Beneficial Effect of Ubiquinol on Hematological and Inflammatory Signaling during Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Supplementation Protocol
2.2. Strenuous Exercise Performance Programme
2.3. Blood Sampling
2.4. Inflammatory Parameters
2.5. Hematological Parameters
2.6. Statistical Analysis
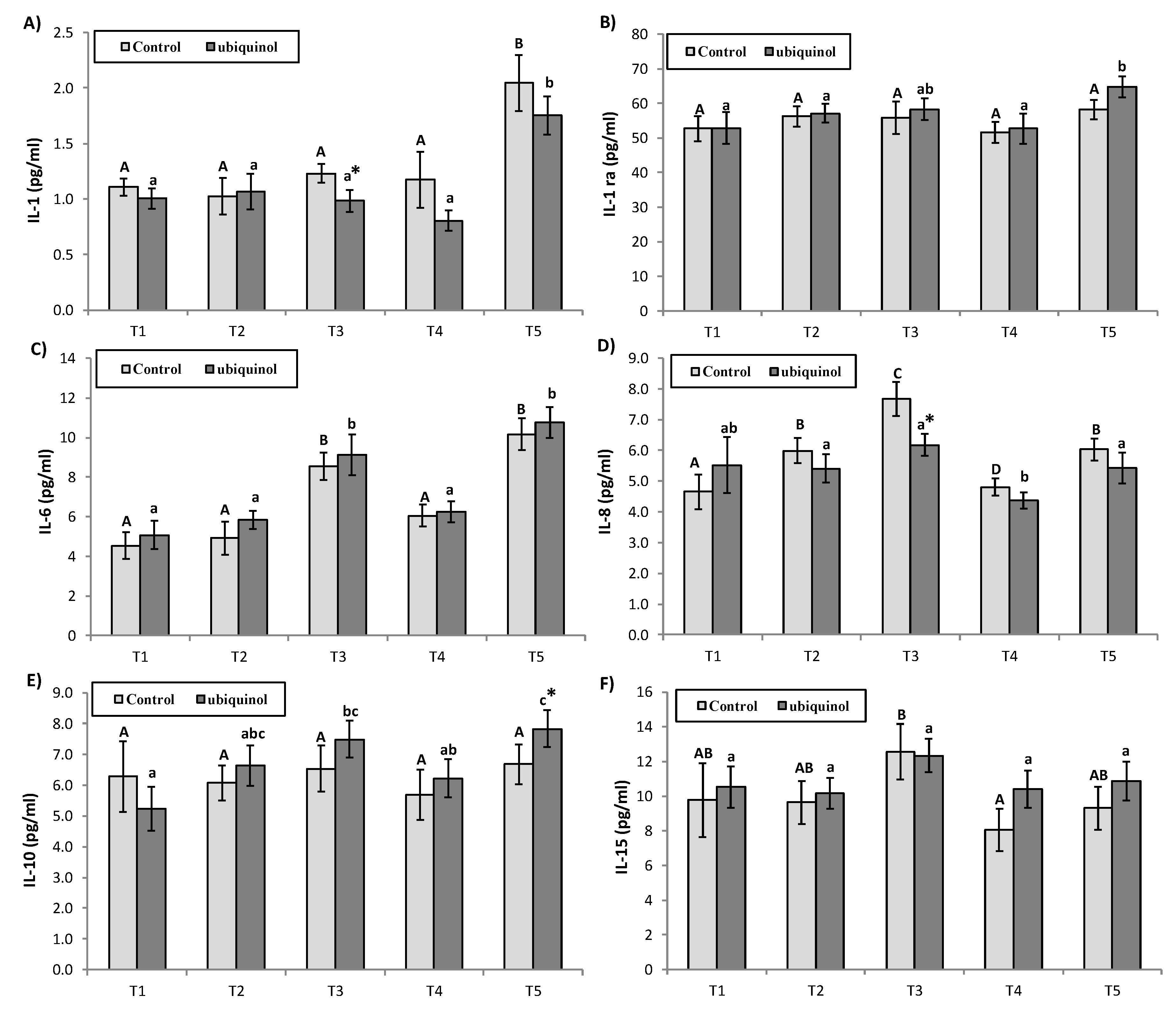
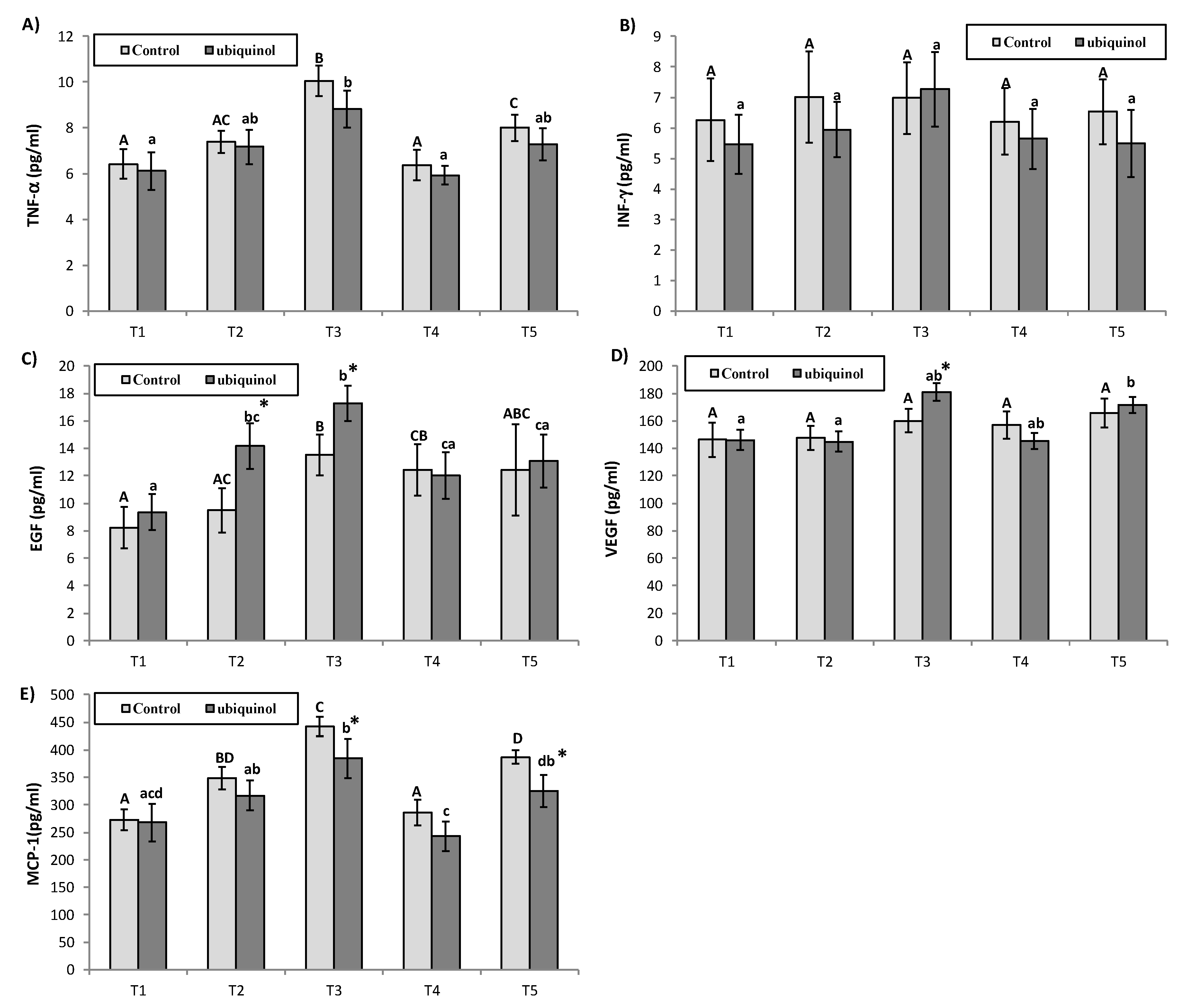
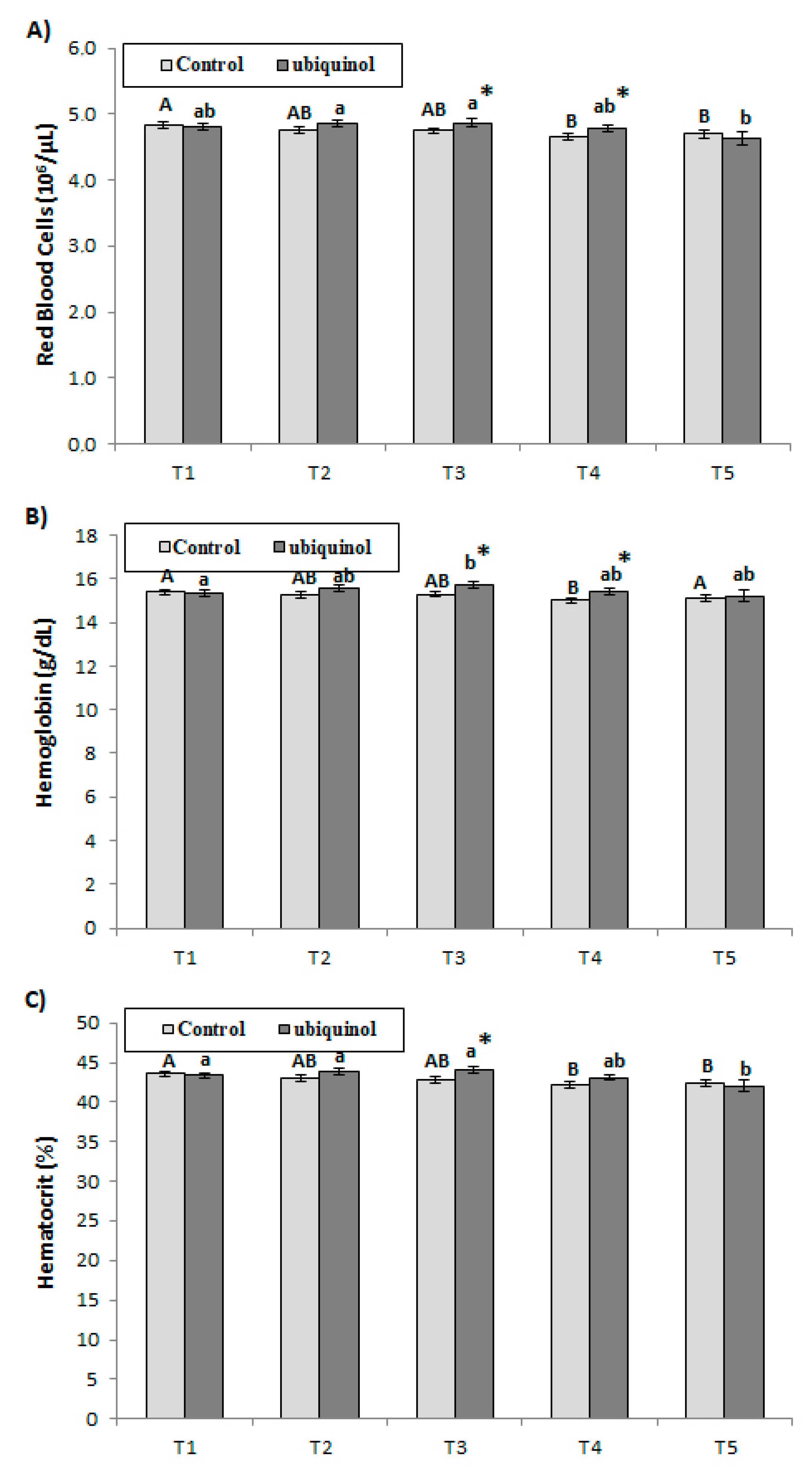
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Matheson, G.O.; Klugl, M.; Dvorak, J.; Engebretsen, L.; Meeuwisse, W.H.; Schwellnus, M.; Blair, S.N.; van Mechelen, W.; Derman, W.; Borjesson, M.; et al. Responsibility of sport and exercise medicine in preventing and managing chronic disease: Applying our knowledge and skill is overdue. Br. J. Sports Med. 2011, 45, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Gillon, A.; Nielsen, K.; Steel, C.; Cornwall, J.; Sheard, P. Exercise attenuates age-associated changes in motoneuron number, nucleocytoplasmic transport proteins and neuromuscular health. GeroScience 2018, 40, 177–192. [Google Scholar] [CrossRef] [PubMed]
- LaRoche, D.P.; Melanson, E.L.; Baumgartner, M.P.; Bozzuto, B.M.; Libby, V.M.; Marshall, B.N. Physiological determinants of walking effort in older adults: Should they be targets for physical activity intervention? GeroScience 2018, 40, 305–315. [Google Scholar] [CrossRef]
- Norling, A.M.; Gerstenecker, A.T.; Buford, T.W.; Khan, B.; Oparil, S.; Lazar, R.M. The role of exercise in the reversal of IGF-1 deficiencies in microvascular rarefaction and hypertension. GeroScience 2019. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Hauth, M.; Walter, M.; Hudemann, J.; Wank, V.; Niess, A.M.; Northoff, H. Exhaustive exercise modifies different gene expression profiles and pathways in LPS-stimulated and un-stimulated whole blood cultures. Brain Behav. Immun. 2014, 39, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Roitt, I.; Delves, P. Roitts Immunology, 12th ed.; Blackwell Science: London, UK, 2013. [Google Scholar]
- Suzuki, K.; Yamada, M.; Kurakake, S.; Okamura, N.; Yamaya, K.; Liu, Q.; Kudoh, S.; Kowatari, K.; Nakaji, S.; Sugawara, K. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur. J. Appl. Physiol. 2000, 81, 281–287. [Google Scholar] [CrossRef]
- Simon, H.B. Exercise and Health: Dose and Response, Considering Both Ends of the Curve. Am. J. Med. 2015, 128, 1171–1177. [Google Scholar] [CrossRef]
- El-Sayed, M.S. Effects of exercise on blood coagulation, fibrinolysis and platelet aggregation. Sports Med. Auckl. N. Z. 1996, 22, 282–298. [Google Scholar] [CrossRef]
- Kong, W.N.; Gao, G.; Chang, Y.Z. Hepcidin and sports anemia. Cell Biosci. 2014, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Sanchez-Oliver, A.J.; Mata-Ordonez, F.; Feria-Madueno, A.; Grimaldi-Puyana, M.; Lopez-Samanes, A.; Perez-Lopez, A. Effects of an Acute Exercise Bout on Serum Hepcidin Levels. Nutrients 2018, 10, 209. [Google Scholar] [CrossRef]
- Chang, C.W.; Chen, Y.M.; Hsu, Y.J.; Huang, C.C.; Wu, Y.T.; Hsu, M.C. Protective effects of the roots of Angelica sinensis on strenuous exercise-induced sports anemia in rats. J. Ethnopharmacol. 2016, 193, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Qi, R.; Wu, H.; Shi, W.; Xu, Y.; Li, M. Reduction of hemoglobin, not iron, inhibited maturation of red blood cells in male rats exposed to high intensity endurance exercises. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2019, 52, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.; Tobin, B. Iron status and exercise. Am. J. Clin. Nutr. 2000, 72, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Saris, W.H.; Senden, J.M.; Brouns, F. What is a normal red-blood cell mass for professional cyclists? Lancet Lond. Engl. 1998, 352, 1758. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Training surface and intensity: Inflammation, hemolysis, and hepcidin expression. Med. Sci. Sports Exerc. 2009, 41, 1138–1145. [Google Scholar] [CrossRef]
- Oh, J.; Sinha, I.; Tan, K.Y.; Rosner, B.; Dreyfuss, J.M.; Gjata, O.; Tran, P.; Shoelson, S.E.; Wagers, A.J. Age-associated NF-kappaB signaling in myofibers alters the satellite cell niche and re-strains muscle stem cell function. Aging 2016, 8, 2871–2896. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Swiderski, K.; Thakur, S.S.; Naim, T.; Trieu, J.; Chee, A.; Stapleton, D.I.; Koopman, R.; Lynch, G.S. Muscle-specific deletion of SOCS3 increases the early inflammatory response but does not affect regeneration after myotoxic injury. Skelet. Muscle 2016, 6, 36. [Google Scholar] [CrossRef]
- Buford, T.W.; Carter, C.S.; VanDerPol, W.J.; Chen, D.; Lefkowitz, E.J.; Eipers, P.; Morrow, C.D.; Bamman, M.M. Composition and richness of the serum microbiome differ by age and link to systemic inflammation. GeroScience 2018, 40, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Dulson, D. T-cells and their cytokine production: The anti-inflammatory and immunosuppressive effects of strenuous exercise. Cytokine 2018, 104, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Feng, Y.; Chen, G.C.; Qin, L.Q.; Fu, C.L.; Chen, L.H. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017, 119, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Abou-Khalil, R.; Mounier, R.; Chazaud, B. Regulation of myogenic stem cell behavior by vessel cells: The "menage a trois" of satellite cells, periendothelial cells and endothelial cells. Cell Cycle (Georget. Tex.) 2010, 9, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.; Pilegaard, H.; Neufer, P.D.; Hellsten, Y. Effect of acute exercise and exercise training on VEGF splice variants in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, 397–402. [Google Scholar] [CrossRef]
- Vina, J.; Gomez-Cabrera, M.C.; Lloret, A.; Marquez, R.; Minana, J.B.; Pallardo, F.V.; Sastre, J. Free radicals in exhaustive physical exercise: Mechanism of production, and protection by antioxidants. IUBMB Life 2000, 50, 271–277. [Google Scholar] [CrossRef]
- Slattery, K.; Bentley, D.; Coutts, A.J. The role of oxidative, inflammatory and neuroendocrinological systems during exercise stress in athletes: Implications of antioxidant supplementation on physiological adaptation during intensified physical training. Sports Med. (Auckl. N. Z.) 2015, 45, 453–471. [Google Scholar] [CrossRef]
- Sarmiento, A.; Diaz-Castro, J.; Pulido-Moran, M.; Kajarabille, N.; Guisado, R.; Ochoa, J.J. Coenzyme Q10 Supplementation and Exercise in Healthy Humans: A Systematic Review. Curr. Drug Metab. 2016, 17, 345–358. [Google Scholar] [CrossRef]
- Cooke, M.; Iosia, M.; Buford, T.; Shelmadine, B.; Hudson, G.; Kerksick, C.; Rasmussen, C.; Greenwood, M.; Leutholtz, B.; Willoughby, D.; et al. Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. J. Int. Soc. Sports Nutr. 2008, 5, 8. [Google Scholar] [CrossRef]
- Littarru, G.P.; Tiano, L. Bioenergetic and antioxidant properties of coenzyme Q10: Recent developments. Mol. Biotechnol. 2007, 37, 31–37. [Google Scholar] [CrossRef]
- Sarmiento, A.; Diaz-Castro, J.; Pulido-Moran, M.; Moreno-Fernandez, J.; Kajarabille, N.; Chirosa, I.; Guisado, I.M.; Javier Chirosa, L.; Guisado, R.; Ochoa, J.J. Short-term ubiquinol supplementation reduces oxidative stress associated with strenuous exercise in healthy adults: A randomized trial. BioFactors (Oxf. Engl.) 2016, 42, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, G.; Georgoudis, G.; Georgakopoulos, D.; Katsouras, C.; Kalfakakou, V.; Evangelou, A. Criterion-related validity of the short International Physical Activity Questionnaire against exercise capacity in young adults. Eur. J. Cardiovasc. Prev. Rehabil. Off. J. Eur. Soc. Cardiol. Work. Groups Epidemiol. Prev. Card. Rehabil. Exerc. Physiol. 2010, 17, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Aboodarda, S.J.; George, J.; Mokhtar, A.H.; Thompson, M. Muscle Strength and Damage Following Two Modes of Variable Resistance Training. J. Sports Sci. Med. 2011, 10, 635–642. [Google Scholar] [PubMed]
- Aniceto, R.R.; Ritti-Dias, R.M.; Dos Prazeres, T.M.; Farah, B.Q.; de Lima, F.F.; do Prado, W.L. Rating of Perceived Exertion During Circuit Weight Training: A Concurrent Validation Study. J. Strength Cond. Res. 2015, 29, 3336–3342. [Google Scholar] [CrossRef]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef]
- Kraus, W.E.; Slentz, C.A. Exercise training, lipid regulation, and insulin action: A tangled web of cause and effect. Obesity 2009, 17, 21–26. [Google Scholar] [CrossRef]
- Warburton, D.E.; Bredin, S.S. Reflections on Physical Activity and Health: What Should We Recommend? Can. J. Cardiol. 2016, 32, 495–504. [Google Scholar] [CrossRef]
- Littarru, G.P.; Tiano, L. Clinical aspects of coenzyme Q10: An update. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 641–646. [Google Scholar] [CrossRef]
- Saha, S.P.; Whayne, T.F., Jr. Coenzyme Q-10 in Human Health: Supporting Evidence? South. Med. J. 2016, 109, 17–21. [Google Scholar] [CrossRef]
- Tidball, J.G. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, 345–353. [Google Scholar] [CrossRef]
- Ostrowski, K.; Schjerling, P.; Pedersen, B.K. Physical activity and plasma interleukin-6 in humans--effect of intensity of exercise. Eur. J. Appl. Physiol. 2000, 83, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Zaldivar, F.; Wang-Rodriguez, J.; Nemet, D.; Schwindt, C.; Galassetti, P.; Mills, P.J.; Wilson, L.D.; Cooper, D.M. Constitutive pro- and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J. Appl. Physiol. (Bethesda Md. 1985) 2006, 100, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Taga, T.; Kishimoto, T. Interleukin-6 in biology and medicine. Adv. Immunol. 1993, 54, 1–78. [Google Scholar] [PubMed]
- Stoner, L.; Lucero, A.A.; Palmer, B.R.; Jones, L.M.; Young, J.M.; Faulkner, J. Inflammatory biomarkers for predicting cardiovascular disease. Clin. Biochem. 2013, 46, 1353–1371. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Fischer, C.P.; Keller, C.; Moller, K.; Pedersen, B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E433–E437. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.R.; Mounier, R.; Plomgaard, P.; Mortensen, O.H.; Penkowa, M.; Speerschneider, T.; Pilegaard, H.; Pedersen, B.K. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J. Physiol. 2007, 584, 305–312. [Google Scholar] [CrossRef]
- Diaz-Castro, J.; Guisado, R.; Kajarabille, N.; Garcia, C.; Guisado, I.M.; de Teresa, C.; Ochoa, J.J. Coenzyme Q(10) supplementation ameliorates inflammatory signaling and oxidative stress associated with strenuous exercise. Eur. J. Nutr. 2012, 51, 791–799. [Google Scholar] [CrossRef]
- Kullo, I.J.; Khaleghi, M.; Hensrud, D.D. Markers of inflammation are inversely associated with VO2 max in asymptomatic men. J. Appl. Physiol. (Bethesda Md. 1985) 2007, 102, 1374–1379. [Google Scholar] [CrossRef]
- Utsal, L.; Tillmann, V.; Zilmer, M.; Maestu, J.; Purge, P.; Saar, M.; Latt, E.; Maasalu, K.; Jurimae, T.; Jurimae, J. Negative correlation between serum IL-6 level and cardiorespiratory fitness in 10- to 11-year-old boys with increased BMI. J. Pediatric Endocrinol. Metab. 2013, 26, 503–508. [Google Scholar] [CrossRef]
- Jurimae, J.; Tillmann, V.; Purge, P.; Jurimae, T. Body composition, maximal aerobic performance and inflammatory biomarkers in endurance-trained athletes. Clin. Physiol. Funct. Imaging 2015. [Google Scholar] [CrossRef]
- Accattato, F.; Greco, M.; Pullano, S.A.; Care, I.; Fiorillo, A.S.; Pujia, A.; Montalcini, T.; Foti, D.P.; Brunetti, A.; Gulletta, E. Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects. PLoS ONE 2017, 12, e0178900. [Google Scholar] [CrossRef] [PubMed]
- Benatti, F.B.; Pedersen, B.K. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat. Rev. Rheumatol. 2015, 11, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.L.; Lloyd, P.G.; Yang, H.T.; Terjung, R.L. Exercise training and peripheral arterial disease. Compr. Physiol. 2012, 2, 2933–3017. [Google Scholar] [CrossRef] [PubMed]
- Tossige-Gomes, R.; Ottone, V.O.; Oliveira, P.N.; Viana, D.J.S.; Araújo, T.L.; Gripp, F.J.; Rocha-Vieira, E. Leukocytosis, muscle damage and increased lymphocyte proliferative response after an adventure sprint race. Braz. J. Med. Biol. Res. 2014, 47, 492–498. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Belardinelli, R.; Mucaj, A.; Lacalaprice, F.; Solenghi, M.; Seddaiu, G.; Principi, F.; Tiano, L.; Littarru, G.P. Coenzyme Q10 and exercise training in chronic heart failure. Eur. Heart J. 2006, 27, 2675–2681. [Google Scholar] [CrossRef]
- Chavakis, E.; Dernbach, E.; Hermann, C.; Mondorf, U.F.; Zeiher, A.M.; Dimmeler, S. Oxidized LDL inhibits vascular endothelial growth factor-induced endothelial cell migration by an inhibitory effect on the Akt/endothelial nitric oxide synthase pathway. Circulation 2001, 103, 2102–2107. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, H.J.; Nishida, M.; Lee, M.S.; Tamura, R.; Yamashita, S.; Matsuzawa, Y.; Lee, I.K.; Koh, G.Y. Betacellulin and amphiregulin induce upregulation of cyclin D1 and DNA synthesis activity through differential signaling pathways in vascular smooth muscle cells. Circ. Res. 2003, 93, 302–310. [Google Scholar] [CrossRef]
- Mifune, M.; Ohtsu, H.; Suzuki, H.; Frank, G.D.; Inagami, T.; Utsunomiya, H.; Dempsey, P.J.; Eguchi, S. Signal transduction of betacellulin in growth and migration of vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2004, 287, C807–C813. [Google Scholar] [CrossRef][Green Version]
- Ojalvo, A.G.; Acosta, J.B.; Mari, Y.M.; Mayola, M.F.; Perez, C.V.; Gutierrez, W.S.; Marichal, I.I.; Seijas, E.A.; Kautzman, A.M.; Pacheco, A.E.; et al. Healing enhancement of diabetic wounds by locally infiltrated epidermal growth factor is associated with systemic oxidative stress reduction. Int. Wound J. 2017, 14, 214–225. [Google Scholar] [CrossRef]
| Age (Years) | Height (cm) | Weight (kg) | BMI (kg/m2) | SBP (mmHg) | DBP (mmHg) | RHR (Beats/min) | |
|---|---|---|---|---|---|---|---|
| Ubiquinol | 38.9 ± 1.4 | 175.4 ± 0.8 | 76.8 ± 1.5 | 25.0 ± 0.4 | 137.0 ± 2.2 | 81.4 ± 1.5 | 57.4 ± 1.8 |
| Control | 38.2 ± 1.2 | 174.5 ± 1.2 | 76.3 ± 2.0 | 25.0 ± 0.5 | 134.1 ± 2.1 | 79.1 ± 1.9 | 57.1 ± 1.5 |
| T1 | T2 | T3 | T4 | T5 | ||
|---|---|---|---|---|---|---|
| Leukocytes (103/µL) | ||||||
| Control | 5.9 ± 0.2 A | 5.9 ± 0.2 A | 6.2 ± 0.2 A | 5.9 ± 0.2 A | 6.2 ± 0.2 A | |
| Ubiquinol | 5.8 ± 0.2 a,c | 6.1 ± 0.2 a,b,c | 6.8 ± 0.3 b | 5.9 ± 0.2 c | 6.4 ± 0.3 a,b,c | |
| Neutrophils (%) | ||||||
| Control | 52.0 ± 1.1 A,C | 52.0 ± 1.1 A,C | 57.1 ± 1.3 B | 52.0 ± 1.1 C | 56.0 ± 1.1 B | |
| Ubiquinol | 59.9 ± 1.1 a | 54.2 ± 1.0 b | 57.9 ± 1.0 c | 56.5 ± 1.5 b,c,d,* | 60.1 ± 1.5 d | |
| Lymphocytes (%) | ||||||
| Control | 34.7 ± 1.1 A,B,C,D | 35.4 ± 0.9 A,C,D | 32.1 ± 1.3 B,D | 36.8 ± 1.0 C | 32.8 ± 1.3 D | |
| Ubiquinol | 36.2 ± 1.1 a | 34.5 ± 1.1 a,b | 32.3 ± 1.0 b,c | 33.6 ± 1.3 a,b,c,* | 30.6 ± 1.3c | |
| Monocytes (%) | ||||||
| Control | 8.1 ± 0.3 A | 7.1 ± 0.3 B | 6.6 ± 0.3 B | 7.4 ± 0.3 A,B | 6.7 ± 0.3 B | |
| Ubiquinol | 8.5 ± 0.3 a | 7.0 ± 0.3 b | 6.5 ± 0.3 b | 6.6 ± 0.3 b,* | 6.4 ± 0.3 b | |
| Eosinophils (%) | ||||||
| Control | 4.8 ± 0.5 A | 4.5 ± 0.6 A,B | 3.5 ± 0.5 B | 4.1 ± 0.5 A,B | 3.8 ± 0.5 A,B | |
| Ubiquinol | 4.1 ± 0.4 a,c | 3.6 ± 0.3 a,c | 2.7 ± 0.3 b | 3.5 ± 0.3 c | 3.2 ± 0.3 a,b,c | |
| Basophils (%) | ||||||
| Control | 0.4 ± 0.04 A,C | 0.6 ± 0.05 B,C,D | 0.7 ± 0.04 C,D | 0.5 ± 0.04 A,B | 0.7 ± 0.07 D | |
| Ubiquinol | 0.4 ± 0.03 a | 0.7 ± 0.05 b | 0.6 ± 0.04 b | 0.5 ± 0.04 c | 0.6 ± 0.06 b,c | |
| Platelets (103/µL) | ||||||
| Control | 240.0 ± 6.3 A | 232.9 ± 6.1 A | 248.9 ± 7.9 A | 231.9 ± 6.6 A | 251.6 ± 12.6 A | |
| Ubiquinol | 239.9 ± 5.8 a | 239.8 ± 7.0 a | 250.3 ± 6.2 a | 244.7 ± 6.6 a | 244.1 ± 5.6 a | |
| MCV (fL) | ||||||
| Control | 90.4 ± 0.5 A | 90.5 ± 0.6 A | 90.5 ± 0.6 A | 90.8 ± 0.7 A | 90.5 ± 0.7 A | |
| Ubiquinol | 90.4 ± 0.7 a | 90.5 ± 0.7 a | 90.7 ± 0.7 a | 90.4 ± 0.8 a | 90.8 ± 0.8 a | |
| MCH (pg) | ||||||
| Control | 31.9 ± 0.3 A | 32.0 ± 0.3 A | 32.3 ± 0.3 A | 32.3 ± 0.3 A | 32.3 ± 0.3 A | |
| Ubiquinol | 32.0 ± 0.3 a | 32.1 ± 0.3 a | 32.3 ± 0.3 a | 32.3 ± 0.3 a | 32.7 ± 0.3 a | |
| MCHC (g/dL) | ||||||
| Control | 35.3 ± 0.1 A | 35.4 ± 0.1 A,B | 35.6 ± 0.1 B | 35.6 ± 0.1 B | 35.6 ± 0.1 B | |
| Ubiquinol | 35.4 ± 0.1 a | 35.4 ± 0.1 a | 35.6 ± 0.1 a,b | 35.7 ± 0.1 b | 36.0 ± 0.1 c,* | |
| RDW (%) | ||||||
| Control | 13.2 ± 0.1 A | 13.1 ± 0.1 A | 13.1 ± 0.1 A | 13.2 ± 0.1 A | 13.2 ± 0.1 A | |
| Ubiquinol | 13.3 ± 0.1 a | 13.2 ± 0.1 a,b | 13.0 ± 0.1 b | 13.2 ± 0.1 a,b | 13.1 ± 0.1 a,b | |
| MPV (fL) | ||||||
| Control | 8.6 ± 0.1 A | 8.8 ± 0.1 A | 8.7 ± 0.1 A | 8.7 ± 0.1 A | 8.7 ± 0.1 A | |
| Ubiquinol | 8.4 ± 0.1 a | 8.6 ± 0.2 a | 8.4 ± 0.1 a | 8.5 ± 0.1 a | 8.5 ± 0.1 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Castro, J.; Moreno-Fernandez, J.; Chirosa, I.; Chirosa, L.J.; Guisado, R.; Ochoa, J.J. Beneficial Effect of Ubiquinol on Hematological and Inflammatory Signaling during Exercise. Nutrients 2020, 12, 424. https://doi.org/10.3390/nu12020424
Diaz-Castro J, Moreno-Fernandez J, Chirosa I, Chirosa LJ, Guisado R, Ochoa JJ. Beneficial Effect of Ubiquinol on Hematological and Inflammatory Signaling during Exercise. Nutrients. 2020; 12(2):424. https://doi.org/10.3390/nu12020424
Chicago/Turabian StyleDiaz-Castro, Javier, Jorge Moreno-Fernandez, Ignacio Chirosa, Luis Javier Chirosa, Rafael Guisado, and Julio J. Ochoa. 2020. "Beneficial Effect of Ubiquinol on Hematological and Inflammatory Signaling during Exercise" Nutrients 12, no. 2: 424. https://doi.org/10.3390/nu12020424
APA StyleDiaz-Castro, J., Moreno-Fernandez, J., Chirosa, I., Chirosa, L. J., Guisado, R., & Ochoa, J. J. (2020). Beneficial Effect of Ubiquinol on Hematological and Inflammatory Signaling during Exercise. Nutrients, 12(2), 424. https://doi.org/10.3390/nu12020424










