Impact of a Three-Week in-Hospital Multidisciplinary Body Weight Reduction Program on Body Composition, Muscle Performance and Fatigue in a Pediatric Obese Population with or without Metabolic Syndrome
Abstract
1. Introduction
2. Material and Methods
2.1. Patients and Body Weight Reduction Program
- -
- OLSB (one-leg standing balance);
- -
- SCT (stair climbing test);
- -
- FSS (fatigue severity scale).
2.2. Anthropometric Measurements
2.3. Metabolic Variables
2.4. Evaluation of Blood Pressure
2.5. Definition of Metabolic Syndrome
2.6. Functional Tests
- (1)
- One-leg standing balance (OLSB). The subjects were invited to stand on one leg with the other flexed for as long as possible, looking straight ahead. The test was considered to be terminated with the ground contact of the flexed leg or with an overt loss of equilibrium, although compensatory movements of arms and lifted leg were allowed. An operator registered the value in seconds with a digital stopwatch. The test was repeated for both legs (right and left) in order to obtain two OLSB values (i.e., OLSBR and OLSBL).
- (2)
- Stair climbing test (SCT). The subjects were invited to climb up ordinary stairs (13 steps of 15.3 cm each, for a total vertical distance of 1.99 m) at the highest possible speed, according with their own capabilities. An operator measured the time employed to cover the test with a digital stopwatch. The test was considered to start at the moment when the first foot was lifted and to terminate with the contact of the same foot on the last step.
2.7. Fatigue Severity Scale
2.8. Statistical Analysis
3. Results
3.1. Patients’ Parameters
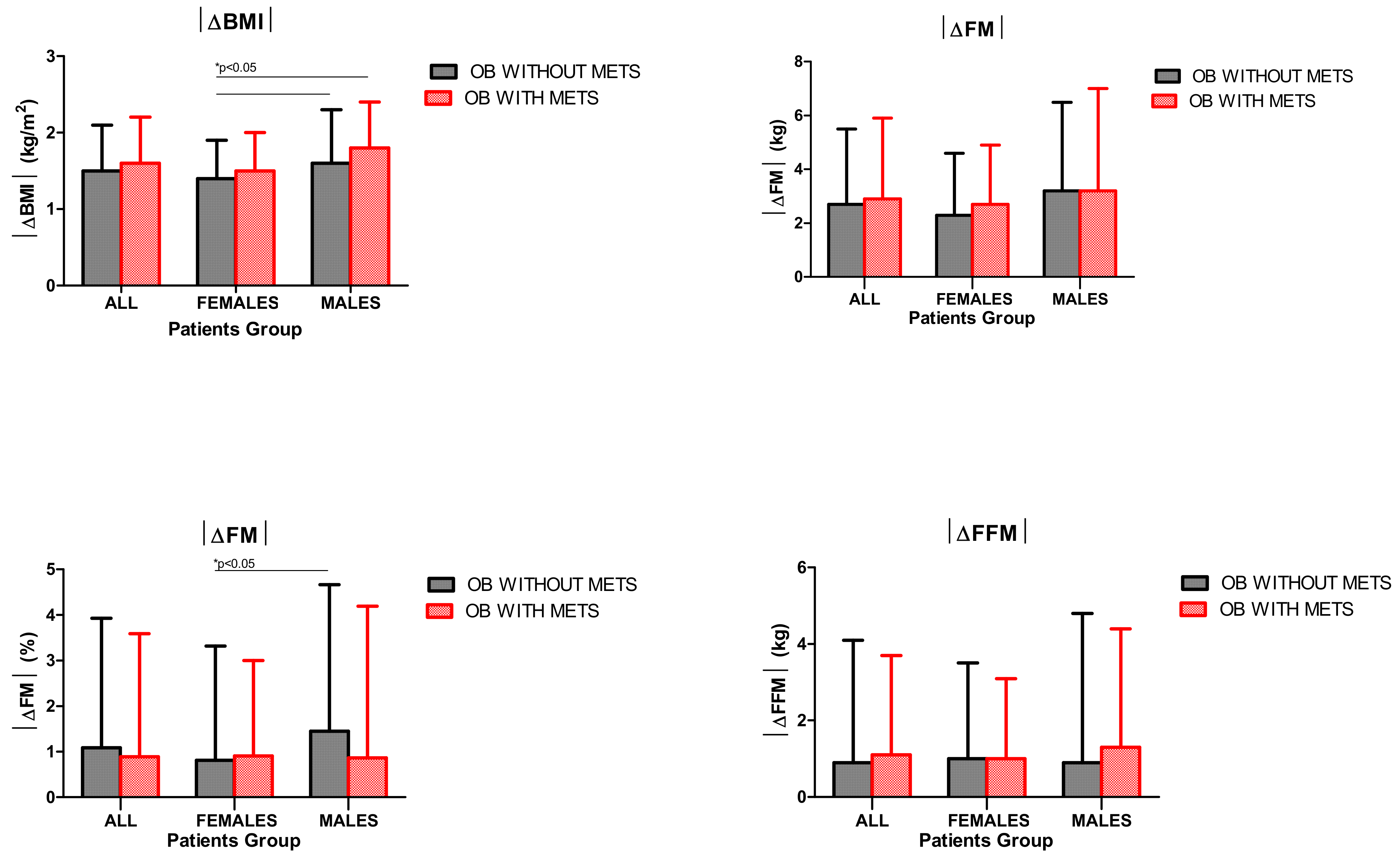
3.2. BMI and Body Composition
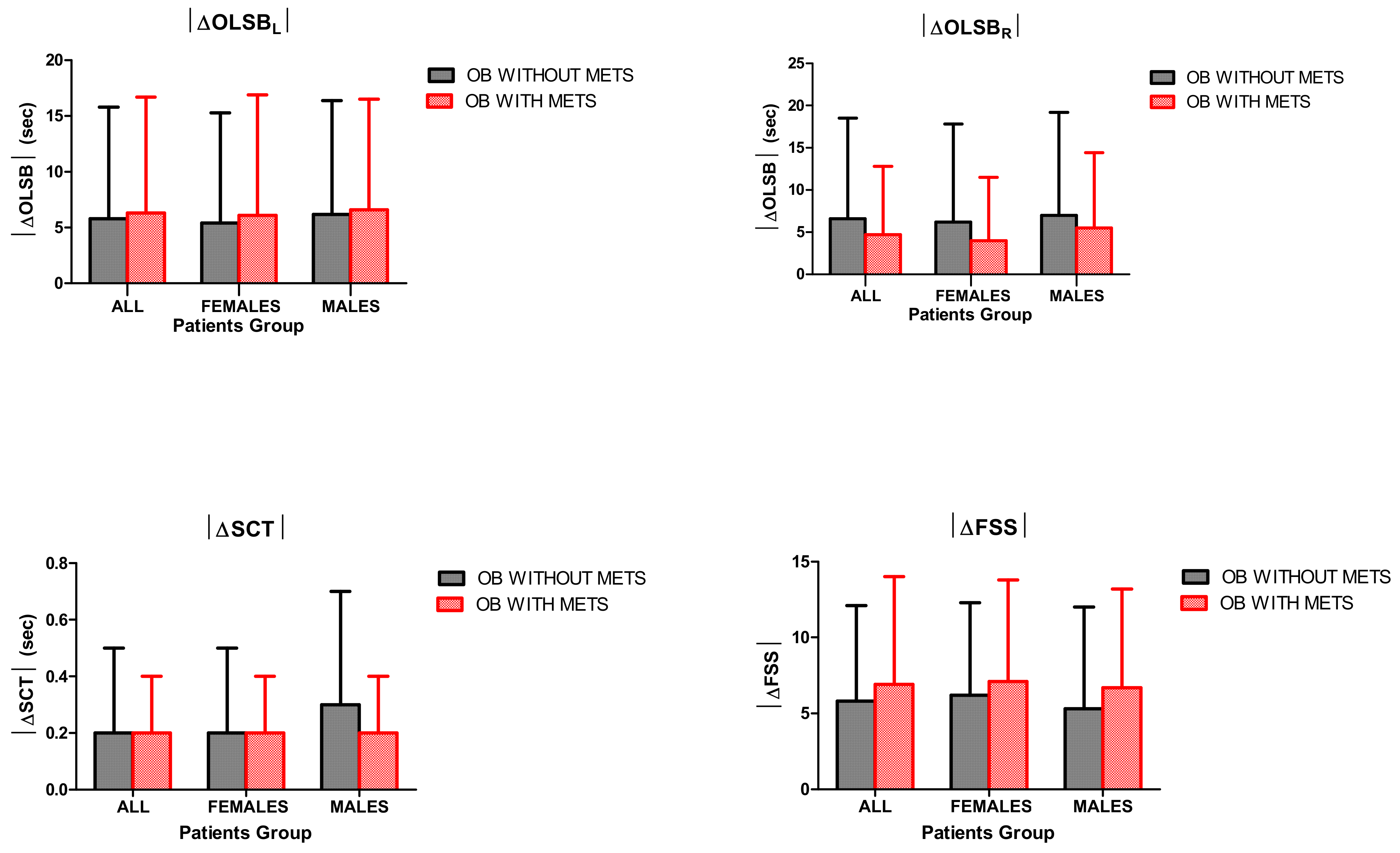
3.3. OLSB
3.4. SCT
3.5. FSS
4. Discussion
- (1)
- BWRP significantly reduced BMI and FM (expressed as %) in all subgroups of obese subjects (females/males and with/without metabolic syndrome), with preservation of FFM;
- (2)
- BWRP significantly improved motor control, muscle performance and fatigue perception in all subgroups of obese subjects (females/males and with/without metabolic syndrome);
- (3)
- the pre–post-BWRP change of each outcome (i.e., |ΔBMI|, |ΔFM|, |ΔOLSB|, |ΔSCT| and |ΔFSS|) was similar between the two obese groups with and without metabolic syndrome;
- (4)
- obese females without metabolic syndrome reached a lower weight loss after BWRP when compared to obese males with and without metabolic syndrome, as demonstrated by the significantly lower values of |ΔBMI| and |ΔFM| (expressed as %).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nittari, G.; Scuri, S.; Petrelli, F.; Pirillo, I.; di Luca, N.M.; Grappasonni, I. Fighting obesity in children from European World Health Organization member states. Epidemiological data, medical-social aspects, and prevention programs. Clin. Ther. 2019, 170, e223–e230. [Google Scholar]
- Kelishadi, R. Childhood overweight, obesity, and the metabolic syndrome in developing countries. Epidemiol. Rev. 2007, 29, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M.; Tuomilehto, J.; Rydén, L. The metabolic syndrome—What is it and how should it be managed? Eur. J. Prev. Cardiol. 2019, 26, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Molnár, D. The prevalence of the metabolic syndrome and type 2 diabetes mellitus in children and adolescents. Int. J. Obes. Relat. Metab. Disord. 2004, 28, S70–S74. [Google Scholar] [CrossRef]
- Ford, E.S.; Li, C. Defining the metabolic syndrome in children and adolescents: Will the real definition please stand up? J. Pediatr. 2008, 152, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Friedman, L.A.; Gray-McGuire, C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The Princeton Lipid Research Clinics Follow-up Study. Pediatrics 2007, 120, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Zuccotti, G.V.; Carnovale, C.; Galli, E.; Nannini, P.; Cervia, D.; Perrotta, C. An update on the assessment and management of metabolic syndrome, a growing medical emergency in paediatric populations. Pharmacol. Res. 2017, 119, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Albert Pérez, E.; Mateu Olivares, V.; Martínez-Espinosa, R.M.; Molina Vila, M.D.; Reig García-Galbis, M. New Insights about How to Make an Intervention in Children and Adolescents with Metabolic Syndrome: Diet, Exercise vs. Changes in Body Composition. A Systematic Review of RCT. Nutrients 2018, 10, 878. [Google Scholar] [CrossRef]
- Garrow, J.S. Treatment of morbid obesity by nonsurgical means: Diet, drugs, behavior modification, exercise. Gastroenterol. Clin. N. Am. 1987, 16, 443–449. [Google Scholar]
- Bryant, M.; Ashton, L.; Nixon, J.; Jebb, S.; Wright, J.; Roberts, K.; Brown, J.; CoOR Scientific Advisory Group. Framework of outcome measures recommended for use in the evaluation of childhood obesity treatment interventions: The CoOR framework. Pediatr. Obes. 2014, 9, e116–e131. [Google Scholar] [CrossRef]
- Bryant, M.; Ashton, L.; Brown, J.; Jebb, S.; Wright, J.; Roberts, K.; Nixon, J. Systematic review to identify and appraise outcome measures used to evaluate childhood obesity treatment interventions (CoOR): Evidence of purpose, application, validity, reliability and sensitivity. Health Technol. Assess. 2014, 18, 1–380. [Google Scholar] [CrossRef] [PubMed]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Invest. 2006, 29, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, P. International consensus conference on impedance in body composition. Age Nutr. 1994, 5, 142–145. [Google Scholar]
- Zimmet, P.; Alberti, K.G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- McCharty, H.D.; Jarret, K.V.; Crawley, H.F. The development of waist circumference percentiles in British children aged 5.0–6.9 y. Eur. J. Clin. Nutr. 2001, 55, 902e7. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, L. The measurement of fatigue in chronic illness: A systematic review of unidimensional and multidimensional fatigue measures. J. Pain Symptom Manag. 2009, 37, 107–128. [Google Scholar] [CrossRef]
- Impellizzeri, F.M.; Agosti, F.; De Col, A.; Sartorio, A. Psychometric properties of the Fatigue Severity Scale in obese patients. Health Qual. Life Outcomes 2013, 11, 32. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; De Col, A.; Tamini, S.; Cicolini, S.; Caroli, D.; De Micheli, R.; Tringali, G.; Abbruzzese, L.; Marazzi, N.; Cella, S.G.; et al. Multidisciplinary Integrated Metabolic Rehabilitation in Elderly Obese Patients: Effects on Cardiovascular Risk Factors, Fatigue and Muscle Performance. Nutrients 2019, 11, 1240. [Google Scholar] [CrossRef]
- Sartorio, A.; Fontana, P.; Trecate, L.; Lafortuna, C.L. Short-term changes of fatigability and muscle performance in severe obese patients after an integrated body mass reduction program. Diabetes Nutr. Metab. 2003, 16, 88–93. [Google Scholar]
- Vellas, B.J.; Rubenstein, L.Z.; Ousset, P.J.; Faisant, C.; Kostek, V.; Nourhashemi, F.; Allard, M.; Albarede, J.L. One-leg standing balance and functional status in a population of 512 community-living elderly persons. Aging 1997, 9, 95–98. [Google Scholar] [CrossRef]
- Cohen, H.; Blatchly, C.A.; Gombash, L.L. A study of the clinical test of sensory interaction and balance. Phys. Ther. 1993, 73, 346–351, discussion 351–354. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, A.; Lafortuna, C.L.; Conte, G.; Faglia, G.; Narici, M.V. Changes in motor control and muscle performance after a short-term body mass reduction program in obese subjects. J. Endocrinol. Invest. 2001, 24, 393–398. [Google Scholar] [CrossRef]
- Drusini, A.G.; Eleazer, G.P.; Caiazzo, M.; Veronese, E.; Carrara, N.; Ranzato, C.; Businaro, F.; Boland, R.; Wieland, D. One-leg standing balance and functional status in an elderly community-dwelling population in northeast Italy. Aging Clin. Exp. Res. 2002, 14, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Owens, S.; Galloway, R. Childhood obesity and the metabolic syndrome. Curr. Atheroscler. Rep. 2014, 16, 436. [Google Scholar] [CrossRef][Green Version]
- Taverno Ross, S.E.; Byun, W.; Dowda, M.; McIver, K.L.; Saunders, R.P.; Pate, R.R. Sedentary behaviors in fifth-grade boys and girls: Where, with whom, and why? Child Obes. 2013, 9, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Francis, K. Methods of anaerobic power assessment (a statistical program for the IBM PC). Phys. Ther. 1987, 67, 270–275. [Google Scholar]
- Lafortuna, C.L.; Fumagalli, E.; Vangeli, V.; Sartorio, A. Lower limb alactic anaerobic power output assessed with different techniques in morbid obesity. J. Endocrinol. Invest. 2002, 25, 134–141. [Google Scholar] [CrossRef]
- Lazzer, S.; Bravo, G.; Tringali, G.; De Micheli, R.; De Col, A.; Sartorio, A. A 3-week multidisciplinary body weight reduction program improves body composition and lower limb power output in 3778 severely obese children and adolescents. Front. Physiol. 2020, in press. [Google Scholar]
- Kanehisa, H.; Ikegawa, S.; Tsunoda, N.; Fukunaga, T. Cross-sectional areas of fat and muscle in limbs during growth and middle age. Int. J. Sports Med. 1994, 15, 420–425. [Google Scholar] [CrossRef]
- Moreh, E.; Jacobs, J.M.; Stessman, J. Fatigue, function, and mortality in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 887–895. [Google Scholar] [CrossRef]
- McWhorter, J.W.; Wallmann, H.W.; Alpert, P.T. The obese child: Motivation as a tool for exercise. J. Pediatr. Health Care 2003, 17, 11–17. [Google Scholar] [CrossRef]
- Vantieghem, S.; Bautmans, I.; Tresignie, J.; Provyn, S. Self-perceived fatigue in adolescents in relation to body composition and physical outcomes. Pediatr. Res. 2018, 83, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Ament, W.; Verkerke, G.J. Exercise and fatigue. Sports Med. 2009, 39, 389–422. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.M.; Bray, G.A. Dietary management of the metabolic syndrome—One size fits all? Proc. Nutr. Soc. 2013, 72, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Runkel, N.; Brydniak, R. Surgical Treatment of Metabolic Syndrome. Visc. Med. 2016, 32, 352–356. [Google Scholar] [CrossRef]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef]
- Di Cesare, M.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.P.; Bentham, J. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 2019, 17, 212. [Google Scholar] [CrossRef]
- Fiorini, G.; Cerri, C.; Bini, S.; Rigamonti, A.E.; Perlini, S.; Marazzi, N.; Sartorio, A.; Cella, S.G. The burden of chronic noncommunicable diseases in undocumented migrants: A 1-year survey of drugs dispensation by a non-governmental organization in Italy. Public Health 2016, 141, 26–31. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Cella, S.G.; Bonomo, S.M.; Mancia, G.; Grassi, G.; Perotti, M.; Agosti, F.; Sartorio, A.; Müller, E.E.; Pincelli, A.I. Effect of somatostatin infusion on peptide YY secretion: Studies in the acute and recovery phase of anorexia nervosa and in obesity. Eur. J. Endocrinol. 2011, 165, 421–427. [Google Scholar] [CrossRef]
- Sartorio, A.; Lafortuna, C.L.; Maffiuletti, N.A.; Agosti, F.; Marazzi, N.; Rastelli, F.; Rigamonti, A.E.; Muller, E.E. GH responses to two consecutive bouts of whole body vibration, maximal voluntary contractions or vibration alternated with maximal voluntary contractions administered at 2-h intervals in healthy adults. Growth Horm. IGF Res. 2010, 20, 416–421. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Grugni, G.; Marazzi, N.; Bini, S.; Bidlingmaier, M.; Sartorio, A. Unaltered ratio of circulating levels of growth hormone/GH isoforms in adults with Prader-Willi syndrome after GHRH plus arginine administration. Growth Horm. IGF Res. 2015, 25, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Giunta, M.; Rigamonti, A.E.; Agosti, F.; Patrizi, A.; Compri, E.; Cardinale, M.; Sartorio, A. Combination of external load and whole body vibration potentiates the GH-releasing effect of squatting in healthy females. Horm. Metab. Res. 2013, 45, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, A.; Lafortuna, C.L.; Vangeli, V.; Tavani, A.; Bosetti, C.; La Vecchia, C. Short-term changes of cardiovascular risk factors after a non-pharmacological body weight reduction program. Eur. J. Clin. Nutr. 2001, 55, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Curioni, C.; Lourenco, P. Long-term weight loss after diet and exercise: A systematic review. Int. J. Obes. 2005, 29, 1168–1174. [Google Scholar] [CrossRef]
- Douketis, J.; Macie, C.; Thabane, L.; Williamson, D. Systematic review of long-term weight loss studies in obese adults: Clinical significance and applicability to clinical practice. Int. J. Obes. 2005, 29, 1153–1167. [Google Scholar] [CrossRef]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef]
- Hutchesson, M.J.; Hulst, J.; Collins, C.E. Weight management interventions targeting young women: A systematic review. J. Acad. Nutr. Diet 2013, 113, 795–802. [Google Scholar] [CrossRef]
- Young, M.; Morgan, P.; Plotnikoff, R.; Callister, R.; Collins, C. Effectiveness of male-only weight loss and weight loss maintenance interventions: A systematic review with meta-analysis. Obes. Rev. 2012, 13, 393–408. [Google Scholar] [CrossRef]
- Williams, R.L.; Wood, L.G.; Collins, C.E.; Callister, R. Effectiveness of weight loss interventions—Is there a difference between men and women: A systematic review. Obes. Rev. 2015, 16, 171–186. [Google Scholar] [CrossRef]
- Wu, B.; O’Sullivan, A. Sex differences in energy metabolism need to be considered with lifestyle modifications in humans. J. Nutr. Metab. 2011, 2011. [Google Scholar] [CrossRef]
- Cunningham, J.J. Body composition as a determinant of energy expenditure: A synthetic review and a proposed general prediction equation. Am. J. Clin. Nutr. 1991, 54, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Hill, J.O.; Jacobsen, D.J.; Potteiger, J.; Sullivan, D.K.; Johnson, S.L.; Heelan, K.; Hise, M.; Fennessey, P.V.; Sonko, B.; et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: The Midwest Exercise Trial. Arch. Intern. Med. 2003, 163, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Oude Luttikhuis, H.; Baur, L.; Jansen, H.; Shrewsbury, V.A.; O’Malley, C.; Stolk, R.P.; Summerbell, C.D. Interventions for treating obesity in children. Cochrane Database Syst. Rev. 2009, 21, CD001872. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T. Effectiveness of lifestyle intervention in overweight children. Proc. Nutr. Soc. 2011, 70, 494–505. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Obese without Metabolic Syndrome | Obese with Metabolic Syndrome | ||||
|---|---|---|---|---|---|---|
| All | Females | Males | All | Females | Males | |
| N. | 548 | 312 | 236 | 96 | 53 | 43 |
| Age (years) | 14.4 ± 2.3 | 14.7 ± 2.3 | 14.0 ± 2.3 | 14.7 ± 2.3 | 14.5 ± 2.5 | 15.0 ± 1.9 |
| Height (m) | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 |
| Weight (kg) | 96.1 ± 23.4 a | 92.2 ± 18.6 b,c,d | 101.2 ± 27.7 | 104.1 ± 23.9 | 101.6 ± 21.4 | 107.2 ± 26.6 |
| BMI (kg/m2) | 36.3 ± 6.7 a | 35.9 ± 5.8 | 36.7 ± 7.7 | 38.3 ± 6.9 | 38.3 ± 6.7 | 38.4 ± 7.2 |
| Waist (cm) | 109.7 ± 15.3 a | 106.2 ± 13.4 b,c,d | 114.9 ± 16.4 | 123.7 ± 17.8 | 121.5 ± 18.7 | 126.3 ± 16.8 |
| WHR | 0.9 ± 0.1 a | 0.9 ± 0.1 b,c,d | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Glucose (mg/dL) | 86.3 ± 6.6 a | 85.4 ± 6.8 d | 87.7 ± 6.1 | 94.7 ± 39.1 | 97.5 ± 53.1 | 91.4 ± 8.3 |
| Triglycerides (mg/dL) | 96.0 ± 36.9 a | 94.5 ± 32.0 c,d | 98.2 ± 43.3 c,d | 158.7 ± 63.2 | 158.7 ± 53.4 | 158.8 ± 74.6 |
| HDL-cholesterol (mg/dL) | 46.0 ± 0.7 a | 46.5 ± 9.5 c,d | 45.3 ± 7.4 c,d | 38.7 ± 7.7 | 39.9 ± 7.0 | 37.3 ± 8.3 |
| HR (bpm) | 83.9 ± 12.9 a | 84.8 ± 12.8 | 82.6 ± 13.0 c | 87.1 ± 13.7 | 88.4 ± 11.7 | 85.4 ± 15.8 |
| DBP (mmHg) | 75.5 ± 5.8 | 75.8 ± 5.6 | 75.0 ± 6.2 | 80.5 ± 8.5 | 86.0 ± 5.5 | 75.8 ± 8.0 |
| SBP (mmHg) | 120.9 ± 10.1 a | 119.5 ± 8.8 c,d | 123.0 ± 11.5 | 130.9 ± 10.7 | 130.0 ± 11.5 | 131.9 ± 9.8 |
| Parameter | Obese without Metabolic Syndrome | Obese with Metabolic Syndrome | ||||
|---|---|---|---|---|---|---|
| All | Females | Males | All | Females | Males | |
| BMI (kg/m2) | ||||||
| Pre | 36.3 ± 6.7 b | 35.9 ± 5.8 | 36.7 ± 7.7 | 38.3 ± 6.9 | 38.3 ± 6.7 | 38.4 ± 7.2 |
| Post | 34.8 ± 6.4 a,b | 34.5 ± 5.6 a | 35.1 ± 7.3 a | 36.7 ± 6.6 a | 36.8 ± 6.5 a | 36.6 ± 6.9 a |
| FM (kg) | ||||||
| Pre | 43.7 ± 8.2 | 43.4 ± 7.2 | 44.3 ± 9.9 | 41.4 ± 12.0 | 43.2 ± 4.5 | 40.1 ± 16.3 |
| Post | 40.8 ± 7.2 a | 41.7 ± 6.8 a | 39.4 ± 8.0 a | 39.0 ± 10.7 a | 41.2 ± 4.3 | 37.3 ± 14.4 |
| FM (%) | ||||||
| Pre | 43.8 ± 5.5 | 45.1 ± 5.1 c | 42.1 ± 5.5 e | 43.9 ± 6.1 | 46.1 ± 5.1 d | 41.2 ± 6.1 |
| Post | 42.7 ± 5.8 a | 44.3 ± 5.3 a,c,d | 40.7 ± 5.9 a,e | 43.0 ± 5.0 a | 45.2 ± 5.1 a,d | 40.3 ± 5.5 a |
| FFM (kg) | ||||||
| Pre | 54.3 ± 9.5 | |||||
| Post | 53.6 ± 9.9 | 48.0 ± 6.4 c | 63.3 ± 6.8 | 54.1 ± 7.2 | 49.2 ± 3.5 d | 58.1 ± 7.1 |
| OLSBL (s) | ||||||
| Pre | 45.6 ± 19.2 | 47.4 ± 18.2 | 43.4 ± 20.2 | 42.8 ± 21.1 | 43.0 ± 21.0 | 42.6 ± 21.4 |
| Post | 51.4 ± 15.1 a | 52.8 ± 14.0 a | 49.5 ± 16.4 a | 49.1 ± 18.0 a | 49.1 ± 18.7 a | 49.2 ± 17.2 a |
| OLSBR (s) | ||||||
| Pre | 45.2 ± 19.3 | 47.5 ± 18.4 | 42.3 ± 20.1 | 43.7 ± 21.0 | 46.3 ± 20.3 | 40.6 ± 21.7 |
| Post | 51.8 ± 15.2 a | 53.7 ± 13.4 a | 49.3 ± 17.0 a | 48.4 ± 17.8 a | 50.3 ± 17.3 a | 46.1 ± 18.2 a |
| SCT (s) | ||||||
| Pre | 3.1 ± 0.6 | 3.1 ± 0.5 c,d | 3.0 ± 0.7 e | 3.1 ± 0.5 | 3.3 ± 0.6 d | 2.9 ± 0.4 |
| Post | 2.8 ± 0.5 a | 2.9 ± 0.4 a | 2.8 ± 0.5 a,e | 2.9 ± 0.5 a | 3.0 ± 0.6 a,d | 2.7 ± 0.4 a |
| FSS | ||||||
| Pre | 26.0 ± 6.4 | 23.8 ± 5.0 | 29.7 ± 7.1 a | 27.3 ± 10.2 | 28.5 ± 9.5 | 26.4 ± 11.8 |
| Post | 22.7 ± 6.4 a | 20.5 ± 4.4 a | 26.2±7.7 a | 20.6 ± 9.2 a | 20.3 ± 9.2 a | 20.8 ± 10.3 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigamonti, A.E.; Tringali, G.; De Micheli, R.; De Col, A.; Tamini, S.; Saezza, A.; Cella, S.G.; Sartorio, A. Impact of a Three-Week in-Hospital Multidisciplinary Body Weight Reduction Program on Body Composition, Muscle Performance and Fatigue in a Pediatric Obese Population with or without Metabolic Syndrome. Nutrients 2020, 12, 208. https://doi.org/10.3390/nu12010208
Rigamonti AE, Tringali G, De Micheli R, De Col A, Tamini S, Saezza A, Cella SG, Sartorio A. Impact of a Three-Week in-Hospital Multidisciplinary Body Weight Reduction Program on Body Composition, Muscle Performance and Fatigue in a Pediatric Obese Population with or without Metabolic Syndrome. Nutrients. 2020; 12(1):208. https://doi.org/10.3390/nu12010208
Chicago/Turabian StyleRigamonti, Antonello Emilio, Gabriella Tringali, Roberta De Micheli, Alessandra De Col, Sofia Tamini, Antonella Saezza, Silvano G. Cella, and Alessandro Sartorio. 2020. "Impact of a Three-Week in-Hospital Multidisciplinary Body Weight Reduction Program on Body Composition, Muscle Performance and Fatigue in a Pediatric Obese Population with or without Metabolic Syndrome" Nutrients 12, no. 1: 208. https://doi.org/10.3390/nu12010208
APA StyleRigamonti, A. E., Tringali, G., De Micheli, R., De Col, A., Tamini, S., Saezza, A., Cella, S. G., & Sartorio, A. (2020). Impact of a Three-Week in-Hospital Multidisciplinary Body Weight Reduction Program on Body Composition, Muscle Performance and Fatigue in a Pediatric Obese Population with or without Metabolic Syndrome. Nutrients, 12(1), 208. https://doi.org/10.3390/nu12010208






