Is There a Link between Zinc Intake and Status with Plasma Fatty Acid Profile and Desaturase Activities in Dyslipidemic Subjects?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Considerations
2.3. Blood Samples Collection and Analysis
2.4. Anthropometric Measurements
2.5. Assessment of Dietary Zn Intake
2.6. Fatty Acid Determination and Estimation of Desaturase Activity
2.7. Mineral Analysis
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Associations between Plasma Zn, Fe, and Cu Status with Anthropometrical and Biochemical Measures
3.3. The Link between the Plasma Zn, Cu, and Fe Status with Total Phospholipid FA Composition and Desaturase Enzyme Activities
3.4. The Link between the Status of the Individual FAs with Certain Components of Dyslipidemic Status (Anthropometrical and Biochemical Parameters)
3.5. Correlations between LA:DGLA Ratio and Estimated Desaturase Activities with FAs, Anthropometrical, and Biochemical Indices
3.6. Dietary Zn Intake of Participants
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. 2018; N°311 WFS. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 4 June 2019).
- Alberti, K.G.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Bettger, W.J.; Reeves, P.G.; Moscatelli, E.A.; Reynolds, G.; O’Dell, B.L. Interaction of zinc and essential fatty acids in the rat. J. Nutr. 1979, 109, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Franssen, R.; Monajemi, H.; Stroes, E.S.; Kastelein, J.J. Obesity and dyslipidemia. Med. Clin. N. Am. 2011, 95, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Doyle, J.T.; Ostfeld, A.M.; Jenkins, C.D.; Kuller, L.; Podell, R.N.; Stamler, J. Optimal resources for primary prevention of atherosclerotic diseases. Atherosclerosis study group. Circulation 1984, 70, 155a–205a. [Google Scholar]
- Hauner, H.; Stangl, K.; Schmatz, C.; Burger, K.; Blomer, H.; Pfeiffer, E.F. Body fat distribution in men with angiographically confirmed coronary artery disease. Atherosclerosis 1990, 85, 203–210. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018aha/acc/aacvpr/aapa/abc/acpm/ada/ags/apha/aspc/nla/pcna guideline on the management of blood cholesterol. J. Am. Coll. Cardiol. 2018, 25, 3168–3209. [Google Scholar]
- Laurant, P.; Kantelip, J.P.; Berthelot, A. Dietary magnesium supplementation modifies blood pressure and cardiovascular function in mineralocorticoid-salt hypertensive rats but not in normotensive rats. J. Nutr. 1995, 125, 830–841. [Google Scholar]
- Castillo-Duran, C.; Cassorla, F. Trace minerals in human growth and development. JPEM 1999, 12, 589–601. [Google Scholar] [CrossRef]
- Prashanth, L.; Kattapagari, K.K.; Chitturi, R.T.; Baddam, V.R.; Prasad, L.K. A review on role of essential trace elements in health and disease. J. NTR Univ. Health Sci. 2015, 4, 75–85. [Google Scholar]
- Prasad, A.S. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp. Gerontol. 2008, 43, 370–377. [Google Scholar] [CrossRef]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998, 67, 952s–959s. [Google Scholar] [CrossRef] [PubMed]
- Turnlund, J.R. Copper, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 286–299. [Google Scholar]
- Kruse-Jarres, J.D.; Rükgauer, M. Trace elements in diabetes mellitus. Peculiarities and clinical validity of determinations in blood cells. J. Trace Elem. Med. Biol. 2000, 14, 21–27. [Google Scholar] [CrossRef]
- Aguilar, M.; Saavedra, P.; Arrieta, F.; Mateos, C.; González-Muñoz, M.J.; Meseguer, I.; Martinez-Para, M.C. Plasma mineral content in type-2 diabetic patients and their association with the metabolic syndrome. Ann. Nutr. Metab. 2007, 51, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Garcia, O.P.; Long, K.Z.; Rosado, J.L. Impact of micronutrient deficiencies on obesity. Nutr. Rev. 2009, 67, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Samman, S. The effect of zinc supplementation in humans on plasma lipids, antioxidant status and thrombogenesis. J. Am. Coll. Nutr. 2006, 25, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Altekin, E.; Coker, C.; Sisman, A.R.; Onvural, B.; Kuralay, F.; Kirimli, O. The relationship between trace elements and cardiac markers in acute coronary syndromes. J. Trace Elem. Med. Biol. 2005, 18, 235–242. [Google Scholar] [CrossRef]
- Reunanen, A.; Knekt, P.; Marniemi, J.; Maki, J.; Maatela, J.; Aromaa, A. Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur. J. Clin. Nutr. 1996, 50, 431–437. [Google Scholar]
- Stadler, N.; Lindner, R.A.; Davies, M.J. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: Evidence for the presence of elevated levels of iron and copper. Arterioscle. Thromb. Vasc. Biol. 2004, 24, 949–954. [Google Scholar] [CrossRef]
- Ma, J.; Folsom, A.R.; Shahar, E.; Eckfeldt, J.H. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The atherosclerosis risk in communities (aric) study investigators. Am. J. Clin. Nutr. 1995, 62, 564–571. [Google Scholar] [CrossRef]
- Vessby, B.; Gustafsson, I.B.; Tengblad, S.; Boberg, M.; Andersson, A. Desaturation and elongation of fatty acids and insulin action. Ann. N. Y. Acad. Sci. 2002, 967, 183–195. [Google Scholar] [CrossRef]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, Y.A.; Portois, L.; Malaisse, W.J. N-3 fatty acids and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 83, 1499s–1504s. [Google Scholar] [CrossRef] [PubMed]
- Warensjo, E.; Riserus, U.; Vessby, B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia 2005, 48, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Kabagambe, E.K.; Tsai, M.Y.; Hopkins, P.N.; Ordovas, J.M.; Peacock, J.M.; Borecki, I.B.; Arnett, D.K. Erythrocyte fatty acid composition and the metabolic syndrome: A national heart, lung, and blood institute goldn study. Clin. Chem. 2008, 54, 154. [Google Scholar] [CrossRef]
- Damsgaard, C.T.; Stark, K.D.; Hjorth, M.F.; Biltoft-Jensen, A.; Astrup, A.; Michaelsen, K.F.; Lauritzen, L. N-3 pufa status in school children is associated with beneficial lipid profile, reduced physical activity and increased blood pressure in boys. Br. J. Nutr. 2013, 110, 1304–1312. [Google Scholar] [CrossRef]
- Kurotani, K.; Sato, M.; Ejima, Y.; Nanri, A.; Yi, S.; Pham, N.M.; Akter, S.; Poudel-Tandukar, K.; Kimura, Y.; Imaizumi, K.; et al. High levels of stearic acid, palmitoleic acid, and dihomo-gamma-linolenic acid and low levels of linoleic acid in serum cholesterol ester are associated with high insulin resistance. Nutr. Res. 2012, 32, 669–675.e3. [Google Scholar] [CrossRef]
- Schiller, K.; Jacobs, S.; Jansen, E.; Weikert, C.; di Giuseppe, R.; Boeing, H.; Schulze, M.B.; Kroger, J. Associated factors of estimated desaturase activity in the epic-potsdam study. NMCD 2014, 24, 503–510. [Google Scholar] [CrossRef]
- Cunnane, S.C. Modulation of long chain fatty acid unsaturation by dietary copper. Adv. Exp. Med. Biol. 1989, 258, 183–195. [Google Scholar]
- Eder, K.; Kirchgessner, M. The effect of dietary fat on activities of lipogenic enzymes in liver and adipose tissue of zinc-adequate and zinc-deficient rats. J. Nutr. Biochem. 1996, 7, 190–195. [Google Scholar] [CrossRef]
- Reed, S.; Qin, X.; Ran-Ressler, R.; Brenna, J.T.; Glahn, R.P.; Tako, E. Dietary zinc deficiency affects blood linoleic acid: Dihomo-γ-linolenic acid (LA:DGLA) ratio; a sensitive physiological marker of zinc status in vivo (Gallus Gallus). Nutrients 2014, 6, 1164–1180. [Google Scholar] [CrossRef]
- Knez, M.; Stangoulis, J.C.R.; Zec, M.; Debeljak-Martacic, J.; Pavlovic, Z.; Gurinovic, M.; Glibetic, M. An initial evaluation of newly proposed biomarker of zinc status in humans-linoleic acid: Dihomo-γ-linolenic acid (LA:DGLA) ratio. Clin. Nutr. ESPEN 2016, 15, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Knez, M.; Stangoulis, J.C.R.; Glibetic, M.; Tako, E. The linoleic acid: Dihomo-γ-linolenic acid ratio (la:Dgla)-an emerging biomarker of zn status. Nutrients 2017, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Chimhashu, T.; Malan, L.; Baumgartner, J.; van Jaarsveld, P.J.; Galetti, V.; Moretti, D.; Smuts, C.M.; Zimmermann, M.B. Sensitivity of fatty acid desaturation and elongation to plasma zinc concentration: A randomised controlled trial in beninese children. Br. J. Nutr. 2018, 119, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C. Differential regulation of essential fatty acid metabolism to the prostaglandins: Possible basis for the interaction of zinc and copper in biological systems. Prog. Lipid Res. 1982, 21, 73–90. [Google Scholar] [CrossRef]
- Das, U.N. A defect in the activity of delta 6 and delta 5 desaturases may be a factor predisposing to the development of insulin resistance syndrome. Prostaglandins Leukot. Essent. Fatty Acids 2005, 72, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Yary, T.; Voutilainen, S.; Tuomainen, T.P.; Ruusunen, A.; Nurmi, T.; Virtanen, J.K. Serum n-6 polyunsaturated fatty acids, delta5- and delta6-desaturase activities, and risk of incident type 2 diabetes in men: The kuopio ischaemic heart disease risk factor study. Am. J. Clin. Nutr. 2016, 103, 1337–1343. [Google Scholar] [CrossRef]
- Knez, M.; Tako, E.; Glahn, R.P.; Kolba, N.; de Courcy-Ireland, E.; Stangoulis, J.C.R. Linoleic acid:Dihomo-γ-linolenic acid ratio predicts the efficacy of Zn-biofortified wheat in chicken (Gallus Gallus). J. Agric. Food Chem. 2018, 66, 1394–1400. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Nikolić, M.; Milešević, J.; Zeković, M.; Gurinović, M.; Glibetić, M. The development and validation of food atlas for portion size estimation in the balkan region. Front. Nutr. 2018, 5, 78. [Google Scholar] [CrossRef]
- Gavrieli, A.; Naska, A.; Konstantinidi, C.; Berry, R.; Roe, M.; Harvey, L.; Finglas, P.; Glibetic, M.; Gurinovic, M.; Trichopoulou, A. Dietary monitoring tools for risk assessment. EFSA Support. Publ. 2014, 11, 607E. [Google Scholar] [CrossRef]
- Gurinović, M.; Milešević, J.; Kadvan, A.; Nikolić, M.; Zeković, M.; Djekić-Ivanković, M.; Dupouy, E.; Finglas, P.; Glibetić, M. Development, features and application of diet assess & plan (dap) software in supporting public health nutrition research in central eastern european countries (ceec). Food Chem. 2018, 238, 186–194. [Google Scholar]
- Gurinovic, M.; Milesevic, J.; Kadvan, A.; Djekic-Ivankovic, M.; Debeljak-Martacic, J.; Takic, M.; Nikolic, M.; Rankovic, S.; Finglas, P.; Glibetic, M. Establishment and advances in the online serbian food and recipe data base harmonized with eurofir standards. Food Chem. 2016, 193, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Pokimica, B.; Garcia-Conesa, M.T.; Zec, M.; Debeljak-Martacic, J.; Rankovic, S.; Vidovic, N.; Petrovic-Oggiano, G.; Konic-Ristic, A.; Glibetic, M. Chokeberry juice containing polyphenols does not affect cholesterol or blood pressure but modifies the composition of plasma phospholipids fatty acids in individuals at cardiovascular risk. Nutrients 2019, 11, 850. [Google Scholar] [CrossRef]
- Veselinovic, M.; Vasiljevic, D.; Vucic, V.; Arsic, A.; Petrovic, S.; Tomic-Lucic, A.; Savic, M.; Zivanovic, S.; Stojic, V.; Jakovljevic, V. Clinical benefits of n-3 pufa and -linolenic acid in patients with rheumatoid arthritis. Nutrients 2017, 9, 325. [Google Scholar] [CrossRef]
- Burdge, G. Alpha-linolenic acid metabolism in men and women: Nutritional and biological implications. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 137–144. [Google Scholar] [CrossRef]
- Jian, X.Q. Determination of Cu, Zn, Fe, Ca, Mg, na and K in serum flame by atomic absorption spectroscopy. In Varian Instruments at Work’s; Varian: Sao Paolo, Brazil, 1990; pp. 1–2. [Google Scholar]
- Rükgauer, M.; Klein, J.; Kruse-Jarres, J.D. Reference values for the trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. J. Trace Elem. Med. Biol. 1997, 11, 92–98. [Google Scholar] [CrossRef]
- Van den Bosch, G.; Van den Bossche, J.; Wagner, C.; De Schouwer, P.; Van De Vyvere, M.; Neels, H. Determination of iron metabolism-related reference values in a healthy adult population. Clin. Chem. 2001, 47, 1465. [Google Scholar]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lonnerdal, B.; Ruel, M.T.; Sandtrom, B.; Wasantwisut, E.; Hotz, C. International zinc nutrition consultative group (izincg) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar]
- Team, R. Rstudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Institute of Medicine Panel on Micronutrients. Dietary reference intakes for vitamin a, vitamin k, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. In Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001; Copyright 2001 by the National Academy of Sciences. [Google Scholar]
- Obeid, O.; Elfakhani, M.; Hlais, S.; Iskandar, M.; Batal, M.; Mouneimne, Y.; Adra, N.; Hwalla, N. Plasma copper, zinc, and selenium levels and correlates with metabolic syndrome components of lebanese adults. Biol. Trace Elem. Res. 2008, 123, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.A.; Song, S.W.; Han, K.; Lee, K.J.; Kim, H.N. The associations between serum zinc levels and metabolic syndrome in the korean population: Findings from the 2010 Korean national health and nutrition examination survey. PLoS ONE 2014, 9, e105990. [Google Scholar] [CrossRef] [PubMed]
- Daradkeh, G. Zinc status among type (2) diabetes mellitus in the state of qatar. Public Health Front. 2014, 3, 4–10. [Google Scholar] [CrossRef]
- Sanchez, C.; Lopez-Jurado, M.; Planells, E.; Llopis, J.; Aranda, P. Assessment of iron and zinc intake and related biochemical parameters in an adult mediterranean population from southern spain: Influence of lifestyle factors. J. Nutr. Biochem. 2009, 20, 125–131. [Google Scholar] [CrossRef]
- Jain, V.K.; Mohan, G. Serum zinc and copper in myocardial infarction with particular reference to prognosis. Biol. Trace Elem. Res. 1991, 31, 317–322. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, Z.; Zheng, J.; Chen, J.; Zhang, X.; Huang, X.F.; Li, D. Serum levels of polyunsaturated fatty acids are low in chinese men with metabolic syndrome, whereas serum levels of saturated fatty acids, zinc, and magnesium are high. Nutr. Res. 2012, 32, 71–77. [Google Scholar] [CrossRef]
- Chang, S.; El Arifeen, S.; Bari, S.; Wahed, M.A.; Rahman, K.M.; Rahman, M.T.; Mahmud, A.B.; Begum, N.; Zaman, K.; Baqui, A.H.; et al. Supplementing iron and zinc: Double blind, randomized evaluation of separate or combined delivery. Eur. J. Clin. Nutr. 2010, 64, 153–160. [Google Scholar] [CrossRef]
- Knez, M.; Graham, R.D.; Welch, R.M.; Stangoulis, J.C.R. New perspectives on the regulation of iron absorption via cellular zinc concentrations in humans. Crit. Rev. Food Sci. Nutr. 2017, 57, 2128–2143. [Google Scholar] [CrossRef]
- Knez, M.; Nikolic, M.; Zekovic, M.; Stangoulis, J.C.R.; Gurinovic, M.; Glibetic, M. The influence of food consumption and socio-economic factors on the relationship between zinc and iron intake and status in a healthy population. Pub. Health Nut. 2017, 20, 2486–2498. [Google Scholar] [CrossRef]
- Bárány, E.; Bergdahl, I.A.; Bratteby, L.E.; Lundh, T.; Samuelson, G.; Schütz, A.; Skerfving, S.; Oskarsson, A. Relationships between trace element concentrations in human blood and serum. Toxicol. Lett. 2002, 134, 177–184. [Google Scholar] [CrossRef]
- Grungreiff, K.; Reinhold, D.; Wedemeyer, H. The role of zinc in liver cirrhosis. Ann. Hept. 2016, 15, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Florianczyk, B. Copper in the organism—Transport and storage in the cells. Ann. Univ. Mariae Curie-Sklodowska Sectio D Med. 2003, 58, 85–88. [Google Scholar]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum copper to zinc ratio: Relationship with aging and health status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Klevay, L.M. Coronary heart disease: The zinc/copper hypothesis. Am. J. Clin. Nutr. 1975, 28, 764–774. [Google Scholar] [CrossRef]
- Bialkowska, M.; Hoser, A.; Szostak, W.B.; Dybczynski, R.; Sterlinski, S.; Nowicka, G.; Majchrzak, J.; Kaczorowski, J.; Danko, B. Hair zinc and copper concentration in survivors of myocardial infarction. Ann. Nutr. Metab. 1987, 31, 327–332. [Google Scholar]
- Bonaventura, P.; Benedetti, G.; Albarede, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Malavolta, M.; Giacconi, R.; Piacenza, F.; Santarelli, L.; Cipriano, C.; Costarelli, L.; Tesei, S.; Pierpaoli, S.; Basso, A.; Galeazzi, R.; et al. Plasma copper/zinc ratio: An inflammatory/nutritional biomarker as predictor of all-cause mortality in elderly population. Biogerontology 2010, 11, 309–319. [Google Scholar] [CrossRef]
- Arnaud, J.; Faure, H.; Bourlard, P.; Denis, B.; Favier, A.E. Longitudinal changes in serum zinc concentration and distribution after acute myocardial infarction. Clin. Chim. Acta Int. J. Clin. Chem. 1994, 230, 147–156. [Google Scholar] [CrossRef]
- Bailey, A.L.; Maisey, S.; Southon, S.; Wright, A.J.; Finglas, P.M.; Fulcher, R.A. Relationships between micronutrient intake and biochemical indicators of nutrient adequacy in a ‘free-living’ elderly UK population. Br. J. Nutr. 1997, 77, 225–242. [Google Scholar] [CrossRef]
- Gibson, R.S. Zinc: The missing link in combating micronutrient malnutrition in developing countries. Proc. Nutr. Soc. 2006, 65, 51–60. [Google Scholar] [CrossRef]
- Coulibaly, A.; Kouakou, B.; Chen, J. Phytic acid in cereal grains: Structure, healthy or harmful ways to reduce phytic acid in cereal grains and their effects on nutritional quality. Am. J. Plant Nutr. Fertil. Tech. 2011, 1, 1–22. [Google Scholar] [CrossRef]
- Jati, I.R.; Widmer, C.; Purwestri, R.C.; Wirawan, N.N.; Gola, U.; Lambert, C.; Biesalski, H.K. Design and validation of a program to identify inadequate intake of iron, zinc, and vitamin a. Nutrients 2014, 30, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Chehrei, A.; Sadrnia, S.; Keshteli, A.H.; Daneshmand, M.A.; Rezaei, J. Correlation of dyslipidemia with waist to height ratio, waist circumference, and body mass index in iranian adults. Asia Pac. J. Clin. Nutr. 2007, 16, 248–253. [Google Scholar] [PubMed]
- Ghayour-Mobarhan, M.; Taylor, A.; Kazemi-Bajestani, S.M.; Lanham-New, S.; Lamb, D.J.; Vaidya, N.; Livingstone, C.; Wang, T.; Ferns, G.A. Serum zinc and copper status in dyslipidaemic patients with and without established coronary artery disease. Clin. Lab. 2008, 54, 321–329. [Google Scholar] [PubMed]
- Romero, C.D.; Sánchez, P.H.; Blanco, F.L.; Rodríguez, E.R.; Majem, L.S. Serum copper and zinc concentrations in a representative sample of the canarian population. J. Trace Elem. Med. Biol. 2002, 16, 75–81. [Google Scholar] [CrossRef]
- Villalpando, S.; Garcia-Guerra, A.; Ramirez-Silva, C.I.; Mejia-Rodriguez, F.; Matute, G.; Shamah-Levy, T.; Rivera, J.A. Iron, zinc and iodide status in mexican children under 12 years and women 12–49 years of age. A probabilistic national survey. Salud Publica Mex. 2003, 45 (Suppl. 4), 520–529. [Google Scholar] [CrossRef]
- Al-Sabaawy, O.M. The relationship between serum lipid profile and selected trace elements for adult men in mosul city. Oman Med J. 2012, 27, 300–303. [Google Scholar] [CrossRef]
- Yerlikaya, F.H.; Toker, A.; Aribas, A. Serum trace elements in obese women with or without diabetes. Indian J. Med. Res. 2013, 137, 339–345. [Google Scholar]
- Di Martino, G.; Matera, M.G.; De Martino, B.; Vacca, C.; Di Martino, S.; Rossi, F. Relationship between zinc and obesity. J. Med. 1993, 24, 177–183. [Google Scholar]
- Choi, M.K.; Bae, Y.J. Relationship between dietary magnesium, manganese, and copper and metabolic syndrome risk in korean adults: The korea national health and nutrition examination survey (2007–2008). Biol. Trace Elem. Res. 2013, 156, 56–66. [Google Scholar] [CrossRef]
- Selva, D.M.; Lecube, A.; Hernandez, C.; Baena, J.A.; Fort, J.M.; Simo, R. Lower zinc-alpha2-glycoprotein production by adipose tissue and liver in obese patients unrelated to insulin resistance. J. Clin. Endocrinol. Metab. 2009, 94, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Neggers, Y.; Bindon, J.; Dressler, W. The relationship between zinc and copper status and lipid levels in african-americans. Biol. Trace Elem. Res. 2001, 79, 1–13. [Google Scholar] [CrossRef]
- Gunasekara, P.; Hettiarachchi, M.; Liyanage, C.; Lekamwasam, S. Effects of zinc and multimineral vitamin supplementation on glycemic and lipid control in adult diabetes. Diabetes Metab. Syndr. Obes. 2011, 4, 53–60. [Google Scholar] [PubMed]
- Lima, S.C.; Arrais, R.F.; Sales, C.H.; Almeida, M.G.; de Sena, K.C.; Oliveira, V.T.; de Andrade, A.S.; Pedrosa, L.F. Assessment of copper and lipid profile in obese children and adolescents. Biol. Trace Elem. Res. 2006, 114, 19–29. [Google Scholar] [CrossRef]
- Carpenter, W.E.; Lam, D.; Toney, G.M.; Weintraub, N.L.; Qin, Z. Zinc, copper, and blood pressure: Human population studies. Medical science monitor: International medical journal of experimental and clinical research. Med. Sci. Monit. 2013, 19, 1–8. [Google Scholar] [CrossRef]
- Atari-Hajipirloo, S.; Valizadeh, N.; Khadem-Ansari, M.-H.; Rasmi, Y.; Kheradmand, F. Altered concentrations of copper, zinc, and iron are associated with increased levels of glycated hemoglobin in patients with type 2 diabetes mellitus and their first-degree relatives. Int. J. Endocrinol. Metab. 2016, 14, e33273. [Google Scholar] [CrossRef]
- Tubek, S. Role of zinc in regulation of arterial blood pressure and in the etiopathogenesis of arterial hypertension. Biol. Trace Elem. Res. 2007, 117, 39–51. [Google Scholar] [CrossRef]
- Hennig, B.; Toborek, M.; McClain, C.J. High-energy diets, fatty acids and endothelial cell function: Implications for atherosclerosis. J. Am. Coll. Nutr. 2001, 20, 97–105. [Google Scholar] [CrossRef]
- Paillard, F.; Catheline, D.; Duff, F.L.; Bouriel, M.; Deugnier, Y.; Pouchard, M.; Daubert, J.C.; Legrand, P. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 436–440. [Google Scholar] [CrossRef]
- Warensjo, E.; Rosell, M.; Hellenius, M.L.; Vessby, B.; De Faire, U.; Riserus, U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: Links to obesity and insulin resistance. Lipids Health Dis. 2009, 8, 37. [Google Scholar] [CrossRef]
- Do, H.J.; Chung, H.K.; Moon, J.; Shin, M.J. Relationship between the estimates of desaturase activities and cardiometabolic phenotypes in koreans. J. Clin. Biochem. Nutr. 2011, 49, 131–135. [Google Scholar] [PubMed]
- Kathiresan, S.; Willer, C.J.; Peloso, G.M.; Demissie, S.; Musunuru, K.; Schadt, E.E.; Kaplan, L.; Bennett, D.; Li, Y.; Tanaka, T.; et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 2009, 41, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Chilton, F.H.; Murphy, R.C.; Wilson, B.A.; Sergeant, S.; Ainsworth, H.; Seeds, M.C.; Mathias, R.A. Diet-gene interactions and pufa metabolism: A potential contributor to health disparities and human diseases. Nutrients 2014, 6, 1993–2022. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C. Iron, copper, zinc and selenium: The other brain selective minerals. In Survival of the Fattest; World Scientific Publishing Company: Singapore, 2005; pp. 131–150. [Google Scholar]
- Krishnamurthy, P.; Wadhwani, A. Antioxidant Enzymes and Human Health. Available online: https://www.intechopen.com/books/antioxidant-enzyme/antioxidant-enzymes-and-human-health (accessed on 27 December 2019).
- Fan, Y.; Zhang, C.; Bu, J. Relationship between selected serum metallic elements and obesity in children and adolescent in the U.S. Nutrients 2017, 9, 104. [Google Scholar] [CrossRef]
- Wahle, E.W.; Davies, N.T. Effect of dietary copper deficiency. In the rat on fatty acid composition of adipose tissue and desaturase activity of liver microsomes. Br. J. Nutr. 1975, 34, 105–112. [Google Scholar] [CrossRef]
- Ho, S.K.; Elliot, J.I.; Jones, G.M. Effects of copper on performance, fatty acid composition of depot fat and fatty acyl desaturase activities in pigs fed a diet with or without supplemental copper. Can. J. Anim. Sci. 1975, 55, 587–594. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Skeaff, C.M.; Crowe, F.L.; Green, T.J.; Hodson, L. Serum fatty acid reference ranges: Percentiles from a new zealand national nutrition survey. Nutrients 2011, 3, 152–163. [Google Scholar] [CrossRef]
- Rasic-Milutinovic, Z.; Popovic, T.; Perunicic-Pekovic, G.; Arsic, A.; Borozan, S.; Glibetic, M. Lower serum paraoxonase-1 activity is related to linoleic and docosahexanoic fatty acids in type 2 diabetic patients. Arch. Med. Res. 2012, 43, 75–82. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, F. N-3 polyunsaturated fatty acids and inflammation in obesity: Local effect and systemic benefit. BioMed. Res. Int. 2015, 2015, 581469. [Google Scholar] [CrossRef]
- Cha, S.H.; Fukushima, A.; Sakuma, K.; Kagawa, Y. Chronic docosahexaenoic acid intake enhances expression of the gene for uncoupling protein 3 and affects pleiotropic mrna levels in skeletal muscle of aged c57bl/6njcl mice. J. Nutr. 2001, 131, 2636–2642. [Google Scholar] [CrossRef]
- Oliver, E.; McGillicuddy, F.; Phillips, C.; Toomey, S.; Roche, H.M. The role of inflammation and macrophage accumulation in the development of obesity-induced type 2 diabetes mellitus and the possible therapeutic effects of long-chain n-3 pufa. Proc. Nutr. Soc. 2010, 69, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.H.; Harris, W.S. Blood fatty acid profiles: New biomarkers for cardiometabolic disease risk. Curr. Atheroscler. Rep. 2018, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.; Gleason, K.; Griffin, B.; Miller, G.J. Influence of an algal triacylglycerol containing docosahexaenoic acid (22:6 n-3) and docosapentaenoic acid (22:5 n-6) on cardiovascular risk factors in healthy men and women. Br. J. Nutr. 2006, 95, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, J.D.; Mensink, R.P.; Schaart, G.; Plat, J. Arachidonic acid but not eicosapentaenoic acid (epa) and oleic acid activates nf-kappab and elevates icam-1 expression in caco-2 cells. Lipids 2007, 42, 687–698. [Google Scholar] [CrossRef]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. Ikk-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005, 11, 191–198. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. OCL 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Esrey, K.L.; Joseph, L.; Grover, S.A. Relationship between dietary intake and coronary heart disease mortality: Lipid research clinics prevalence follow-up study. J. Clin. Epidemiol. 1996, 49, 211–216. [Google Scholar] [CrossRef]
- Boniface, D.R.; Tefft, M.E. Dietary fats and 16-year coronary heart disease mortality in a cohort of men and women in great britain. Eur. J. Clin. Nutr. 2002, 56, 786–792. [Google Scholar] [CrossRef]
- Peter, A.; Cegan, A.; Wagner, S.; Lehmann, R.; Stefan, N.; Konigsrainer, A.; Konigsrainer, I.; Haring, H.U.; Schleicher, E. Hepatic lipid composition and stearoyl-coenzyme a desaturase 1 mrna expression can be estimated from plasma vldl fatty acid ratios. Clin. Chem. 2009, 55, 2113–2120. [Google Scholar] [CrossRef]
| Whole Sample (n = 27) | 1st Zn Tertile (n = 8) | 2nd Zn Tertile (n = 7) | 3rd Zn Tertile (n = 8) | p-Value | |
|---|---|---|---|---|---|
| Clinical Parameters | |||||
| Age, Years | 57.00 [16.00] | 59.50 [15.00] | 63.00 [24.00] | 53.00 [14.00] | p = 0.781 |
| Weight, Kg | 77.50 [31.30] | 68.65 [18.37] | 76.4 [22.70] | 95.90 [21.10] | p = 0.001 |
| BMI, Kg/M2 | 27.10 [8.30] | 24.85 [8.97] | 26.60 [3.80] | 34.10 [9.82] | p = 0.009 |
| Plasma Zn, Mg/L | 0.75 [0.14] | 0.64 [0.09] | 0.75 [0.05] | 0.86 [0.08] | p < 0.001 |
| Plasma Fe, Mg/L | 0.85 [0.65] | 0.82 [0.23] | 1.10 [0.91] | 0.90 [0.76] | p = 0.328 |
| Plasma Cu, Mg/L | 0.98 [0.16] | 1.01 [0.06] | 0.94 [0.11] | 0.95 [0.18] | p = 0.289 |
| Cu:Zn | 1.27 [0.39] | 1.60 [0.36] | 1.26 [0.10] | 1.05 [0.31] | p < 0.001 |
| Total Cholesterol, Mmol/L | 5.87 [1.26] | 5.87 [0.67] | 6.65 [1.29] | 5.95 [0.96] | p = 0.383 |
| HDL-C, Mmol/L | 1.41 [0.64] | 1.69 [0.35] | 1.28 [0.60] | 1.22 [0.59] | p = 0.318 |
| LDL-C, Mmol/L | 3.95 [0.79] | 3.59 [0.60] | 4.33 [1.00] | 3.83 [0.58] | p = 0.076 |
| TG, Mmol/L | 1.20 [0.69] | 1.00 [0.33] | 1.56 [0.49] | 1.37 [0.77] | p = 0.928 |
| FPG, Nmol/L | 5.17 [0.98] | 4.96 [0.57] | 5.76 [1.11] | 5.53 [1.16] | p = 0.166 |
| Systolic BP, MmHg | 131.00 [32.00] | 137.00 [51.75] | 120.00 [29.00] | 136.50 [13.00] | p = 0.295 |
| Diastolic BP, MmHg | 81.00 [22.00] | 76.50 [17.75] | 70.00 [19.00] | 90.50 [14.50] | p = 0.075 |
| Fatty Acids in Plasma Phospholipids% | |||||
| 18:1 n-9 | 8.11 [1.89] | 8.04 [1.27] | 8.12 [2.40] | 8.02 [1.59] | p = 0.951 |
| 18:2 n-6 | 24.01 [5.09] | 26.62 [5.51] | 25.69 [6.79] | 21.99 [4.10] | p = 0.385 |
| 18:3 n-3 | 0.08 [0.068] | 0.11 [0.05] | 0.07 [0.05] | 0.08 [0.08] | p = 0.214 |
| 20:3 n-6 | 2.83 [1.28] | 2.71 [1.17] | 2.83 [1.29] | 2.71 [1.32] | p = 0.958 |
| 20:4 n-6 | 11.67 [2.84] | 10.86 [4.90] | 11.58 [2.72] | 12.27 [1.03] | p = 0.330 |
| 20:5 n-3 | 0.27 [0.21] | 0.27 [0.57] | 0.23 [0.09] | 0.42 [0.16] | p = 0.239 |
| 22:4 n-6 | 0.36 [0.17] | 0.33 [0.22] | 0.40 [0.16] | 0.37 [0.15] | p = 0.775 |
| 22:5 n-3 | 0.55 [0.15] | 0.27 [0.57] | 0.50 [0.16] | 0.56 [0.19] | p = 0.436 |
| 22:6 n-3 | 2.49 [1.42] | 2.44 [1.95] | 2.40 [0.17] | 3.32 [1.33] | p = 0.403 |
| Δ5-Desaturase | 3.68 [1.80] | 3.47 [0.61] | 3.82 [1.53] | 4.52 [2.88] | p = 0.406 |
| Δ6-Desaturase | 0.009 [0.003] | 0.007 [0.005] | 0.009 [0.003] | 0.009 [0.003] | p = 0.866 |
| LA: DGLA | 8.81 [3.85] | 9.45 [6.18] | 8.93 [3.94] | 8.47 [2.94] | p = 0.856 |
| Δ9-Desaturase | 0.14 [0.01] | 0.013 [0.004] | 0.014 [0.010] | 0.012 [0.005] | p = 0.145 |
| BMI | Body Fat% | FPG | TG | Total Cholesterol | LDL-C | HDL-C | Cho/HDL | LDL/HDL | Non-HDL/HDL | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | r = −0.147 p = 0.504 504504 | r = 0.177 p = 0.417 | r = −0.156 p = 0.477 | r = −0.161 p = 0.463 | r = 0.040 p = 0.856 | r = −0.006 p = 0.978 | r = 0.346 p = 0.106 | r = −0.335 p = 0.118 | r = −0.269 p = 0.215 | r = −0.335 p = 0.118 |
| Fe | r = −0.161 p = 0.463 | r = −0.497 p = 0.016 | r = −0.097 p = 0.660 | r = 0.128 p = 0.562 | r = −0.198 p = 0.364 | r = −0.116 p = 0.943 | r = −0.272 p = 0.210 | r = 0.195 p = 0.373 | r = 0.212 p = 0.331 | r = 0.195 p = 0.373 |
| Zn | r = 0.536 p = 0.021 | r = 0.161 p = 0.798 | r = 0.386 p = 0.069 | r = 0.269 p = 0.215 | r = 0.192 p = 0.379 | r = 0.289 p = 0.181 | r = −0.307 p = 0.155 | r = 0.469 p = 0.024 | r = 0.499 p = 0.015 | r = 0.469 p = 0.024 |
| Cu:Zn | r = −0.488 p = 0.018 | r = −0.065 p = 0.769 | r = −0.349 p = 0.102 | r = −0.298 p = 0.167 | r = −0.109 p = 0.622 | r = −0.203 p = 0.354 | r = 0.430 p = 0.041 | r = −0.541 p = 0.008 | r = −0.518 p = 0.011 | r = −0.542 p = 0.008 |
| 18:1 n-9 | 18:2 n-6 | 18:3 n-3 | 20:3 n-6 | 20:4 n-6 | 20:5 n-3 | 22:4 n-6 | 22:5 n-3 | 22:6 n-3 | LA: DGLA | Δ5-Desaturase | Δ6-Desaturase | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | r = 0.433 p = 0.039 | r = 0.265 p = 0.222 | r = 0.542 p < 0.001 | r = −0.194 p = 0.060 | r = −0.267 p = 0.217 | r = 0.231 p = 0.290 | r = −0.357 p = 0.095 | r = −0.108 p = 0.624 | r = 0.162 p = 0.461 | r = 0.293 p = 0.175 | r = −0.010 p = 0.963 | r = −0.196 p = 0.370 |
| Fe | r = 0.415 p = 0.049 | r = 0.059 p = 0.788 | r = −0.503 p = 0.014 | r = 0.157 p = 0.474 | r = −0.092 p = 0.677 | r = −0.021 p = 0.925 | r = 0.113 p = 0.609 | r = 0.045 p = 0.837 | r = −0.049 p = 0.823 | r = −0.092 p = 0.677 | r = −0.145 p = 0.508 | r = 0.137 p = 0.532 |
| Zn | r = 0.118 p = 0.330 | r = −0.182 p = 0.380 | r = −0.152 p = 0.696 | r = 0.007 p = 0.975 | r = 0.118 p = 0.391 | r = 0.131 p = 0.553 | r = 0.108 p = 0.622 | r = 0.077 p = 0.728 | r = 0.214 p = 0.327 | r = −0.132 p = 0.058 | r = 0.168 p = 0.471 | r = 0.168 p = 0.443 |
| Cu:Zn | r = −0.130 p = 0.517 | r = 0.297 p = 0.168 | r = 0.449 p = 0.026 | r = −0.118 p = 0.593 | r = −0.305 p = 0.157 | r = 0.051 p = 0.816 | r = −0.345 p = 0.107 | r = −0.111 p = 0.615 | r = −0.036 p = 0.872 | r = −0.243 p = 0.244 | r = −0.120 p = 0.586 | r = −0.243 p = 0.264 |
| BMI | Body Fat% | TG | Cholesterol | LDL-C | HDL-C | |
|---|---|---|---|---|---|---|
| 18:1 n-9 | r = −0.065 p = 0.746 | r = 0.193 p = 0.334 | r = −0.330 p = 0.093 | r = 0.474 p = 0.012 | r = 0.266 p = 0.180 | r = 0.660 p < 0.001 |
| 18:2 n-6 | r = −0.458 p = 0.016 | r = −0.317 p = 0.107 | r = −0.028 p = 0.888 | r = −0.362 p = 0.063 | r = −0.310 p = 0.116 | r = −0.107 p = 0.597 |
| 18:3 n-3 | r = −0.057 p = 0.777 | r = 0.301 p = 0.127 | r = −0.127 p = 0.527 | r = 0.005 p = 0.979 | r = −0.181 p = 0.367 | r = 0.233 p = 0.243 |
| 20:3 n-6 | r = 0.270 p = 0.173 | r = 0.168 p = 0.403 | r = 0.560 p = 0.002 | r = 0.185 p = 0.355 | r = 0.243 p = 0.222 | r = −0.339 p = 0.083 |
| 20:4 n-6 | r = 0.416 p = 0.031 | r = 0.245 p = 0.217 | r = −0.135 p = 0.502 | r = 0.118 p = 0.557 | r = 0.089 p = 0.658 | r = 0.109 p = 0.614 |
| 20:5 n-3 | r = 0.223 p > 0.05 | r = 0.261 p > 0.05 | r = −0.106 p > 0.05 | r = 0.029 p > 0.05 | r = −0.016 p > 0.05 | r = 0.134 p > 0.05 |
| 22:4 n-6 | r = −0.080 p = 0.970 | r = −0.204 p = 0.306 | r = 0.102 p = 0.612 | r = −0.036 p = 0.860 | r = −0.168 p = 0.967 | r = −0.162 p = 0.421 |
| 22:5 n-3 | r = 0.009 p = 0.965 | r = 0.011 p = 0.957 | r = −0.107 p = 0.597 | r = 0.011 p = 0.955 | r = −0.028 p = 0.692 | r = 0.080 p = 0.889 |
| 22:6 n-3 | r = 0.294 p = 0.136 | r = 0.201 p = 0.314 | r = 0.024 p = 0.905 | r = 0.029 p = 0.885 | r = 0.153 p = 0.447 | r = −0.143 p = 0.477 |
| 18:2 n-6 | 20:3 n-6 | 20:4 n-6 | 22:6 n-3 | BMI | TG | Cho/HDL | LDL/HDL | Non-HDL/HDL | |
|---|---|---|---|---|---|---|---|---|---|
| LA:DGLA Ratio | r = 0.470 p = 0.013 | r = −0.889 p < 0.001 | r = −0.209 p = 0.296 | r = −0.008 p = 0.969 | r = −0.418 p = 0.030 | r = −0.506 p = 0.007 | r = −0.433 p = 0.024 | r = −0.362 p = 0.063 | r = −0.433 p = 0.024 |
| Δ5-Desaturase | r = −0.406 p = 0.035 | r = −0.710 p < 0.001 | r = 0.694 p < 0.001 | r = 0.441 p = 0.021 | r = 0.085 p = 0.672 | r = −0.392 p = 0.043 | r = −0.301 p = 0.243 | r = −0.240 p = 0.454 | r = −0.301 p = 240 |
| Δ 6-Desaturase | r = −0.627 p < 0.001 | r = 0.200 p = 0.317 | r = 0.311 p = 0.114 | r = −0.075 p = 0.710 | r = 0.398 p = 0.040 | r = 0.194 p = 0.334 | r = 0.125 p = 0.533 | r = 0.132 p = 0.510 | r = 0.127 p = 0.527 |
| Δ 9-Desaturase | r = −0.402 p = 0.037 | r = 0.212 p = 0.289 | r = 0.056 p = 0.781 | r = −0.086 p = 0.669 | r = 0.284 p = 0.152 | r = 0.239 p = 0.230 | r = 0.112 p = 0.578 | r = 0.091 p = 0.653 | r = 0.111 p = 0.581 |
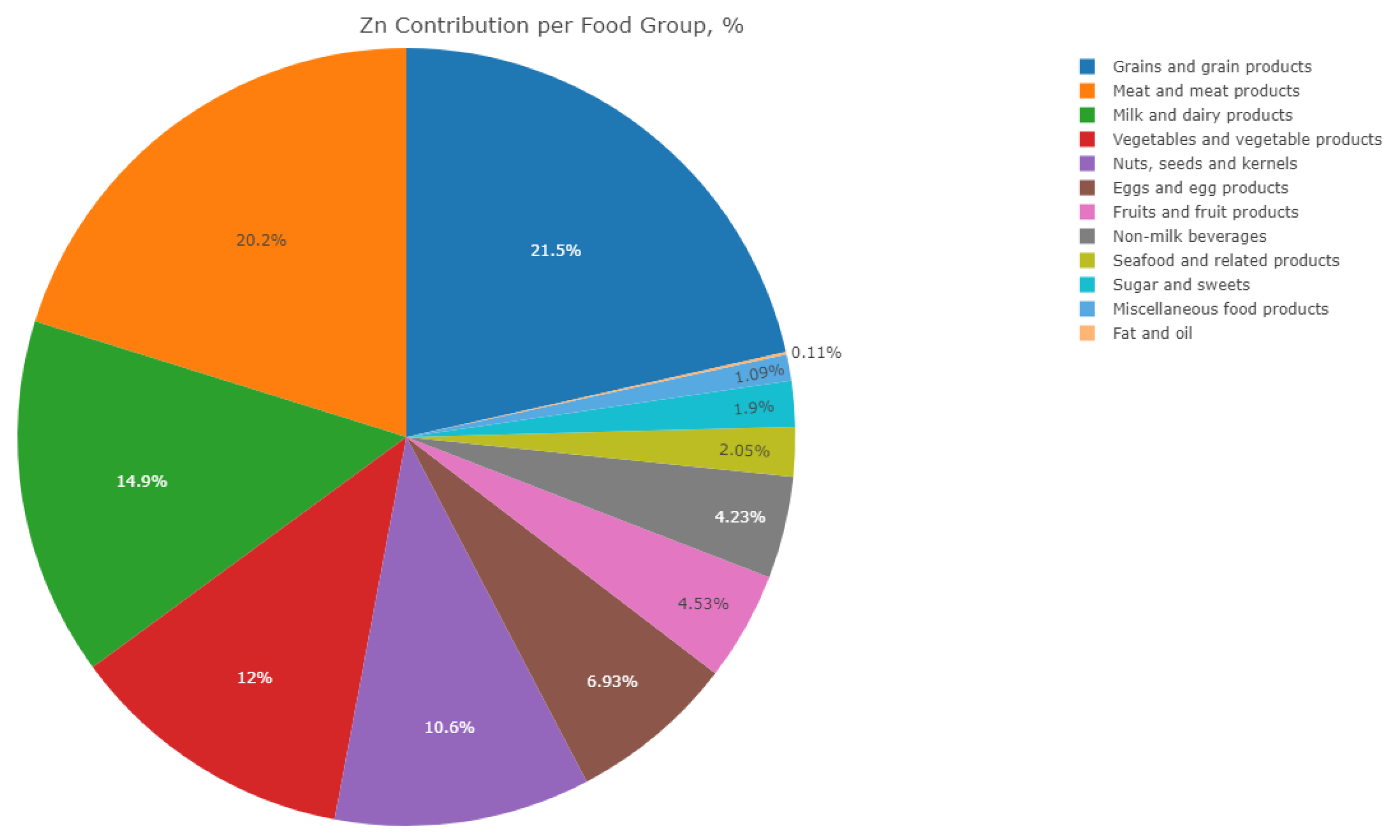
| Food Group | Average Zn Intake, Mg | Contribution to Zn Intake, % |
|---|---|---|
| Grains and Grain Products | 1.595 | 21.49 |
| Meat and Meat Products | 1.499 | 20.20 |
| Milk and Dairy Products | 1.104 | 14.88 |
| Vegetables and Vegetable Products | 0.888 | 11.97 |
| Nuts, Seeds, and Kernels | 0.789 | 10.63 |
| Eggs and Egg Products | 0.514 | 6.93 |
| Fruit and Fruit Products | 0.336 | 4.53 |
| Non-Milk Beverages | 0.314 | 4.23 |
| Sugar and Sweets | 0.141 | 1.90 |
| Seafood and Related Products | 0.152 | 2.05 |
| Miscellaneous Food Products | 0.081 | 1.09 |
| Fat and Oil | 0.008 | 0.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knez, M.; Pantovic, A.; Zekovic, M.; Pavlovic, Z.; Glibetic, M.; Zec, M. Is There a Link between Zinc Intake and Status with Plasma Fatty Acid Profile and Desaturase Activities in Dyslipidemic Subjects? Nutrients 2020, 12, 93. https://doi.org/10.3390/nu12010093
Knez M, Pantovic A, Zekovic M, Pavlovic Z, Glibetic M, Zec M. Is There a Link between Zinc Intake and Status with Plasma Fatty Acid Profile and Desaturase Activities in Dyslipidemic Subjects? Nutrients. 2020; 12(1):93. https://doi.org/10.3390/nu12010093
Chicago/Turabian StyleKnez, Marija, Ana Pantovic, Milica Zekovic, Zoran Pavlovic, Maria Glibetic, and Manja Zec. 2020. "Is There a Link between Zinc Intake and Status with Plasma Fatty Acid Profile and Desaturase Activities in Dyslipidemic Subjects?" Nutrients 12, no. 1: 93. https://doi.org/10.3390/nu12010093
APA StyleKnez, M., Pantovic, A., Zekovic, M., Pavlovic, Z., Glibetic, M., & Zec, M. (2020). Is There a Link between Zinc Intake and Status with Plasma Fatty Acid Profile and Desaturase Activities in Dyslipidemic Subjects? Nutrients, 12(1), 93. https://doi.org/10.3390/nu12010093







