Iron Status of Infants in the First Year of Life in Northern Taiwan
Abstract
1. Introduction
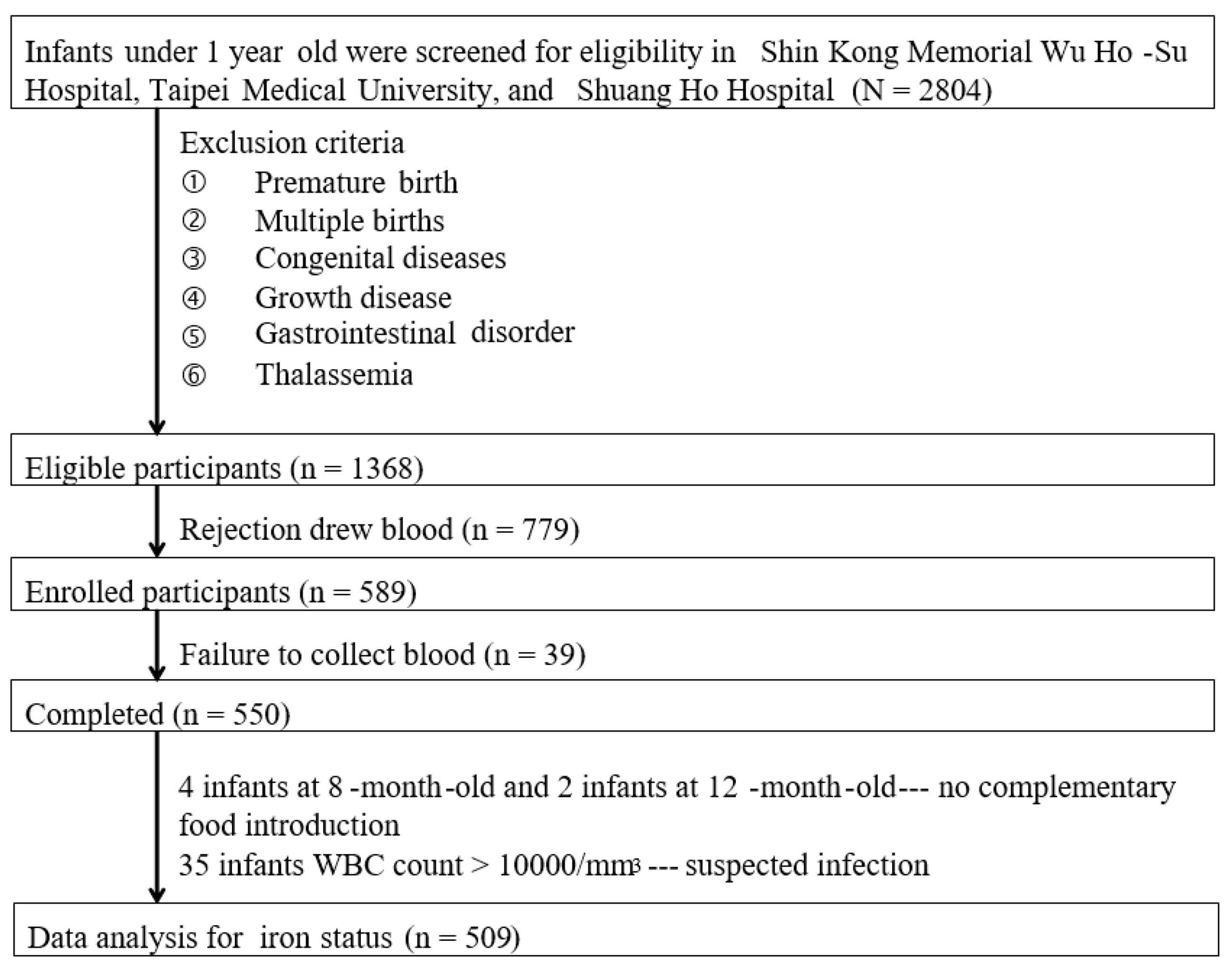
2. Participants and Methods
2.1. Study Subjects
2.2. Basic Characteristics and Dietary Iron Intake Assessment
2.3. Breast Milk and Blood Collection
2.4. Biochemical Analyses
2.5. Breast Milk Iron Content Analysis
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics and Infant Anemia Diagnosis
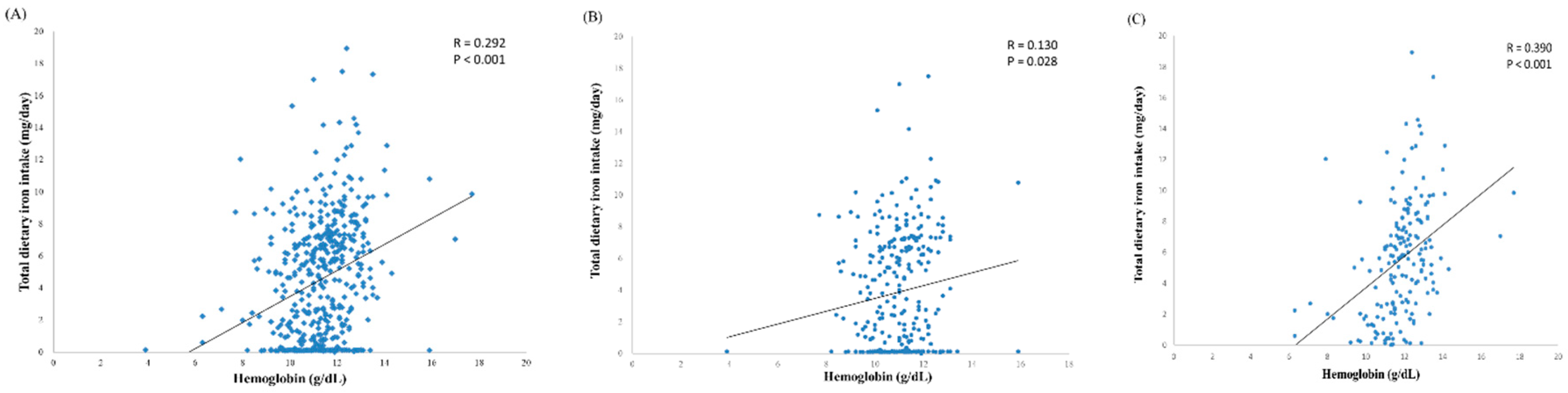
3.2. Analysis of Iron Status
3.3. Iron Intake of Infants
3.4. Association between Feeding Type and Iron Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lessen, R.; Kavanagh, K. Position of the Academy of Nutrition and Dietetics: Promoting and Supporting Breastfeeding. J. Acad. Nutr. Diet. 2015, 115, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.; Wilson, D.C.; Booth, I.; Lucas, A. Six months of exclusive breast feeding: How good is the evidence? BMJ 2011, 342, c5955. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Monajemzadeh, S.; Zarkesh, M. Iron deficiency anemia in infants aged 12-15 months in Ahwaz, Iran. Indian J. Pathol. Microbiol. 2009, 52, 182–184. [Google Scholar] [CrossRef]
- Özdemir, N. Iron deficiency anemia from diagnosis to treatment in children. Turk Pediatri Ars. 2015, 50, 11–19. [Google Scholar] [CrossRef]
- Qasem, W.A.; Friel, J.K. An Overview of Iron in Term Breast-Fed Infants. Clin. Med. Insights Pediatr. 2015, 9, 79–84. [Google Scholar] [CrossRef]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef]
- Steinbicker, A.U.; Muckenthaler, M.U. Out of Balance—Systemic Iron Homeostasis in Iron-Related Disorders. Nutrients 2013, 5, 3034–3061. [Google Scholar] [CrossRef]
- Lozoff, B.; Smith, J.B.; Clark, K.M.; Perales, C.G.; Rivera, F.; Castillo, M. Home intervention improves cognitive and social-emotional scores in iron-deficient anemic infants. Pediatrics 2010, 126, e884–e894. [Google Scholar] [CrossRef]
- Allen, L.H. Anemia and iron deficiency: Effects on pregnancy outcome. Am. J. Clin. Nutr. 2000, 71, 1280S–1284S. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.C.; Wall, C.R.; Brewster, D.; Nicholson, R.; Whitehall, J.; Super, L.; Pitcher, L. Policy statement on iron deficiency in pre-school-aged children. J. Paediatr. Child Health 2007, 43, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; O’brien, K.O. Pregnancy and iron homeostasis: An update. Nutr. Rev. 2013, 71, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Ejezie, F.; Nwagha, U.; Ikekpeazu, E.; Ozoemena, O.; Onwusi, E. Assessment of iron content of breast milk in preterm and term mothers in enugu urban. Ann. Med. Health Sci. Res. 2011, 1, 85–90. [Google Scholar] [PubMed]
- Friel, J.; Qasem, W.; Cai, C. Iron and the Breastfed Infant. Antioxidants 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Erick, M. Breast milk is conditionally perfect. Med. Hypotheses 2018, 111, 82–89. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Wan, Y.; Li, J.; Chen, Y.; Zheng, J.; Huang, T.; Li, D. Prolonged Exclusive Breastfeeding Duration Is Positively Associated with Risk of Anemia in Infants Aged 12 Months. J. Nutr. 2016, 146, 1707–1713. [Google Scholar] [CrossRef]
- Tsai, S.-F.; Chen, S.-J.; Yen, H.-J.; Hung, G.-Y.; Tsao, P.-C.; Jeng, M.-J.; Lee, Y.-S.; Soong, W.-J.; Tang, R.-B. Iron Deficiency Anemia in Predominantly Breastfed Young Children. Pediatr. Neonatol. 2014, 55, 466–469. [Google Scholar] [CrossRef]
- Lyu, L.C.; Lee, F.J.; Chen, H.Y.; Fang, L.J.; Chiang, H.J. Test-weighing and nutrient intakes of breast milk feeding for Taiwanese infants from 1 to 12 months of age. Nutr. Sci. J. 2011, 3, 87–98. [Google Scholar]
- Cheng, X.Z.; Jin, C.; Zhang, K.C. Determination of trace elements in waste beer yeasts by ICP-MS with microwave digestion. Guang Pu Xue Yu Guang Pu Fen Xi = Guang Pu 2008, 28, 2421–2424. [Google Scholar]
- Krishnaswamy, S.; Bhattarai, D.; Bharti, B.; Bhatia, P.; Das, R.; Bansal, D. Iron deficiency and iron deficiency anemia in 3–5 months-old, Breastfed Healthy Infants. Indian J. Pediatr. 2017, 84, 505–508. [Google Scholar] [CrossRef]
- Arvas, A.; Elgormus, Y.; Gur, E.; Alikasifoglu, M.; Celebi, A. Iron status in breast-fed full-term infants. Turk. J. Pediatr. 2000, 42, 22–26. [Google Scholar]
- Dube, K.; Schwartz, J.; Mueller, M.J.; Kalhoff, H.; Kersting, M. Iron intake and iron status in breastfed infants during the first year of life. Clin. Nutr. 2010, 29, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.F.; Taddei, J.A.; Lopez, F.A.; Braga, J.A. Breastfeeding exclusively and iron deficiency anemia during the first 6 months of age. Rev. Assoc. Médica Bras. 2014, 60, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Trave, T.D.; Vélaz, L.D. Prevalence of iron deficiency in healthy 12-month-old infants. An. Esp. Pediatr. 2002, 57, 209–214. [Google Scholar]
- Vendt, N.; Grünberg, H.; Leedo, S.; Tillmann, V.; Talvik, T. Prevalence and causes of iron deficiency anemias in infants aged 9 to 12 months in Estonia. Medicina 2007, 43, 947. [Google Scholar] [CrossRef]
- Al Hawsawi, Z.M.; Al-Rehali, S.A.; Mahros, A.M.; Al-Sisi, A.M.; Al-Harbi, K.D.; Yousef, A.M. High prevalence of iron deficiency anemia in infants attending a well-baby clinic in northwestern Saudi Arabia. Saudi Med. J. 2015, 36, 1067–1070. [Google Scholar] [CrossRef]
- Hong, J.; Chang, J.Y.; Shin, S.; Oh, S. Breastfeeding and Red Meat Intake Are Associated with Iron Status in Healthy Korean Weaning-age Infants. J. Korean Med Sci. 2017, 32, 974–984. [Google Scholar] [CrossRef]
- Clark, K.M.; Li, M.; Zhu, B.; Liang, F.; Shao, J.; Zhang, Y.; Ji, C.; Zhao, Z.; Kaciroti, N.; Lozoff, B. Breastfeeding, mixed, or formula feeding at 9 months of age and the prevalence of iron deficiency and iron deficiency anemia in two cohorts of infants in China. J. Pediatr. 2017, 181, 56–61. [Google Scholar] [CrossRef]
- Cai, C.; Harding, S.; Friel, J. Breast milk iron concentrations may be lower than previously reported: Implications for exclusively breastfed infants. Matern. Pediatr. Nutr. 2015, 2, 2. [Google Scholar]
- Domellof, M.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Iron requirements of infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 119–129. [Google Scholar] [CrossRef]
- Mukhopadhyay, K.; Yadav, R.K.; Kishore, S.S.; Garewal, G.; Jain, V.; Narang, A. Iron status at birth and at 4 weeks in term small-for-gestation infants in comparison with appropriate-for-gestation infants. J. Matern.-Fetal Neonatal Med. 2011, 24, 886–890. [Google Scholar] [CrossRef]
- Rao, R.; Georgieff, M.K. Iron therapy for preterm infants. Clin. Perinatol. 2009, 36, 27–42. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.K.; Kenny, L.C.; Hourihane, J.O.B.; Irvine, A.D.; Murray, D.M.; Kiely, M.E. Impact of maternal, antenatal and birth-associated factors on iron stores at birth: Data from a prospective maternal–infant birth cohort. Eur. J. Clin. Nutr. 2017, 71, 782. [Google Scholar] [CrossRef] [PubMed]
- Ervasti, M.; Sankilampi, U.; Heinonen, S.; Punnonen, K. Early signs of maternal iron deficiency do not influence the iron status of the newborn, but are associated with higher infant birthweight. Acta Obs. Gynecol. Scand. 2009, 88, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Savage, G.; Tubman, T.R.; Lappin, T.R.; Halliday, H.L. Study of maternal influences on fetal iron status at term using cord blood transferrin receptors. Arch. Dis. Child. Fetal Neonatal. Ed. 2001, 84, F40–F43. [Google Scholar] [CrossRef]
- Chaparro, C.M.; Neufeld, L.M.; Tena Alavez, G.; Eguia-Liz Cedillo, R.; Dewey, K.G. Effect of timing of umbilical cord clamping on iron status in Mexican infants: A randomised controlled trial. Lancet 2006, 367, 1997–2004. [Google Scholar] [CrossRef]
- Qasem, W.; Fenton, T.; Friel, J. Age of introduction of first complementary feeding for infants: A systematic review. BMC Pediatr. 2015, 15, 107. [Google Scholar] [CrossRef]
- Baker, R.D.; Greer, F.R. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics 2010, 126, 1040–1050. [Google Scholar] [CrossRef]
- Chiou, S.-T.; Chen, L.-C.; Yeh, H.; Wu, S.-R.; Chien, L.-Y. Early Skin-to-Skin Contact, Rooming-in, and Breastfeeding: A Comparison of the 2004 and 2011 National Surveys in Taiwan. Birth 2014, 41, 33–38. [Google Scholar] [CrossRef]
- Schulman, I. Iron Requirements in Infancy. JAMA J. Am. Med. Assoc. 1961, 175, 118–123. [Google Scholar] [CrossRef]
- Carter, R.C.; Jacobson, J.L.; Burden, M.J.; Armony-Sivan, R.; Dodge, N.C.; Angelilli, M.L.; Lozoff, B.; Jacobson, S.W. Iron deficiency anemia and cognitive function in infancy. Pediatrics 2010, 126, e427–e434. [Google Scholar] [CrossRef]
| Characteristics | Normal N = 439 | ID b N = 49 | IDA N = 21 | p Value |
|---|---|---|---|---|
| Infant | ||||
| Number (%) | 439 (86) | 49 (10) | 21 (4) | |
| Male (%) | 236 (53.8) | 24 (49.0) | 12 (57.1) | 0.546 |
| Gestational age | 38.4 ± 1.5 | 38.2 ± 1.6 | 38.2 ± 1.4 | 0.673 |
| Chronological age | 5.9 ± 4.3 | 9.9 ± 3.6 | 9.6 ± 3.4 | <0.001 * |
| Birth weight (kg) | 3.0 ± 0.5 | 3.0 ± 0.6 | 3.0 ± 0.5 | 0.919 |
| Body length (percentile) | 52.9 ± 30.9 | 55.8 ± 31.4 | 37.9 ± 31.3 | 0.118 |
| Body weight (percentile) | 51.2 ± 28.3 | 51.3 ± 30.0 | 43.9 ± 30.4 | 0.568 |
| Head circumference (percentile) | 56.4 ± 30.2 | 50.1 ± 31.2 | 51.9 ± 20.3 | 0.424 |
| Feeding type c | ||||
| Breast-fed (%) | 127 (28.9) | 32 (65.3) | 18 (85.7) | <0.001 * |
| Mix-fed (%) | 80 (18.2) | 6 (12.2) | 2 (9.5) | |
| Formula-fed (%) | 232 (52.8) | 11 (22.4) | 1 (4.8) | |
| Mother | ||||
| Age (year) | 32.6 ± 4.2 | 32.1 ± 3.6 | 32.4 ± 4.7 | 0.791 |
| BMI (kg/m2) | 21.3 ± 3.7 | 20.8 ± 5.5 | 20.7 ± 3.8 | 0.654 |
| Gestational weight gain (kg) | 14.3 ± 7.9 | 16.3 ± 11.9 | 11.6 ± 5.5 | 0.125 |
| Education (year) | 14.6 ± 2.3 | 15.0 ± 2.3 | 15.2 ± 2.0 | 0.382 |
| Classification | Normal N = 439 | ID b N = 49 | IDA N = 21 | p Value c |
|---|---|---|---|---|
| Infant | ||||
| Hb (g/L) | 11.4 ± 1.3 a | 11.6 ± 0.7 a | 9.2 ± 1.4 b | <0.001 |
| Ferritin (ng/mL) | 55.0 (98.4) a | 10.2 (5.7) b | 5.2 (5.7) b | <0.001 |
| TS (%) | 22.5 ± 11.3 a | 11.5 ± 5.5 bc | 7.0 ± 4.5 c | <0.001 |
| Maternal (before delivery) | ||||
| Hb (g/L) | 12.0 ± 1.9 | 12.1 ± 1.4 | 11.3 ± 1.4 | 0.361 |
| Hct (%) | 36.2 ± 4.6 | 36.6 ± 3.2 | 34.5 ± 3.7 | 0.333 |
| MCV (fl) | 86.8 ± 8.2 | 87.1 ± 8.4 | 86.7 ± 8.1 | 0.584 |
| Parameters | Normal | ID b | IDA | p Value |
|---|---|---|---|---|
| 1–6 months | ||||
| Number (%) | 307 (93.6) | 12 (3.7) | 9 (2.7) | |
| Chronological age | 3.4 ± 1.8 | 5.0 ± 1.2 | 4.8 ± 1.1 | 0.007 * |
| Breastfeed (%) | 174 (56.7) | 12(100) | 9 (100) | 0.001 * |
| Iron intake from milk (mg/day) c | 3.43 (6.62) | 0.13 (0.03) | 0.13 (0.17) | 0.003 * |
| Total iron intake (mg/day) d | 3.49 (6.81) | 0.13(0.18) | 0.13(0.17) | 0.007 * |
| 7–12 months | ||||
| Number (%) | 132 (73.0) | 37 (20.4) | 12 (6.6) | |
| Chronological age | 11.3 ± 2.9 | 11.2 ± 2.9 | 11.3 ± 2.0 | 0.976 |
| Breastfeed (%) | 21 (15.9) | 26 (70.3) | 11 (91.7) | <0.001 * |
| Iron intake from milk (mg/day) c | 5.04 (3.92) | 0.14 (2.01) | 0.14 (0.01) | <0.001 * |
| Iron intake from complementary food (mg/day) | 1.04 (1.00) | 1.29 (2.00) | 1.20 (2.00) | 0.840 |
| Total iron intake (mg/day) | 6.47 (4.35) | 2.28 (3.02) | 1.33 (0.98) | <0.001 * |
| Variables | β | SE a | OR | 95% CI | p Value |
|---|---|---|---|---|---|
| Model 1 b | |||||
| ID | 0.999 | 0.201 | 2.715 | 1.830–4.030 | <0.001 * |
| IDA | 1.467 | 0.368 | 4.338 | 2.108–8.927 | <0.001 * |
| Model 2 | |||||
| ID | 0.984 | 0.201 | 2.674 | 1.803–3.966 | <0.001 * |
| IDA | 1.361 | 0.369 | 3.901 | 1.893–8.042 | <0.001 * |
| Model 3 | |||||
| ID | 0.769 | 0.232 | 2.157 | 1.369–3.399 | 0.001 * |
| IDA | 1.434 | 0.437 | 4.196 | 1.780–9.887 | 0.001 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-M.; Mu, S.-C.; Shih, C.-K.; Chen, Y.-L.; Tsai, L.-Y.; Kuo, Y.-T.; Cheong, I.-M.; Chang, M.-L.; Chen, Y.-C.; Li, S.-C. Iron Status of Infants in the First Year of Life in Northern Taiwan. Nutrients 2020, 12, 139. https://doi.org/10.3390/nu12010139
Chen C-M, Mu S-C, Shih C-K, Chen Y-L, Tsai L-Y, Kuo Y-T, Cheong I-M, Chang M-L, Chen Y-C, Li S-C. Iron Status of Infants in the First Year of Life in Northern Taiwan. Nutrients. 2020; 12(1):139. https://doi.org/10.3390/nu12010139
Chicago/Turabian StyleChen, Chiao-Ming, Shu-Ci Mu, Chun-Kuang Shih, Yi-Ling Chen, Li-Yi Tsai, Yung-Ting Kuo, In-Mei Cheong, Mei-Ling Chang, Yi-Chun Chen, and Sing-Chung Li. 2020. "Iron Status of Infants in the First Year of Life in Northern Taiwan" Nutrients 12, no. 1: 139. https://doi.org/10.3390/nu12010139
APA StyleChen, C.-M., Mu, S.-C., Shih, C.-K., Chen, Y.-L., Tsai, L.-Y., Kuo, Y.-T., Cheong, I.-M., Chang, M.-L., Chen, Y.-C., & Li, S.-C. (2020). Iron Status of Infants in the First Year of Life in Northern Taiwan. Nutrients, 12(1), 139. https://doi.org/10.3390/nu12010139







