting of sweet solutions | No (separate tests against baseline) | p < 0.0005, FWE corrected | Sugar elicited responses in more brain regions |
| James et al. 2009 [21] | NeuroReport (1.146) | 9 (6M, 3F) | 29 ± 4.3 | NA | Healthy | Sucrose | Aspartame | Unclear | Passive tasting of sweet solutions | No (separate tests against time) | p < 0.05, uncorrected | Sweetener elicited brain responses of longer duration in the insula |
| Kilpatrick et al. 2014 [22] | Gastroenterology (19.809) | 22 (22F) | 26.3 ± 1.6 | 27.6 ± 0.6 | Obesity | Sucrose | Truvia (stevia-based) | 6 h | Reported brain activity at resting state after drinking sweet beverages | Yes | p < 0.05, FWE corrected | Sugar and sweetener caused increased responses in different brain regions |
| Oberndorfer et al. 2013 [23] | Am J Psychiatry (13.655) | 42 (42F) | 40.7 ± 4.2 | 22.3 ± 2.1 | Anorexia and bulimia | Sucrose | Sucralose | Overnight | Passive tasting of sweet solutions | Yes | p < 0.005 with clusters >32 voxels, FWE corrected | Sugar caused larger brain responses in patients recovered from bulimia. Sweetener caused larger responses in patients recovered from anorexia |
| Parent et al. 2011 [24] | Neuropsychologia (2.872) | 14 (14M) | 24.1, range = 19–34 | NA | Healthy | Glucose | Saccharin | Overnight | Reported brain activity at viewing pictures and recalling them, after drinking sweet solutions | Yes | p < 0.001 with clusters >2 voxels, uncorrected | Sugar caused larger widespread brain responses and connectivity |
| Smeets et al. 2005 [25] | Am J Clin Nutr (6.568) | 5 (5M) | 20.4 ± 5.6 | 21.7 ± 2.5 | Healthy | Glucose | Aspartame | Overnight | Reported brain activity at resting state after drinking sweet beverages | No (separate tests against time) | p = 0.0018, Bonferroni corrected | Sugar elicited prolonged decreased brain responses but not sweetener |
| Smeets et al. 2011 [26] | NeuroImage (5.812) | 10 (10M) | 23.3 ± 2.8 | 22.4 ± 2.0 | Healthy | Sucrose | Aspartame + acesulfame K + cyclamate + saccharin | 2 h | Reported brain activity at passive tasting of sweet beverages, before and after drinking sweet beverages | Yes | p < 0.005, uncorrected | Sugar and sweetener caused larger responses in different brain regions. The differential responses were modulated by pre-loading of sweet beverages |
| Stone et al. 2005 [27] | Neurobiol Learn Mem (3.010) | 8 (5M, 3F) | 38.8 ± 10.7 | 28.6 ± 4.9 | Schizophrenia | Glucose | Saccharin | 8 h | Reported brain activity at verbal encoding task after drinking sweet beverages | Yes | p < 0.005 with clusters >5 voxels, uncorrected | Sugar caused larger brain responses in parahippocampus |
| Tyron et al. 2015 [28] | J Clin Endocrinol Metab (5.605) | 19 (19F) | 26.9 ± 6.5 | 25.7 ± 3.3 | Obesity | Sucrose | Aspartame | Unclear | Reported brain activity at stress task, after drinking sweet beverages for 2 weeks | Yes | p < 0.05, FDR corrected | Sugar treatment caused larger brain responses in hippocampus |
| Van Opstal et al. 2019a [29] | Nutr Neurosci (3.950) | 20 (20M) | 22.2 ± 1.3 | 22.4 ± 1.1 | Healthy | Glucose, fructose | Sucralose, allulose | Overnight | Reported brain activity at resting state before and after drinking sweet beverages | No | p < 0.05, FWE corrected | Sugar caused decreased brain activity in cingulate, insula and basal ganglia |
| Van Opstal et al. 2019b [30] | Nutrition (3.591) | 16 (16M) | 22.4 ± 1.3 | 22 ± 1.2 | Healthy | Glucose, fructose, sucrose | Sucralose | 10 h | Reported brain activity at resting state after drinking sweet beverages | No | p < 0.05, uncorrected | Sugar caused more decreased brain activity in hypothalamus. Sweetener caused more increased brain activity in the ventral tegmental area |
| Van Rijn et al. 2015 [31] | Behav Brain Res (2.770) | 30 (30F) | 22 ± 3 | 22.6 ± 1.4 | Healthy | Maltodextrin + Sucralose (sweet with energy) | Sucralose (sweet without energy) | 3 h | Passive tasting of sweet solutions under hungry and satiated conditions | Yes | p < 0.001 with clusters >8 voxels, uncorrected | In overall, sugar and sweetener did not have significant difference. However, sugar caused larger differential brain response between hunger and satiety states |
| Wagner et al. 2015 [32] | Psychiatry Res Neuroimaging (2.270) | 42 (42F) | 26.7 ± 6.0 | 21.9 ± 2.1 | Anorexia and bulimia | Sucrose | Sucralose | Overnight | Passive tasting of sweet solutions | Yes | p < 0.05 with clusters >30 voxels, FWE corrected | Sugar caused larger brain response upon repeated exposure in patients recovered from bulimia and healthy controls. Sucralose caused larger brain response upon repeated exposure in patients recovered from anorexia |
| Study | Criterion | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Score | |
| Chambers et al. 2009 [13] | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 12 |
| Connolly et al. 2013 [14] | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 12 |
| Di Salle et al. 2013 [15] | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 8 |
| Frank et al. 2008 [16] | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 8 |
| Gramling et al. 2019 [17] | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 12 |
| Green and Murphy 2012 [18] | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 12 |
| Griffioen-Roose et al. 2013 [19] | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 12 |
| Haase et al. 2009 [20] | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 12 |
| James et al. 2009 [21] | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 4 |
| Kilpatrick et al. 2014 [22] | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 12 |
| Oberndorfer et al. 2013 [23] | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 12 |
| Parent et al. 2011 [24] | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 10 |
| Smeets et al. 2005 [25] | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 10 |
| Smeets et al. 2011 [26] | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 10 |
| Stone et al. 2005 [27] | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 12 |
| Tyron et al. 2015 [28] | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 10 |
| Van Opstal et al. 2019a [29] | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 12 |
| Van Opstal et al. 2019b [30] | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 10 |
| Van Rijn et al. 2015 [31] | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 10 |
| Wagner et al. 2015 [32] | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeung, A.W.K.; Wong, N.S.M. How Does Our Brain Process Sugars and Non-Nutritive Sweeteners Differently: A Systematic Review on Functional Magnetic Resonance Imaging Studies. Nutrients 2020, 12, 3010. https://doi.org/10.3390/nu12103010
Yeung AWK, Wong NSM. How Does Our Brain Process Sugars and Non-Nutritive Sweeteners Differently: A Systematic Review on Functional Magnetic Resonance Imaging Studies. Nutrients. 2020; 12(10):3010. https://doi.org/10.3390/nu12103010
Chicago/Turabian StyleYeung, Andy Wai Kan, and Natalie Sui Miu Wong. 2020. "How Does Our Brain Process Sugars and Non-Nutritive Sweeteners Differently: A Systematic Review on Functional Magnetic Resonance Imaging Studies" Nutrients 12, no. 10: 3010. https://doi.org/10.3390/nu12103010
APA StyleYeung, A. W. K., & Wong, N. S. M. (2020). How Does Our Brain Process Sugars and Non-Nutritive Sweeteners Differently: A Systematic Review on Functional Magnetic Resonance Imaging Studies. Nutrients, 12(10), 3010. https://doi.org/10.3390/nu12103010





