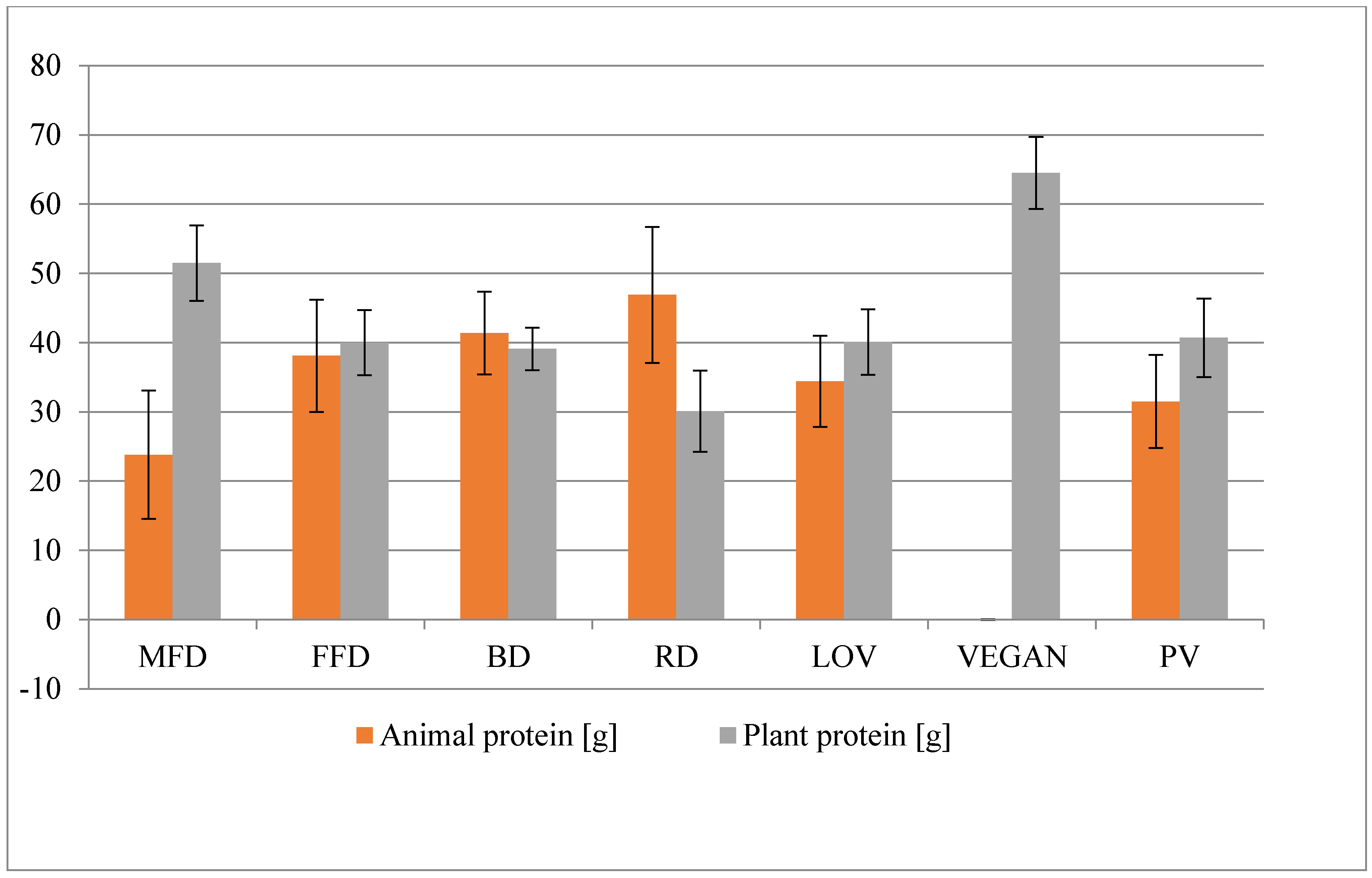5. Discussion
The nutritional habits of Poles deviate in many ways from the rules of rational nutrition. The diets are poor in vegetables, fruit, and wholegrain products, which results in the deficiency of some vitamins and minerals. Despite the same caloricity of the diets, there were differences between them in protein content. The source of proteins in a diet include products of animal and plant origin. The vegan (VEGAN) diet contained no products of animal origin and as such, it had the highest content of plant protein. Higher consumption of plant products is associated with the higher provision of individual constituents, such as fiber, potassium, and magnesium. Therefore, the decrease in the prevalence of CVD results from a number of factors, not just the consumption of plant protein [
38]. The replacement of 1 standard portion of red meat (85 g) with three different plant sources of protein decreases the risk of coronary heart disease (CHD) by 13–30% according to the Nurses ‘Health Study [
39], and by 7–19% in the combined control analyses Nurses ‘Health Study and Health Professionals Follow-Up [
40]. The type of the consumed protein is important due to the various contribution of exogenous amino acids between plant and animal products [
41]. Our study confirmed that the vegan diet and its derivatives pose a risk of insufficient supply of endogenous amino acids, relatively exogenous amino acids, as well as purely exogenous amino acids (leucine, isoleucine, lysine, methionine, threonine, phenylalanine, tryptophan, and valine). In the case of relatively exogenous amino acids, which include histidine, arginine, and serine, these amino acids can be produced in the body, but in exceptional situations, such as various illnesses, stressful events, or the period of quick growth, they should be supplied in appropriate amounts together with food [
42]. In the context of the vegetarian diet, lysine is an important amino acid that requires special attention. In products of plant origin, its content is limited. To increase the supply of lysine, the vegetarian and (especially) vegan diets should be enriched with nuts and/or soy seeds. At the same time, appropriate supply of lysine has an influence on the decrease of the risk of heart diseases and some neoplasms as a result of the limitation of the activity of enzymes responsible for the lipogenesis and synthesis of cholesterol. Lysine also contributes to the reduction of the concentration of insulin-like growth factors (IGF) [
43]. Another amino acid whose decreased supply is observed in the vegetarian diet is methionine. During the course of various transformations, methionine is transformed into taurine and homocysteine. For many people following the vegetarian diet, this amino acid reduces the assimilation of other amino acids [
44]. When it comes to mental health, tryptophan is important. This amino acid is necessary for the production of serotonin, which is responsible for feeling well, the regulation of sleep, and it also prevents hyperactivity in children. Moreover, it is transformed into melatonin, and it also influences the secretion of hormones that support the synthesis of pyridoxine and niacin. The richest source of tryptophan is turkey meat, milk, and dairy. Because most vegetarian diets exclude these products, tryptophan supply is reduced in these diets, and tryptophan is also a limiting amino acid in our menus. It is used in the synthesis of neurotransmitters and, as such, its significant deficiency causes a specific depressive reaction [
45,
46]. There are ways of increasing the biological quality of the consumed plant protein through the organization of menus in a way that would supplement the content of protein products with the missing amino acids. One of the ways is to combine legume products with grain seeds in one meal, e.g., beans with rice. In comparison to animal proteins, plant proteins have lower contents of leucine, lysine, methionine, and tryptophan [
42].
Vegetarian diets are associated with the lower level of cholesterol in the plasma and lower blood pressure. However, this is strongly associated with the lifestyle of vegetarians—these people are usually non-smokers, they do not drink alcohol, and are physically active.
The regular diet of Poles was characterized by the highest percentage of fat, unsaturated fatty acids, and cholesterol in comparison to other menus. This correlates with the increased risk of developing ischemic heart disease, atherosclerosis, and cancers—prostate, breast, or colon. Circulatory system diseases have been the main cause of death in the Polish population in recent decades [
43]. The lowest content of PUFA was observed in FFD, RD, and PV. PUFA have a positive influence on the functioning of the circulatory system, e.g., they contribute to the decrease in the level of cholesterol, they lower arterial blood pressure, prevent the development of clots, and increase the strength of heart contraction [
47].
High content of red meat, processed meat products, and eggs in these menus was the reason for the excessive consumption of cholesterol and saturated fatty acids, which are a significant factor contributing to deaths resulting from CVD. High level of cholesterol is also associated with the development of colorectal cancer [
48]. Excessive levels of cholesterol also contribute to the development and progression of neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease [
49]. Cholesterol is necessary for the proper development of the fetus in the first stages of pregnancy. After being born, about 40-50% of the child’s cholesterol intake comes from mother’s milk. Because of that, the VEGAN diet should not be recommended to pregnant or breastfeeding women [
50].
Assimilable carbohydrates that are digested in the gastrointestinal tract are responsible for the supply of energy to muscles, the brain, heart, intestines, and erythrocytes. Dietary fiber (cellulose, hemicellulose, pectin, lignin) is not digested by enzymes in the gastrointestinal tract. The main functions of dietary fiber in the body are: the reduction of the level of cholesterol, glucose and insulin, the stimulation of fermentation processes in the intestine, the decrease in the time of intestinal passage, and the increase in the volume of stool [
37]. According to WHO/FAO, the daily consumption of 25 g of fiber enables the correct functioning of the body. Basing on our original study, the lowest and insufficient content of fiber was observed in the RD. The insufficient supply of fiber in the diet may be associated with the development of disorders in the functioning of intestines, as well as with the increase in the risk of coronary artery disease and type 2 diabetes [
51]. The main products of fermentation bacteria in the intestines are SCFA, especially acetate, propionate, and butyrate. They have many properties that are beneficial to health, they are responsible for feeling full, and they stimulate the immune system. In the case when there is a deficiency of dietary fiber, microbes shift to less favorable energy sources [
52]. Moreover, prolonged consumption of high-fat and high-saccharose diet may lead to the death of the positive species of gut microflora [
53]. Despite the numerous health benefits related to the high consumption of fiber, its excessive intake can have negative consequences. Products rich in food fiber, i.e., legumes, nuts, tofu, and some cereals are characterized by high content of phytic acid. Phytates may bind with some minerals, e.g., iron, zinc, and calcium, forming insoluble complexes, reducing their assimilation in the digestive tract [
54]. This is why when using diets based on plant products (mainly legumes and cereals as in VEGAN and PV) it is important to control the levels of minerals in the body.
The regular diet was characterized by the highest content of saccharose out of all of the analyzed diets. This was caused by the presence of significant amounts of candy and sugar in the diet, the latter being added, e.g., to coffee, tea, and processed foods. Saccharose, which consists of glucose and fructose, also occurs naturally in honey, fruit, and vegetables, but in significantly lower quantities than in ready-made products prepared by the food industry. Excessive consumption of sugar is associated with many negative health aspects, such as circulatory system diseases, obesity, type 2 diabetes, caries, cirrhosis, and dementia [
55]. Saccharose in the rest of the analyzed diets mainly originates from fruit, vegetables, and honey which, apart from being a source of sugar, include numerous valuable vitamins, minerals, as well as fiber.
The highest average content of lactose was observed in LOV, PV, and FFD. Very small amounts of milk sugar were observed in MFD and VEGAN, which originated from bread added in the dietetics program, though there should be no trace of this sugar in the menus at all. MFD and VEGAN could be appropriate for people with confirmed lactose intolerance and the incorrect assimilation of lactose in the digestive tract. Studies show that the risk of symptoms after the consumption of lactose depends on the dose of lactose, lactose expression, intestinal flora, and the sensitivity of the digestive tract [
56]. When treating lactose intolerance, it is recommended to reduce lactose consumption, not eliminate it from the diet completely because in blind studies, most patients that reported the intolerance tolerated at least 12 g of lactose (which is equivalent to 250 mL of milk), and up to 18 g with the consumed foods [
57]. A study conducted by Staudacher et al. regarding a diet poor in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) indicated the improvement of symptoms in 86% of patients with the irritable bowel syndrome (IBS), in comparison to 49% in the case of a standard dietary intervention [
58]. FODMAP is a diet that includes low contents of fermenting oligo-, di- and monosaccharides, as well as polyols, so fructose, lactose, fructans, galactans, and artificial sweeteners like sorbitol, mannitol, maltitol, and xylitol. All of these constituents are poorly absorbed in the small intestine, they are osmotically active (they can have laxative effects, they influence intestinal motility), and they are quickly fermented by intestinal bacteria. FODMAP aims at reducing or eliminating the presence of such symptoms as flatulence, stomachache, nausea, diarrhea, and constipation [
59].
The regular diet of Poles was characterized by significantly higher consumption of sodium. Only VEGAN menus were characterized by lower levels of this constituent. High-sodium diet significantly increases the risk of developing hypertension, insulin resistance, dyslipidemia, and hipoadiponectemia [
60]. The widespread supply of sodium is considered as one of the main causes of death resulting from circulatory system diseases. Sportsmen require higher intake of sodium, especially those that exercise in high temperatures. Higher sodium intake is also important for patients with insufficiency of the adrenal cortex and thyroid. During intense physical activity, contestants lose this element along with sweat [
61]. Because of that, VEGAN may not be the right choice for people that do intense exercise.
Out of all of the analyzed diets, only RD was characterized by insufficient levels of potassium, when compared to the valid norm. Incorrect supply of this element may be associated with the increased risk of stroke and other circulatory system diseases [
62]. Studies conducted by Zhang et al. indicate that excessive consumption of sodium positively correlated with increased systolic blood pressure and hypertension, and that the consumption of potassium negatively correlate with both of these disorders. Furthermore, the ratio of sodium and potassium was also important in the prevention of these problems [
63].
The analysis of diets in terms of calcium content revealed that MFD, RD, and VEGAN contain insufficient amounts of this element. Well-absorbed sources of calcium are milk and milk products. Other sources include small fish (consumed with bones), beans, kale, parsley leaves, nuts, almonds, sesame seeds, and poppy seeds. It has to be highlighted that calcium originating from plant sources is less efficiently absorbed than calcium from milk and its products, which is associated with, e.g., the presence of lactose, which amplifies the absorption of this element [
64]. However, it is emphasized that the best absorbed sources of calcium are vegetable with low in oxalate [
65]. MFD completely eliminated milk products, this is why the average content of this macroelement was so low despite the use of other products that are its source. Due to the fact that the consumption of minerals with water was not taken into account, deficiencies in all types of diets, especially in terms of calcium, may be much smaller. The right supplementation of calcium and Vitamin D is of key importance for the prevention of the progressing loss of bone mass. In the case of postmenopausal women, it is recommended to consume a daily total of 1200 mg of calcium originating from food and supplements, and to supplement the diet with 800–2000 IU of Vitamin D. The supplementation is insufficient to prevent bone breaking in persons with osteoporosis. However, this is an important addition to a pharmacological intervention [
66].
Phosphorus deficiencies were not observed in the studied diets. However, the proportion of Ca:P should be 1:1 to maintain the proper state of the skeleton. Mineral metabolism dysfunctions are the frequent complications of chronic kidney disease (CKD). A damaged kidney is not able to fully dispose of a phosphorus charge, leading to, e.g., secondary hyperparathyroidism. Studies conducted by Moe et al. indicated that protein products rich in phosphorus, i.e., cereals and legumes, are a better source of protein for people with CKD. The results of this study show that the use of the vegetarian diet in patients with CKD leads to the reduction of the level of phosphorus in the serum, when compared to a diet that includes meat [
67].
The analyzed RD did not fulfill the RDA norm for magnesium with reference to men. Studies conducted by Adebamowo et al. indicated that a magnesium-, potassium-, and calcium-rich diet may contribute to the decrease in the risk of stroke in men [
68]. Generally speaking, no pathological states associated with low magnesium consumption have been observed, but a small to moderate deficiency of this element resulting from chronic stress may significantly contribute to the presence of such illnesses as atherosclerosis, hypertension, osteoporosis, diabetes, and cancer [
69]. Coffee, which is often consumed in large amounts by the Polish population, is a factor that is commonly believed to decrease the assimilation of magnesium, which favors many pathologies [
70,
71].
Iron present in food products has many forms and is usually classified as heme and nonheme iron. All of the analyzed diets fulfilled RDA for iron, but they differed in terms of its origin. In the case of meat-eliminating diets, it is mainly nonheme iron, which can occur in products in the form of various complexes, which may improve or weaken its absorption. An example of substances that significantly reduce the absorption of iron in the digestive tract are phytates and tannins of plant origin [
72]. This is why the content of nonheme iron in the diet should be several times higher than heme iron.
RDA norms for such minerals as zinc, copper, and manganese have been fulfilled by all of the analyzed diets. However, taking into account factors that interrupt absorption and assimilation in the digestive tract, the supply of these constituents may turn out to be too low. A frequent factor facilitating this process is animal protein, which is not present in VEGAN. It has been demonstrated that the supplementation with zinc has a protective effect on the epithelial barrier of intestines and helps in various pathologies, including chronic alcohol consumption, oxidative stress, diarrhea, chronic fatigue syndrome, colitis, other gastrointestinal problems, and even some neurological disorders. However, zinc deficiency may result from the wide use of proton-pump inhibitor medicines, diets including large amounts of products rich in phytates and the decreasing consumption of meat and fish [
50,
73].
The main sources of copper are food products (75%) and drinking water (25%). Genetic illnesses connected to the disturbed metabolism of this element include Menkes disease, associated with bad absorption, and Wilson’s disease, in which the excretion of iron is disturbed. Infants are more vulnerable to the deficiency than adults. This is true especially for premature infants because the fetus absorbs copper in the last months of pregnancy. Children that do not go through breastfeeding require supplementation in the first year of their lives [
74]. However, high copper consumption with trans-fat and saturated fatty acids has been associated with the accelerated decrease in cognitive functions of the elderly [
75].
Manganese is a necessary element that is required for the proper functioning of the immune system, the regulation of the level of sugar in the blood, cellular energy, reproduction, digestion, bone growth, blood coagulation, hemostasis, and protection against reactive oxygen species. Manganese deficiencies are rarely observed because it is available in many food products. The absorption of manganese is strictly regulated in intestines and no toxicity resulting from its excessive intake with the diet was observed. The toxicity of manganese in the world results from environmental pollution, including the pollution of air and drinking water [
76].
All of the analyzed diets were characterized by insufficient iodine content when compared to the norm. However, the addition of salt was not included in the meals. Thanks to fortification (in Poland in the form of potassium iodide), salt is the main source of iodine in the diet of Poles. The main sources of this constituent include sea fish, which were not present in FFD, LOV, VEGAN, and PV. This is why the average content of iodine in these diets is the lowest. The main results of the deficiency are goiter and hypothyroidism. In pregnant women, insufficient consumption of iodine may be associated with impaired psychomotor development of children, the risk of miscarriage or endemic cretinism. The impaired mental and somatic development may result from the deficiency of iodine in children and teenagers [
77].
All of the analyzed diets fulfilled the norm for Vitamin A, sometimes significantly exceeding the recommended values. The lowest content of retinol was observed in VEGAN because the diet completely eliminates products of animal origin, which are its source. RD has the lowest average content of beta-carotene; a carotenoid present in plant products. The reason for the limited assimilation of Vitamin A may be the excessive intake of fiber or alcohol, excess amounts of iron, nitrates, nitrites, and free radicals in the body, as well as insufficient levels of zinc [
41]. The symptoms of Vitamin A deficiency include weaker sight, skin dryness, the weakening of mucous membranes, higher vulnerability to infections.
The diets were properly balanced in terms of Vitamin E, only RD did not fulfill the RDA norm for men. Vitamin E also influences the efficiency of muscles and the production of sperm. Therefore, its appropriate supply is very important in men [
78].
The effects of vitamins from the B family were observed in many aspects, including brain function, energy production, DNA and RNA synthesis and repair, as well as in the synthesis of numerous neurochemical substances and signaling particles. Insufficient amounts of B-family vitamins are associated with inflammatory processes and oxidative stress as indicated by the increased concentration of homocysteine in blood plasma [
79]. The average content of B-family vitamins in the analyzed diets fulfilled the norm of the recommended consumption for the Polish population. The source of these vitamins includes both products of plant and animal origin. The only exception is Vitamin B
12, which can be found only in animal foods. The VEGAN diet achieved the norm for this Vitamin Because the products used in the menus, i.e., soy milk or tofu, were enriched. The group of B vitamins includes folates (Vitamin B
9). The regular diet of Poles turned out to be insufficient in terms of this nutrient. The methylenetetrahydrofolate reductase (MTHFR) 3 gene codes the methylene tetrahydrofolate reductase enzyme, which participates in the metabolism of folates, homocysteine, and methionine. MTHFR transforms folic acid from food into an active form, which can be used by the organism. This way, MTHFR influences the transformation of toxic homocysteine into methionine with the participation of folic acid. The presence of the C677T mutation of the MTHFR gene leads to the deficiency of folic acid and the accumulation of homocysteine. It is estimated that about 15% of the Polish population has the mutation of the MTHFR gene. More efficiently assimilated vitamins are those that originate from the diet, not supplements. The most valuable sources of B-family vitamins are meat, fish, seafood, nuts, liver, green leafy vegetables, yeast, and eggs. People with the MTHFR gene mutation should be supplemented with methylated folic acid because this is the only form that will be assimilated by the body. The C677T polymorphism of methylene tetrahydrofolate reductase is associated with various illnesses, i.e., circulatory system diseases, neoplasms, neurological diseases, diabetes, or psoriasis [
80].
Vitamin C (ascorbic acid) has strong antioxidant properties, is well assimilated from the digestive tract, and its excess is removed with urine. The main sources of ascorbic acid in a diet are fruit and vegetables, but there are huge losses of this constituent during heat treatment and storage, even >75% when compared to fresh, raw product [
41]. The regular diet (RD) was characterized by the lowest content of Vitamin C and, at the same time, it did not fulfill the norms of consumption. The remaining diets (MFD, FFD, BD, LOV, VEGAN, and PV) covered the norm for this vitamin. The symptoms of scurvy or Vitamin C deficiency include swelling of the lower limbs, bleeding of gums, tiredness, and hemorrhages, as well as psychological issues, including depression, hysteria, and social introversion [
81].
Vitamin D deficiency is the main problem of public health in the entire world in all age groups, even in countries where it is generally assumed that UV radiation is sufficient to prevent this deficiency or in industrialized countries where fortification has been conducted for years.
The causes of Vitamin D deficiency:
- -
the use of sunscreen,
- -
elderly age,
- -
obesity,
- -
malabsorption,
- -
kidney and liver diseases,
- -
use of anticonvulsants.
Due to the fact that it is very difficult to supplement proper amounts of Vitamin D with food, none of the analyzed diets fulfilled the norm for this constituent. Regardless of whether the diet eliminated products of animal origin or not, supplementation is necessary, particularly in the autumn-winter period in the temperate climate that is present in Poland. It has been demonstrated that Vitamin D stimulates the absorption of calcium in the intestines [
82]. Vitamin D deficiency is usually manifested through the deformation of bones (rickets) or hypocalcaemia in infancy and childhood, as well as through pain and musculoskeletal weakness in adults. Many other health problems, including circulatory system diseases, type 2 diabetes, several neoplasms, and autoimmune diseases, can be associated with Vitamin D deficiency [
83].
The analysis of menus prepared by qualified dieticians will make it possible to avoid some deficiencies. However, it is important to take into account the fact that most people who follow this type of diet do it rather poorly, which—in most cases—leads to even higher deficiencies, including calorie deficiencies, especially in the VEGE diet. An important assumption of this study was the same caloric value for every type of the analyzed diets.
Moreover, dietary fiber plays a key role in many metabolic processes not only directly related to the function of the intestine. Vegetable fiber is used by the intestinal microbes (stimulating growth of intestinal microbes) to synthesize SCFA, which support healthy colonic epithelial cells [
84]. Fiber consumption directly affects stool bulkiness, fecal pH, and intestinal transit time. The end products (acetate, propionate, and butyrate) produced by microorganisms affect enhancing various blood parameters (glucose, insulin) and the manner of bowel movements. The study showed that the numbers of bifidobacteria, lactobacilli, and methanogens were significantly decreased in the colon of patients with mixed refractory constipation [
84,
85]. Taking into account the above considerations, conclusions were drawn. Periodic implementation of vegetarian diets may help in the treatment of several diseases and symptoms associated with the metabolic syndrome, such as hypertension, hyperlipidemia, obesity, type 2 diabetes, and cardiovascular diseases. The implementation of diets that eliminate products of animal origin can be risky for pregnant or breastfeeding women, children, and the elderly.