Pre-Sleep Casein Protein Ingestion Does Not Impact Next-Day Appetite, Energy Intake and Metabolism in Older Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Preliminary Visit
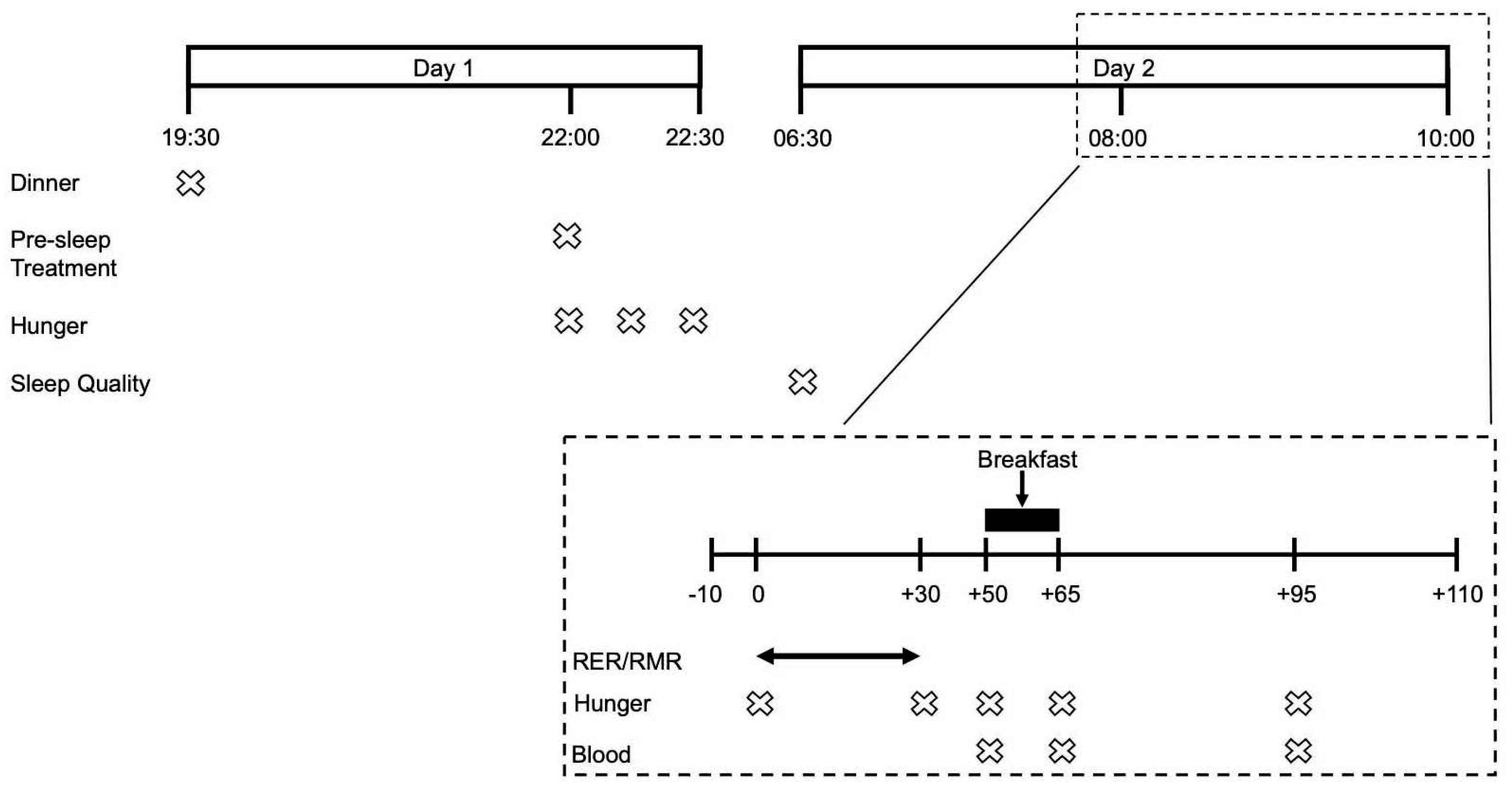
2.4. Experimental Visits
2.4.1. Standardization of Evening Meal
2.4.2. Pre-Sleep Beverages
2.4.3. Subjective Measurement of Appetite and Sleep Quality
2.4.4. Resting Metabolic Rate Measurement
2.4.5. Ad Libitum Breakfast and Assessment of Energy Intake
2.4.6. Blood Sampling and Analyses
2.5. Statistics
3. Results
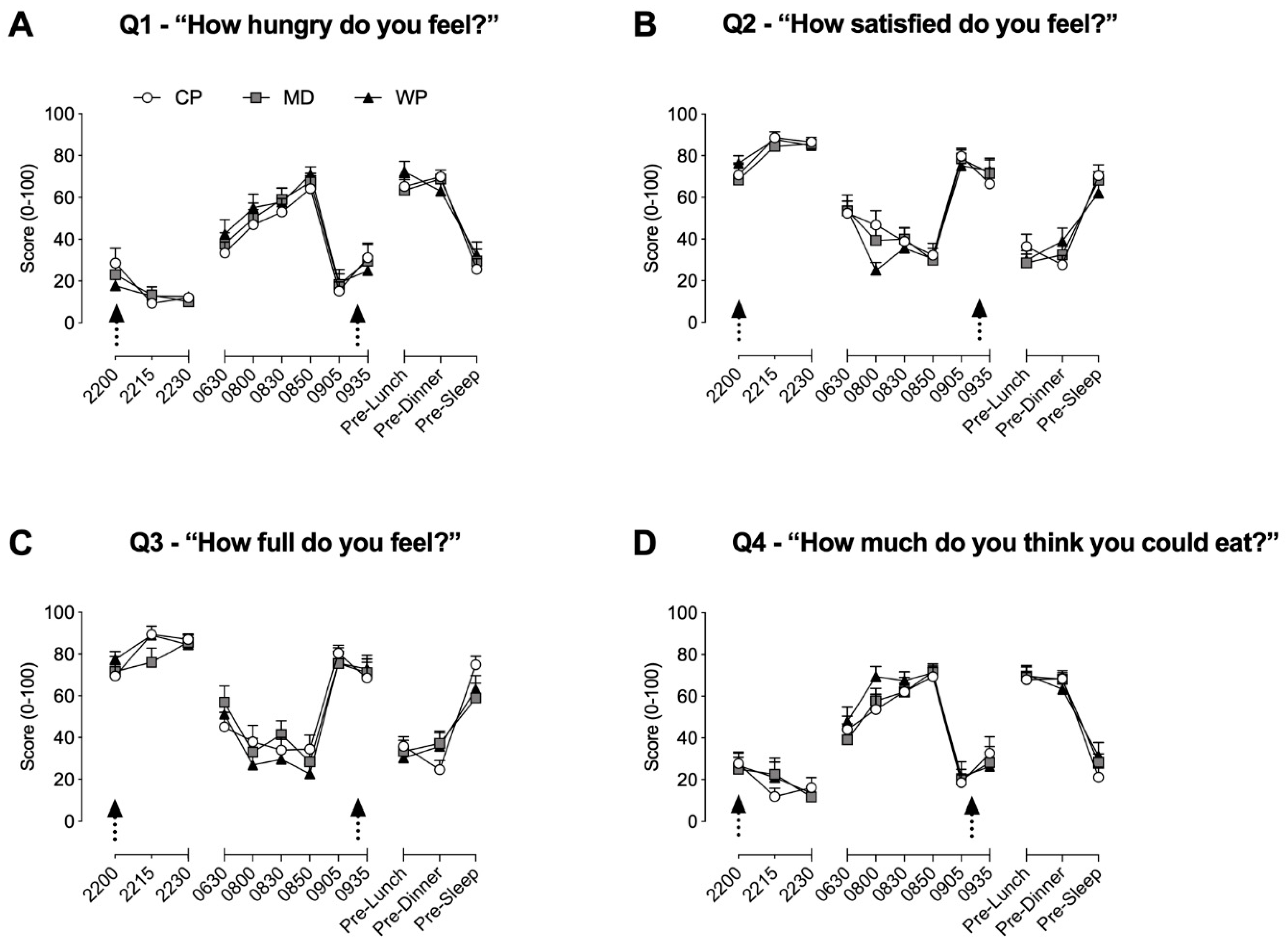
3.1. Appetite and Sleep Quality
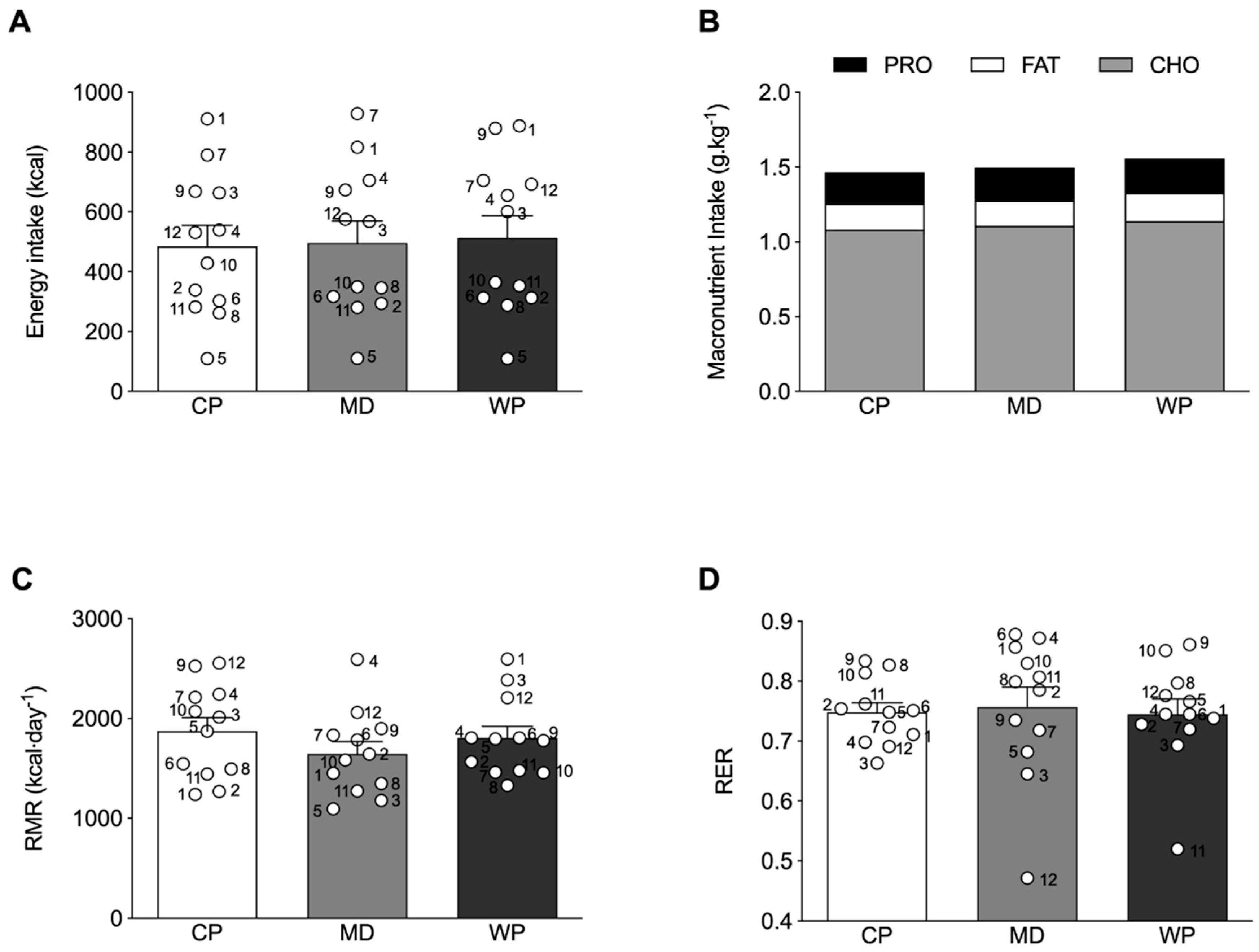
3.2. Energy Intake and Metabolic Measurements
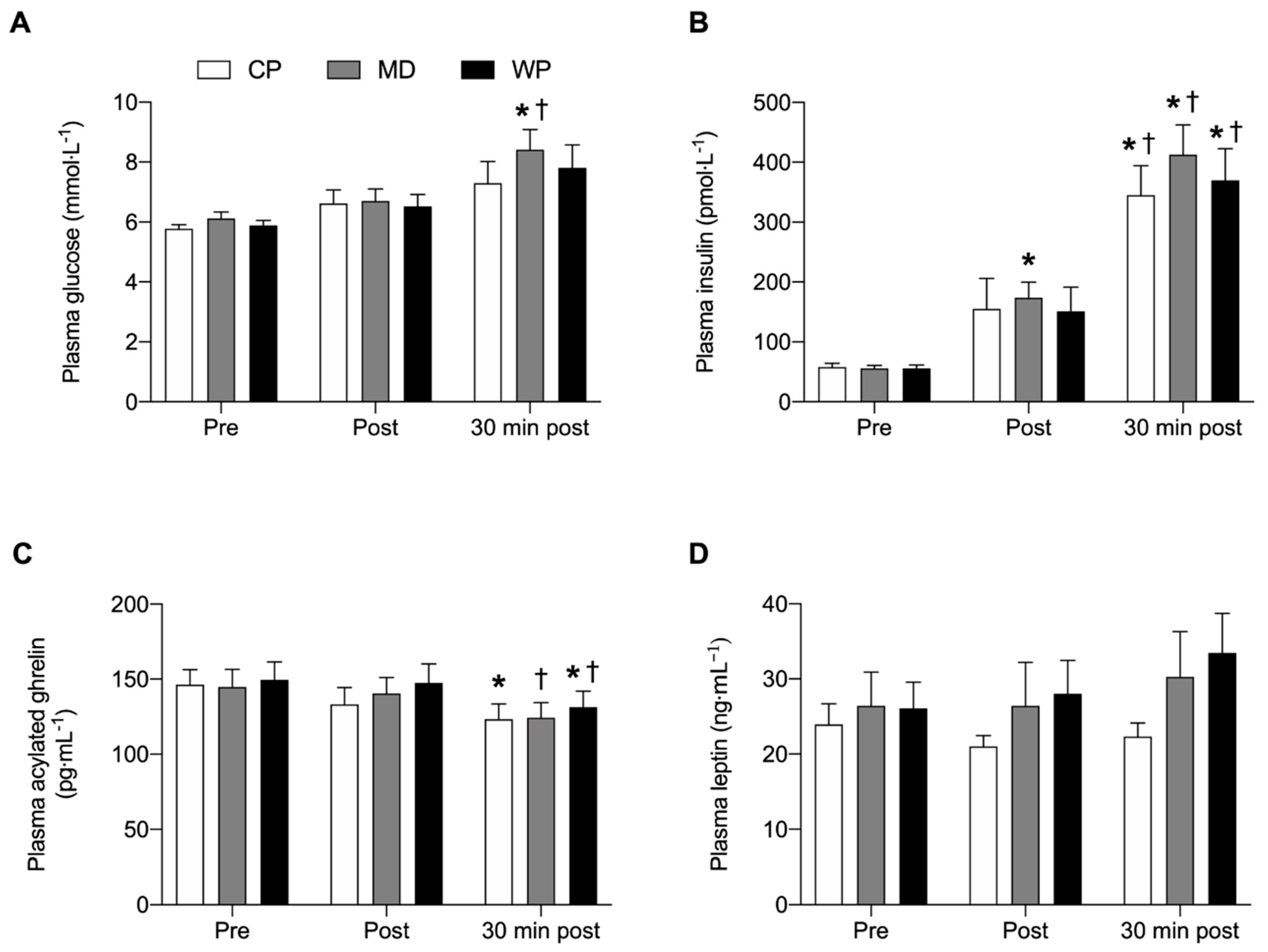
3.3. Glucose, Insulin, Ghrelin and Leptin Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Landi, F.; Liperoti, R.; Fusco, D.; Mastropaolo, S.; Quattrociocchi, D.; Proia, A.; Tosato, M.; Bernabei, R.; Onder, G. Sarcopenia and mortality among older nursing home residents. J. Am. Med. Dir. Assoc. 2012, 13, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.B.; Hannan, M.T.; Murabito, J.M.; Kiel, D.P.; McLean, R.R. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: The framingham study. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.L.; Walston, J.D.; Fried, L.P.; Beamer, B.A. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: The women’s health and aging study. Arch. Intern. Med. 2011, 171, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, Y.P.; Shin, H.J.; Lee, W. Associations of sarcopenia and sarcopenic obesity with metabolic syndrome considering both muscle mass and muscle strength. J. Prev. Med. Public Health 2016, 49, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilsirente study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Mijnarends, D.M.; Koster, A.; Schols, J.M.; Meijers, J.M.; Halfens, R.J.; Gudnason, V.; Eiriksdottir, G.; Siggeirsdottir, K.; Sigurdsson, S.; Jonsson, P.V.; et al. Physical activity and incidence of sarcopenia: The population-based AGES—Reykjavik Study. Age Ageing 2016, 45, 614–620. [Google Scholar] [CrossRef]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B.; et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef]
- Wahlin-Larsson, B.; Wilkinson, D.J.; Strandberg, E.; Hosford-Donovan, A.; Atherton, P.J.; Kadi, F. Mechanistic links underlying the impact of c-reactive protein on muscle mass in elderly. Cell. Physiol. Biochem. 2017, 44, 267–278. [Google Scholar] [CrossRef]
- Ethgen, O.; Beaudart, C.; Buckinx, F.; Bruyere, O.; Reginster, J.Y. The future prevalence of sarcopenia in Europe: A claim for public health action. Calcif. Tissue Int. 2017, 100, 229–234. [Google Scholar] [CrossRef]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef]
- Pinedo-Villanueva, R.; Westbury, L.D.; Syddall, H.E.; Sanchez-Santos, M.T.; Dennison, E.M.; Robinson, S.M.; Cooper, C. Health care costs associated with muscle weakness: A UK population-based estimate. Calcif. Tissue Int. 2019, 104, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the prot-age study group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Sithamparapillai, A.; Brimble, K.S.; Banfield, L.; Morton, R.W.; Phillips, S.M. Changes in kidney function do not differ between healthy adults consuming higher- compared with lower- or normal-protein diets: A systematic review and meta-analysis. J. Nutr. 2018, 148, 1760–1775. [Google Scholar] [CrossRef] [PubMed]
- Kouw, I.W.; Holwerda, A.M.; Trommelen, J.; Kramer, I.F.; Bastiaanse, J.; Halson, S.L.; Wodzig, W.K.; Verdijk, L.B.; van Loon, L.J. Protein ingestion before sleep increases overnight muscle protein synthesis rates in healthy older men: A randomized controlled trial. J. Nutr. 2017, 147, 2252–2261. [Google Scholar] [CrossRef] [PubMed]
- Trommelen, J.; van Loon, L.J. Pre-sleep protein ingestion to improve the skeletal muscle adaptive response to exercise training. Nutrients 2016, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Verdijk, L.B.; van Loon, L.J.C. The impact of pre-sleep protein ingestion on the skeletal muscle adaptive response to exercise in humans: An update. Front. Nutr. 2019, 6, 17. [Google Scholar] [CrossRef]
- Madzima, T.A.; Panton, L.B.; Fretti, S.K.; Kinsey, A.W.; Ormsbee, M.J. Night-time consumption of protein or carbohydrate results in increased morning resting energy expenditure in active college-aged men. Br. J. Nutr. 2014, 111, 71–77. [Google Scholar] [CrossRef]
- Luhovyy, B.L.; Akhavan, T.; Anderson, G.H. Whey proteins in the regulation of food intake and satiety. J. Am. Coll. Nutr. 2007, 26, 704S–712S. [Google Scholar] [CrossRef]
- Anderson, G.H.; Moore, S.E. Dietary proteins in the regulation of food intake and body weight in humans. J. Nutr. 2004, 134, 974S–979S. [Google Scholar] [CrossRef]
- Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Snijders, T.; Halson, S.L.; Rollo, I.; Verdijk, L.B.; van Loon, L.J.C. Presleep dietary protein-derived amino acids are incorporated in myofibrillar protein during postexercise overnight recovery. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E457–E467. [Google Scholar] [CrossRef] [PubMed]
- Caudwell, P.; Finlayson, G.; Gibbons, C.; Hopkins, M.; King, N.; Naslund, E.; Blundell, J.E. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am. J. Clin. Nutr. 2013, 97, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Giezenaar, C.; Chapman, I.; Luscombe-Marsh, N.; Feinle-Bisset, C.; Horowitz, M.; Soenen, S. Ageing is associated with decreases in appetite and energy intake—A meta-analysis in healthy adults. Nutrients 2016, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, A.L.; Robinson, S.M.; Sayer, A.A.; Roberts, H.C. An overview of appetite decline in older people. Nurs. Older People 2015, 27, 29–35. [Google Scholar] [CrossRef]
- Atalayer, D.; Astbury, N.M. Anorexia of aging and gut hormones. Aging Dis. 2013, 4, 264–275. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Parrott, A.C.; Hindmarch, I. Factor analysis of a sleep evaluation questionnaire. Psychol. Med. 1978, 8, 325–329. [Google Scholar] [CrossRef]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zuniga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Borsheim, E.; Bui, Q.U.; Tissier, S.; Kobayashi, H.; Ferrando, A.A.; Wolfe, R.R. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin. Nutr. 2008, 27, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Borsheim, E.; Bui, Q.U.; Tissier, S.; Cree, M.G.; Ronsen, O.; Morio, B.; Ferrando, A.A.; Kobayashi, H.; Newcomer, B.R.; Wolfe, R.R. Amino acid supplementation decreases plasma and liver triacylglycerols in elderly. Nutrition 2009, 25, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Groen, B.B.; Res, P.T.; Pennings, B.; Hertle, E.; Senden, J.M.; Saris, W.H.; van Loon, L.J. Intragastric protein administration stimulates overnight muscle protein synthesis in elderly men. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E52–E60. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.F.; Verdijk, L.B.; Hamer, H.M.; Verlaan, S.; Luiking, Y.C.; Kouw, I.W.K.; Senden, J.M.; van Kranenburg, J.; Gijsen, A.P.; Bierau, J.; et al. Both basal and post-prandial muscle protein synthesis rates, following the ingestion of a leucine-enriched whey protein supplement, are not impaired in sarcopenic older males. Clin. Nutr. 2017, 36, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Madzima, T.A.; Melanson, J.T.; Black, J.R.; Nepocatych, S. Pre-sleep consumption of casein and whey protein: Effects on morning metabolism and resistance exercise performance in active women. Nutrients 2018, 10, 1273. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Gorman, K.A.; Miller, E.A.; Baur, D.A.; Eckel, L.A.; Contreras, R.J.; Panton, L.B.; Spicer, M.T. Nighttime feeding likely alters morning metabolism but not exercise performance in female athletes. Appl. Physiol. Nutr. Metab. 2016, 41, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.; Noakes, M.; Trenerry, C.; Clifton, P.M. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J. Clin. Endocrinol. Metab. 2006, 91, 1477–1483. [Google Scholar] [CrossRef]
- Latner, J.D.; Schwartz, M. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite 1999, 33, 119–128. [Google Scholar] [CrossRef]
- Sharafi, M.; Alamdari, N.; Wilson, M.; Leidy, H.J.; Glynn, E.L. Effect of a high-protein, high-fiber beverage preload on subjective appetite ratings and subsequent ad libitum energy intake in overweight men and women: A randomized, double-blind placebo-controlled, crossover study. Curr. Dev. Nutr. 2018, 2, nzy022. [Google Scholar] [CrossRef]
- Lay, A.H.H.; Crabtree, D.R.; Campbell, T.G.; Dreczkowski, G.M.; Galloway, S.D.R.; Tipton, K.D.; Witard, O.C. A bedtime milk snack does not impact rmr, substrate utilisation and appetite the following morning in mildly overweight males. Br. J. Nutr. 2018, 119, 1355–1365. [Google Scholar] [CrossRef]
- Holt, G.M.; Owen, L.J.; Till, S.; Cheng, Y.; Grant, V.A.; Harden, C.J.; Corfe, B.M. Systematic literature review shows that appetite rating does not predict energy intake. Crit. Rev. Food Sci. Nutr. 2017, 57, 3577–3582. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise-re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Vetter, C.; Dashti, H.S.; Lane, J.M.; Anderson, S.G.; Schernhammer, E.S.; Rutter, M.K.; Saxena, R.; Scheer, F. Night shift work, genetic risk, and type 2 diabetes in the UK biobank. Diabetes Care 2018, 41, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Bescos, R.; Boden, M.J.; Jackson, M.L.; Trewin, A.J.; Marin, E.C.; Levinger, I.; Garnham, A.; Hiam, D.S.; Falcao-Tebas, F.; Conte, F.; et al. Four days of simulated shift work reduces insulin sensitivity in humans. Acta Physiol. (Oxf. Engl.) 2018, 223, e13039. [Google Scholar] [CrossRef]
- Potter, G.D.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian rhythm and sleep disruption: Causes, metabolic consequences, and countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef]
- Crispim, C.A.; Zimberg, I.Z.; dos Reis, B.G.; Diniz, R.M.; Tufik, S.; de Mello, M.T. Relationship between food intake and sleep pattern in healthy individuals. J. Clin. Sleep Med. 2011, 7, 659–664. [Google Scholar] [CrossRef]
- Adam, K. Dietary habits and sleep after bedtime food drinks. Sleep 1980, 3, 47–58. [Google Scholar] [CrossRef]
- Williams, J.; Mobarhan, S. A critical interaction: Leptin and ghrelin. Nutr. Rev. 2003, 61, 391–393. [Google Scholar] [CrossRef]
- Shiiya, T.; Nakazato, M.; Mizuta, M.; Date, Y.; Mondal, M.S.; Tanaka, M.; Nozoe, S.; Hosoda, H.; Kangawa, K.; Matsukura, S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J. Clin. Endocrinol. Metab. 2002, 87, 240–244. [Google Scholar] [CrossRef]
- Monteleone, P.; Bencivenga, R.; Longobardi, N.; Serritella, C.; Maj, M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 5510–5514. [Google Scholar] [CrossRef]
- Smeuninx, B.; McKendry, J.; Wilson, D.; Martin, U.; Breen, L. Age-related anabolic resistance of myofibrillar protein synthesis is exacerbated in obese inactive individuals. J. Clin. Endocrinol. Metab. 2017, 102, 3535–3545. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, K.S.; Given, B.D.; Van Cauter, E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J. Clin. Invest. 1988, 81, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.F.; Bernaba, B.; Hwu, C.M.; Jinagouda, S.; Fahmi, S.; Kogosov, E.; Boyadjian, R. Insulin regulates plasma ghrelin concentration. J. Clin. Endocrinol. Metab. 2002, 87, 3997–4000. [Google Scholar] [CrossRef] [PubMed]
- Romon, M.; Lebel, P.; Velly, C.; Marecaux, N.; Fruchart, J.C.; Dallongeville, J. Leptin response to carbohydrate or fat meal and association with subsequent satiety and energy intake. Am. J. Physiol. 1999, 277, E855–E861. [Google Scholar] [CrossRef]
- Halton, T.L.; Hu, F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef]
- Kinsey, A.W.; Cappadona, S.R.; Panton, L.B.; Allman, B.R.; Contreras, R.J.; Hickner, R.C.; Ormsbee, M.J. The effect of casein protein prior to sleep on fat metabolism in obese men. Nutrients 2016, 8, 452. [Google Scholar] [CrossRef]
- Leyh, S.M.; Willingham, B.D.; Baur, D.A.; Panton, L.B.; Ormsbee, M.J. Pre-sleep protein in casein supplement or whole-food form has no impact on resting energy expenditure or hunger in women. Br. J. Nutr. 2018, 120, 988–994. [Google Scholar] [CrossRef]
- Westerterp, K.R. Diet induced thermogenesis. Nutr. Metab. (Lond.) 2004, 1, 5. [Google Scholar] [CrossRef]
- Spaeth, A.M.; Dinges, D.F.; Goel, N. Resting metabolic rate varies by race and by sleep duration. Obesity (Silver Spring) 2015, 23, 2349–2356. [Google Scholar] [CrossRef]
- Campbell, P.J.; Carlson, M.G.; Hill, J.O.; Nurjhan, N. Regulation of free fatty acid metabolism by insulin in humans: Role of lipolysis and reesterification. Am. J. Physiol. 1992, 263, E1063–E1069. [Google Scholar] [CrossRef]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.L.; Wu, C.L.; Chen, Y.C.; Wang, P.G.; Gonzalez, J.T.; Betts, J.A. Post-exercise carbohydrate-energy replacement attenuates insulin sensitivity and glucose tolerance the following morning in healthy adults. Nutrients 2018, 10, 123. [Google Scholar] [CrossRef] [PubMed]
| Energy (kcal) | Carbohydrate (g) | Protein (g) | Fat (g) | |
|---|---|---|---|---|
| CP | 168 | 1.92 | 40 | 0.72 |
| MD | 168 | 42 | 0 | 0 |
| WP | 0 | 0 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morehen, S.; Smeuninx, B.; Perkins, M.; Morgan, P.; Breen, L. Pre-Sleep Casein Protein Ingestion Does Not Impact Next-Day Appetite, Energy Intake and Metabolism in Older Individuals. Nutrients 2020, 12, 90. https://doi.org/10.3390/nu12010090
Morehen S, Smeuninx B, Perkins M, Morgan P, Breen L. Pre-Sleep Casein Protein Ingestion Does Not Impact Next-Day Appetite, Energy Intake and Metabolism in Older Individuals. Nutrients. 2020; 12(1):90. https://doi.org/10.3390/nu12010090
Chicago/Turabian StyleMorehen, Stephen, Benoit Smeuninx, Molly Perkins, Paul Morgan, and Leigh Breen. 2020. "Pre-Sleep Casein Protein Ingestion Does Not Impact Next-Day Appetite, Energy Intake and Metabolism in Older Individuals" Nutrients 12, no. 1: 90. https://doi.org/10.3390/nu12010090
APA StyleMorehen, S., Smeuninx, B., Perkins, M., Morgan, P., & Breen, L. (2020). Pre-Sleep Casein Protein Ingestion Does Not Impact Next-Day Appetite, Energy Intake and Metabolism in Older Individuals. Nutrients, 12(1), 90. https://doi.org/10.3390/nu12010090





