Extra Virgin Olive Oil: Lesson from Nutrigenomics
Abstract
1. Introduction
2. EVOO Cultivars and Biodiversity: Effect on Health Promotion
3. Protective Role for EVOO on Human Health
3.1. EVOO in Obesity, MetS, and Diabetes
3.2. EVOO in Cardiovascular Diseases
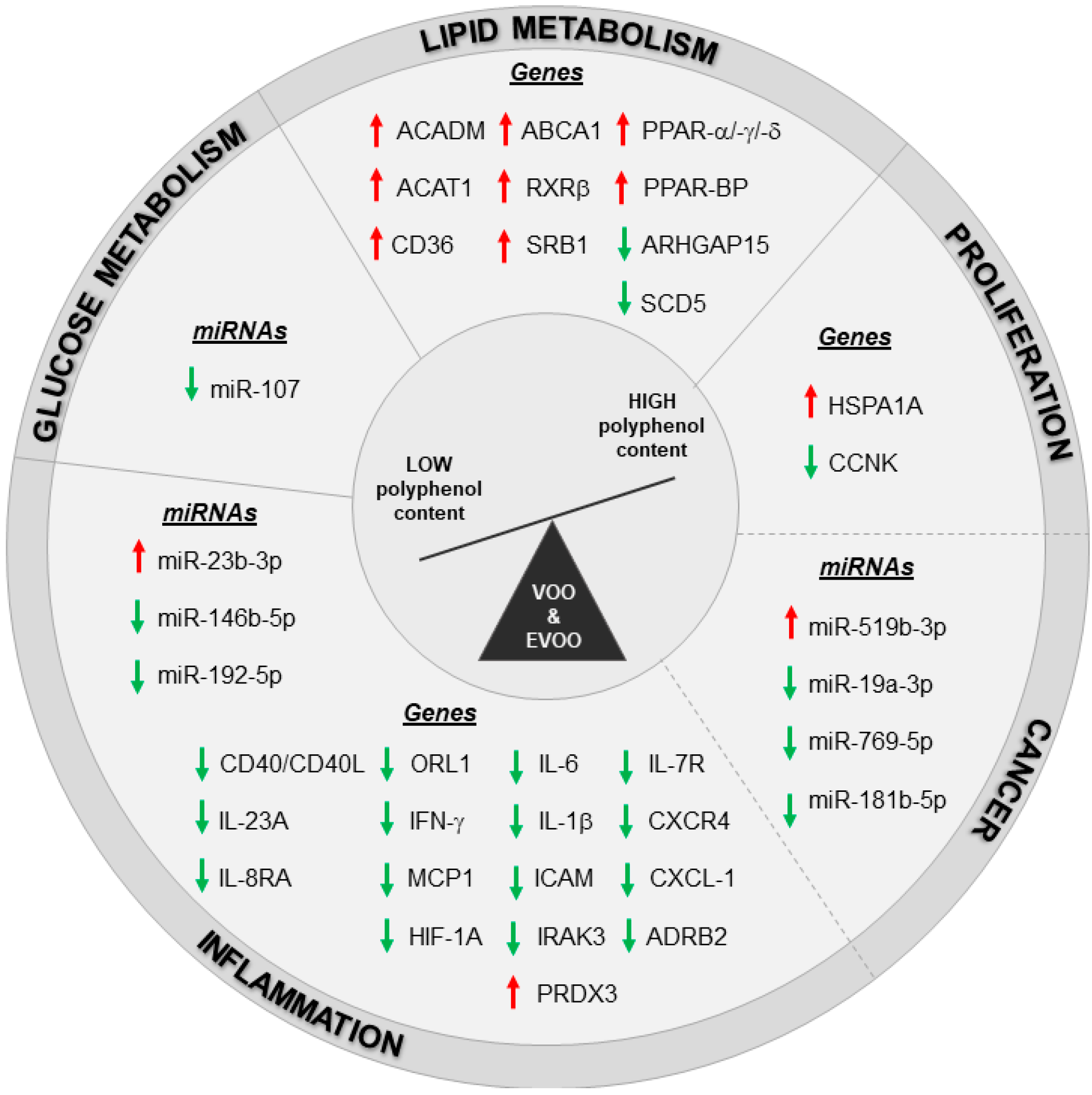
4. Nutrigenomics of Different EVOO Cultivars
| Dietary Intervention | Administration Timing | Type of the Study | Study Population | Background Diet | Method Used to Identify Dietary Pattern | Tissue | Technical Approach | Target Molecule | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| HPC vs. MPC VOO | Post prandial (acute intake) | Randomized, double-blind, crossover, controlled trial | Pre/hypertensive patients from Spain | 2-week washout period (during the week before the intervention: 10% of saturated fatty acids; on the day before: polyphenol-free diet. | 3-day dietary record | WBCs | RT-PCR | Genes | [90] |
| HPC vs. LPC EVOO | Post prandial (acute intake) | Paired study | Healthy subjects and MetS patients from Italy | 1-week washout period (no olive oil); 3 days before the intervention: low-phenolic compound diet. | - | PBMCs | Microarray | Genes/miRNAs | [32] |
| HPC vs. LPC VOO | Post prandial (VOO-based breakfast) | Randomized, double-blind, crossover trial | MetS patients from Spain | 6-week washout period (low fat, CHO diet, no vitamins and soy supplements); on the day before the intervention: no phenol-rich food. | 3-day dietary record and FFQ | PBMCs | Microarray | Genes | [93] |
| HPC vs. LPC VOO | Post prandial (VOO-based breakfast) | Randomized, crossover trial | MetS patients from Spain | 6-week washout period (low fat, CHO diet, no vitamins and soy supplements); on the day before the intervention: no phenol-rich food. | 3-day dietary record and FFQ | PBMCs | RT-PCR | Genes | [94] |
| HPC vs. LPC VOO | Sustained consumption (3 weeks) | Randomized, crossover, controlled trial | Healthy subjects from Finland, Germany, and Spain (subgroup of EUROLIVE study) | 2-week washout period (no olives and olive oil) | 3-day dietary record | PBMCs | RT-PCR | Genes | [87] |
| HPC vs. LPC VOO | Sustained consumption (3 weeks) | Randomized, double-blind, crossover trial | Healthy subjects from Finland, Germany, and Spain (subgroup of EUROLIVE study) | 2-week washout period (no olives and olive oil) | 3-day dietary record | PBMCs | RT-PCR | Genes | [88] |
| TMD+VOO TMD+WOO Control diet | Sustained consumption (12 weeks) | Randomized, parallel, controlled trial | Healthy subjects from Spain | - | FFQ | PBMCs | RT-PCR | Genes | [89] |
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalogiouri, N.P.; Aalizadeh, R.; Thomaidis, N.S. Application of an advanced and wide scope non-target screening workflow with LC-ESI-QTOF-MS and chemometrics for the classification of the Greek olive oil varieties. Food Chem. 2018, 256, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Martinez-Gonzalez, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Loffredo, L.; Pignatelli, P.; Angelico, F.; Bartimoccia, S.; Nocella, C.; Cangemi, R.; Petruccioli, A.; Monticolo, R.; Pastori, D.; et al. Extra virgin olive oil use is associated with improved post-prandial blood glucose and LDL cholesterol in healthy subjects. Nutr. Diabetes 2015, 5, e172. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Gonzalez, C.A. The role of olive oil in disease prevention: A focus on the recent epidemiological evidence from cohort studies and dietary intervention trials. Br. J. Nutr. 2015, 113 (Suppl. S2), S94–S101. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef]
- Zohary, D.; Spiegel-Roy, P. Beginnings of fruit growing in the old world. Science 1975, 187, 319–327. [Google Scholar] [CrossRef]
- Ayed, R.B.; Grati-Kamoun, N.; Moreau, F.; Rebaï, A. Comparative study of microsatellite profiles of DNA from oil and leaves of two Tunisian olive cultivars. Eur. Food Res. Technol. 2009, 229, 757–762. [Google Scholar] [CrossRef]
- Cicatelli, A.; Fortunati, T.; De Feis, I.; Castiglione, S. Oil composition and genetic biodiversity of ancient and new olive (Olea europea L.) varieties and accessions of southern Italy. Plant Sci. 2013, 210, 82–92. [Google Scholar] [CrossRef]
- Merchak, N.; El Bacha, E.; Bou Khouzam, R.; Rizk, T.; Akoka, S.; Bejjani, J. Geoclimatic, morphological, and temporal effects on Lebanese olive oils composition and classification: A (1)H NMR metabolomic study. Food Chem. 2017, 217, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Muzzalupo, I.; Vendramin, G.G.; Chiappetta, A. Genetic biodiversity of Italian olives (Olea europaea) germplasm analyzed by SSR markers. Sci. World J. 2014, 2014, 296590. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, R.; Angiolillo, A.; Guerrero, C.; Pellegrini, M.; Rallo, L.; Besnard, G.; Berville, A.; Martin, A.; Baldoni, L. A first linkage map of olive (Olea europaea L.) cultivars using RAPD, AFLP, RFLP and SSR markers. Theor. Appl. Genet. 2003, 106, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Dabbou, S.; Selvaggini, R.; Urbani, S.; Taticchi, A.; Servili, M.; Hammami, M. Comparison of the chemical composition and the organoleptic profile of virgin olive oil from two wild and two cultivated Tunisian Olea europaea. Chem. Biodivers. 2011, 8, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D. Olive oil. World Rev. Nutr. Diet. 2007, 97, 180–210. [Google Scholar] [PubMed]
- Rotondi, A.; Alfei, B.; Magli, M.; Pannelli, G. Influence of genetic matrix and crop year on chemical and sensory profiles of Italian monovarietal extra-virgin olive oils. J. Sci. Food Agric. 2010, 90, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, S.; Lucini, L.; Angilletta, F.; Rocchetti, G.; Farinelli, D.; Tombesi, S.; Trevisan, M. Discrimination of extra-virgin-olive oils from different cultivars and geographical origins by untargeted metabolomics. Food Res. Int. 2019, 121, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Florez, A.; Sinausia Nieva, L.; Sanchez-Ortiz, A.; Beltran, G.; Perona, J.S. The Fatty Acid Composition of Virgin Olive Oil from Different Cultivars Is Determinant for Foam Cell Formation by Macrophages. J. Agric. Food Chem. 2015, 63, 6731–6738. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, F.; Ranalli, A.; Contento, S.; Casanovas, M.; Antonucci, M.; Simone, G.D. Bioactives and nutraceutical phytochemicals naturally occurring in virgin olive oil. The case study of the Nocellara del Belice Italian olive cultivar. Nat. Prod. Res. 2013, 27, 1686–1690. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary intake of 337 polyphenols in French adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [Google Scholar] [CrossRef]
- Bianco, A.; Melchioni, C.; Ramunno, A.; Romeo, G.; Uccella, N. Phenolic components of Olea europaea—isolation of tyrosol derivatives. Nat. Prod. Res. 2004, 18, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Farag, R.S.; El-Baroty, G.S.; Basuny, A.M. Safety evaluation of olive phenolic compounds as natural antioxidants. Int. J. Food Sci. Nutr. 2003, 54, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Correa, J.A.; Lopez-Villodres, J.A.; Asensi, R.; Espartero, J.L.; Rodriguez-Gutierez, G.; De La Cruz, J.P. Virgin olive oil polyphenol hydroxytyrosol acetate inhibits in vitro platelet aggregation in human whole blood: Comparison with hydroxytyrosol and acetylsalicylic acid. Br. J. Nutr. 2009, 101, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Virruso, C.; Accardi, G.; Colonna-Romano, G.; Candore, G.; Vasto, S.; Caruso, C. Nutraceutical properties of extra-virgin olive oil: A natural remedy for age-related disease? Rejuvenation Res. 2014, 17, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.M.; Ritieni, A.; Sacchi, R.; Skog, K.; Borgen, E.; Fogliano, V. Characterization of phenolic compounds in virgin olive oil and their effect on the formation of carcinogenic/mutagenic heterocyclic amines in a model system. J. Agric. Food Chem. 2001, 49, 3969–3975. [Google Scholar] [CrossRef]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Covas, M.I.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Covas, M.I.; de la Torre, K.; Farre-Albaladejo, M.; Kaikkonen, J.; Fito, M.; Lopez-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventos, R.M.; et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic. Biol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Maschi, O.; Galli, G.V.; Fagnani, R.; Dal Cero, E.; Caruso, D.; Bosisio, E. Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. Br. J. Nutr. 2008, 99, 945–951. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, S.; Vacca, M.; Cariello, M.; Graziano, G.; D’Orazio, A.; Salvia, R.; Sasso, R.C.; Sabba, C.; Palasciano, G.; Moschetta, A. Genes and miRNA expression signatures in peripheral blood mononuclear cells in healthy subjects and patients with metabolic syndrome after acute intake of extra virgin olive oil. Biochim. Biophys. Acta 2016, 1861, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Galletti, P.; Maisto, G.; Cucciolla, V.; D’Angelo, S.; Zappia, V. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEBS Lett. 2000, 470, 341–344. [Google Scholar] [CrossRef]
- Quintero-Florez, A.; Pereira-Caro, G.; Sanchez-Quezada, C.; Moreno-Rojas, J.M.; Gaforio, J.J.; Jimenez, A.; Beltran, G. Effect of olive cultivar on bioaccessibility and antioxidant activity of phenolic fraction of virgin olive oil. Eur. J. Nutr. 2018, 57, 1925–1946. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Incani, A.; Atzeri, A.; Angioni, A.; Campus, M.; Cauli, E.; Zurru, R.; Deiana, M. Antioxidant Effect of Natural Table Olives Phenolic Extract Against Oxidative Stress and Membrane Damage in Enterocyte-Like Cells. J. Food Sci. 2017, 82, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Czerska, M.; Mikolajewska, K.; Zielinski, M.; Gromadzinska, J.; Wasowicz, W. Today’s oxidative stress markers. Med. Pracy 2015, 66, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, R.; Luna, G. Characterisation of monovarietal virgin olive oils. Eur. J. Lipid Sci. Tech. 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Fito, M.; Cladellas, M.; de la Torre, R.; Marti, J.; Munoz, D.; Schroder, H.; Alcantara, M.; Pujadas-Bastardes, M.; Marrugat, J.; Lopez-Sabater, M.C.; et al. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: A randomized, crossover, controlled trial. Eur. J. Clin. Nutr. 2008, 62, 570–574. [Google Scholar] [CrossRef]
- Ambra, R.; Natella, F.; Lucchetti, S.; Forte, V.; Pastore, G. alpha-Tocopherol, beta-carotene, lutein, squalene and secoiridoids in seven monocultivar Italian extra-virgin olive oils. Int. J. Food Sci. Nutr. 2017, 68, 538–545. [Google Scholar] [CrossRef]
- Del Coco, L.; De Pascali, S.A.; Fanizzi, F.P. (1)H NMR Spectroscopy and Multivariate Analysis of Monovarietal EVOOs as a Tool for Modulating Coratina-Based Blends. Foods 2014, 3, 238–249. [Google Scholar] [CrossRef]
- Lopez-Miranda, J.; Perez-Jimenez, F.; Ros, E.; De Caterina, R.; Badimon, L.; Covas, M.I.; Escrich, E.; Ordovas, J.M.; Soriguer, F.; Abia, R.; et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaen and Cordoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar]
- Njajou, O.T.; Kanaya, A.M.; Holvoet, P.; Connelly, S.; Strotmeyer, E.S.; Harris, T.B.; Cummings, S.R.; Hsueh, W.C. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab. Res. Rev. 2009, 25, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Roca, Y.; Bueno, H.; Fernandez-Ortiz, A.; Ordovas, J.M.; Ibanez, B.; Fuster, V.; Rodriguez-Artalejo, F.; Laclaustra, M. Oxidized LDL Is Associated with Metabolic Syndrome Traits Independently of Central Obesity and Insulin Resistance. Diabetes 2017, 66, 474–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruano, J.; Lopez-Miranda, J.; Fuentes, F.; Moreno, J.A.; Bellido, C.; Perez-Martinez, P.; Lozano, A.; Gomez, P.; Jimenez, Y.; Perez Jimenez, F. Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J. Am. Coll. Cardiol. 2005, 46, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Carbot, K.; Chavez-Servin, J.L.; Jauregui, O.; Castellote, A.I.; Lamuela-Raventos, R.M.; Nurmi, T.; Poulsen, H.E.; Gaddi, A.V.; Kaikkonen, J.; Zunft, H.F.; et al. Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL. J. Nutr. 2010, 140, 501–508. [Google Scholar] [CrossRef]
- Visioli, F.; Franco, M.; Toledo, E.; Luchsinger, J.; Willett, W.C.; Hu, F.B.; Martinez-Gonzalez, M.A. Olive oil and prevention of chronic diseases: Summary of an International conference. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 649–656. [Google Scholar] [CrossRef]
- Pitsavos, C.; Panagiotakos, D.; Weinem, M.; Stefanadis, C. Diet, exercise and the metabolic syndrome. Rev. Diabetes Stud. 2006, 3, 118–126. [Google Scholar] [CrossRef]
- Romaguera, D.; Norat, T.; Vergnaud, A.C.; Mouw, T.; May, A.M.; Agudo, A.; Buckland, G.; Slimani, N.; Rinaldi, S.; Couto, E.; et al. Mediterranean dietary patterns and prospective weight change in participants of the EPIC-PANACEA project. Am. J. Clin. Nutr. 2010, 92, 912–921. [Google Scholar] [CrossRef]
- Razquin, C.; Martinez, J.A.; Martinez-Gonzalez, M.A.; Mitjavila, M.T.; Estruch, R.; Marti, A. A 3 years follow-up of a Mediterranean diet rich in virgin olive oil is associated with high plasma antioxidant capacity and reduced body weight gain. Eur. J. Clin. Nutr. 2009, 63, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Fito, M.; Chiva-Blanch, G.; Fiol, M.; Gomez-Gracia, E.; Aros, F.; Lapetra, J.; et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, e6–e17. [Google Scholar] [CrossRef]
- Soriguer, F.; Almaraz, M.C.; Ruiz-de-Adana, M.S.; Esteva, I.; Linares, F.; Garcia-Almeida, J.M.; Morcillo, S.; Garcia-Escobar, E.; Olveira-Fuster, G.; Rojo-Martinez, G. Incidence of obesity is lower in persons who consume olive oil. Eur. J. Clin. Nutr. 2009, 63, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Bach, A.; Serra-Majem, L. Obesity and the Mediterranean diet: A systematic review of observational and intervention studies. Obes. Rev. 2008, 9, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Fu, J.; Astarita, G.; Li, X.; Gaetani, S.; Campolongo, P.; Cuomo, V.; Piomelli, D. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008, 8, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Due, A.; Larsen, T.M.; Hermansen, K.; Stender, S.; Holst, J.J.; Toubro, S.; Martinussen, T.; Astrup, A. Comparison of the effects on insulin resistance and glucose tolerance of 6-mo high-monounsaturated-fat, low-fat, and control diets. Am. J. Clin. Nutr. 2008, 87, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Cummings, S.J.; Mamo, J.C.; Kenrick, M.; Piers, L.S. The acute effects of olive oil v. cream on postprandial thermogenesis and substrate oxidation in postmenopausal women. Br. J. Nutr. 2004, 91, 245–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tortosa, A.; Bes-Rastrollo, M.; Sanchez-Villegas, A.; Basterra-Gortari, F.J.; Nunez-Cordoba, J.M.; Martinez-Gonzalez, M.A. Mediterranean diet inversely associated with the incidence of metabolic syndrome: The SUN prospective cohort. Diabetes Care 2007, 30, 2957–2959. [Google Scholar] [CrossRef]
- Romaguera, D.; Norat, T.; Mouw, T.; May, A.M.; Bamia, C.; Slimani, N.; Travier, N.; Besson, H.; Luan, J.; Wareham, N.; et al. Adherence to the Mediterranean diet is associated with lower abdominal adiposity in European men and women. J. Nutr. 2009, 139, 1728–1737. [Google Scholar] [CrossRef]
- Paniagua, J.A.; Gallego de la Sacristana, A.; Romero, I.; Vidal-Puig, A.; Latre, J.M.; Sanchez, E.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care 2007, 30, 1717–1723. [Google Scholar] [CrossRef]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Teno, C.; Perez-Martinez, P.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Garcia-Rios, A.; Marin, C.; Gomez, P.; Jimenez-Gomez, Y.; Camargo, A.; Rodriguez-Cantalejo, F.; et al. Dietary fat modifies the postprandial inflammatory state in subjects with metabolic syndrome: The LIPGENE study. Mol. Nutr. Food Res. 2012, 56, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gomez, Y.; Marin, C.; Peerez-Martinez, P.; Hartwich, J.; Malczewska-Malec, M.; Golabek, I.; Kiec-Wilk, B.; Cruz-Teno, C.; Rodriguez, F.; Gomez, P.; et al. A low-fat, high-complex carbohydrate diet supplemented with long-chain (n-3) fatty acids alters the postprandial lipoprotein profile in patients with metabolic syndrome. J. Nutr. 2010, 140, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, D.; Guevara, M.; Norat, T.; Langenberg, C.; Forouhi, N.G.; Sharp, S.; Slimani, N.; Schulze, M.B.; Buijsse, B.; Buckland, G.; et al. Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: The InterAct project. Diabetes Care 2011, 34, 1913–1918. [Google Scholar] [PubMed]
- Salas-Salvado, J.; Bullo, M.; Estruch, R.; Ros, E.; Covas, M.I.; Ibarrola-Jurado, N.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lasa, A.; Miranda, J.; Bullo, M.; Casas, R.; Salas-Salvado, J.; Larretxi, I.; Estruch, R.; Ruiz-Gutierrez, V.; Portillo, M.P. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. Eur. J. Clin. Nutr. 2014, 68, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; de la Fuente-Arrillaga, C.; Nunez-Cordoba, J.M.; Basterra-Gortari, F.J.; Beunza, J.J.; Vazquez, Z.; Benito, S.; Tortosa, A.; Bes-Rastrollo, M. Adherence to Mediterranean diet and risk of developing diabetes: Prospective cohort study. BMJ 2008, 336, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Lampousi, A.M.; Portillo, M.P.; Romaguera, D.; Hoffmann, G.; Boeing, H. Olive oil in the prevention and management of type 2 diabetes mellitus: A systematic review and meta-analysis of cohort studies and intervention trials. Nutr. Diabetes 2017, 7, e262. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Hu, F.B.; Martinez-Gonzalez, M.A.; Fito, M.; Bullo, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gomez-Gracia, E.; et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef]
- Buckland, G.; Mayen, A.L.; Agudo, A.; Travier, N.; Navarro, C.; Huerta, J.M.; Chirlaque, M.D.; Barricarte, A.; Ardanaz, E.; Moreno-Iribas, C.; et al. Olive oil intake and mortality within the Spanish population (EPIC-Spain). Am. J. Clin. Nutr. 2012, 96, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Travier, N.; Barricarte, A.; Ardanaz, E.; Moreno-Iribas, C.; Sanchez, M.J.; Molina-Montes, E.; Chirlaque, M.D.; Huerta, J.M.; Navarro, C.; et al. Olive oil intake and CHD in the European Prospective Investigation into Cancer and Nutrition Spanish cohort. Br. J. Nutr. 2012, 108, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Barzi, F.; Woodward, M.; Marfisi, R.M.; Tavazzi, L.; Valagussa, F.; Marchioli, R.; Investigators, G.I.-P. Mediterranean diet and all-causes mortality after myocardial infarction: Results from the GISSI-Prevenzione trial. Eur. J. Clin. Nutr. 2003, 57, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Dominguez, L.J.; Delgado-Rodriguez, M. Olive oil consumption and risk of CHD and/or stroke: A meta-analysis of case-control, cohort and intervention studies. Br. J. Nutr. 2014, 112, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef]
- Covas, M.I.; Nyyssonen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Baumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef]
- Reboredo-Rodriguez, P.; Varela-Lopez, A.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Bompadre, S.; Quiles, J.L.; et al. Phenolic Compounds Isolated from Olive Oil as Nutraceutical Tools for the Prevention and Management of Cancer and Cardiovascular Diseases. Int. J. Mol. Sci. 2018, 19, 2305. [Google Scholar] [CrossRef]
- Sales, N.M.; Pelegrini, P.B.; Goersch, M.C. Nutrigenomics: Definitions and advances of this new science. J. Nutr. Metab. 2014, 2014, 202759. [Google Scholar] [CrossRef]
- Wittwer, J.; Rubio-Aliaga, I.; Hoeft, B.; Bendik, I.; Weber, P.; Daniel, H. Nutrigenomics in human intervention studies: Current status, lessons learned and future perspectives. Mol. Nutr. Food Res. 2011, 55, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M.; et al. Nutrigenomics of extra-virgin olive oil: A review. Biofactors 2017, 43, 17–41. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, S.; Vacca, M.; Graziano, G.; D’Orazio, A.; Cariello, M.; Martelli, N.; Di Tullio, G.; Salvia, R.; Grandaliano, G.; Belfiore, A.; et al. Nuclear receptors expression chart in peripheral blood mononuclear cells identifies patients with Metabolic Syndrome. Biochim. Biophys. Acta 2013, 1832, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, E.; Sugimoto, M.; Kawaguchi, K.; Pu, F.; Uozumi, R.; Yamaguchi, A.; Nishie, M.; Tsuda, M.; Kotake, T.; Morita, S.; et al. Gene expression profile of peripheral blood mononuclear cells may contribute to the identification and immunological classification of breast cancer patients. Breast Cancer 2019, 26, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Castaner, O.; Corella, D.; Covas, M.I.; Sorli, J.V.; Subirana, I.; Flores-Mateo, G.; Nonell, L.; Bullo, M.; de la Torre, R.; Portoles, O.; et al. In vivo transcriptomic profile after a Mediterranean diet in high-cardiovascular risk patients: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 98, 845–853. [Google Scholar] [PubMed]
- Seo, D.; Ginsburg, G.S.; Goldschmidt-Clermont, P.J. Gene expression analysis of cardiovascular diseases: Novel insights into biology and clinical applications. J. Am. Coll. Cardiol. 2006, 48, 227–235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castaner, O.; Covas, M.I.; Khymenets, O.; Nyyssonen, K.; Konstantinidou, V.; Zunft, H.F.; de la Torre, R.; Munoz-Aguayo, D.; Vila, J.; Fito, M. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am. J. Clin. Nutr. 2012, 95, 1238–1244. [Google Scholar] [CrossRef]
- Martin-Pelaez, S.; Castaner, O.; Konstantinidou, V.; Subirana, I.; Munoz-Aguayo, D.; Blanchart, G.; Gaixas, S.; de la Torre, R.; Farre, M.; Saez, G.T.; et al. Effect of olive oil phenolic compounds on the expression of blood pressure-related genes in healthy individuals. Eur. J. Nutr. 2017, 56, 663–670. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.I.; Munoz-Aguayo, D.; Khymenets, O.; de la Torre, R.; Saez, G.; Tormos Mdel, C.; Toledo, E.; Marti, A.; Ruiz-Gutierrez, V.; et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: A randomized controlled trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef]
- Farras, M.; Valls, R.M.; Fernandez-Castillejo, S.; Giralt, M.; Sola, R.; Subirana, I.; Motilva, M.J.; Konstantinidou, V.; Covas, M.I.; Fito, M. Olive oil polyphenols enhance the expression of cholesterol efflux related genes in vivo in humans. A randomized controlled trial. J. Nutr. Biochem. 2013, 24, 1334–1339. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, E.; Morgano, A.; Peres, C.; Contursi, A.; Bertrand-Michel, J.; Arconzo, M.; Guillou, H.; Villani, G.; Moschetta, A. PGC-1alpha induced browning promotes involution and inhibits lactation in mammary glands. Cell Mol. Life Sci. 2019, 1–15. [Google Scholar] [CrossRef]
- Camargo, A.; Ruano, J.; Fernandez, J.M.; Parnell, L.D.; Jimenez, A.; Santos-Gonzalez, M.; Marin, C.; Perez-Martinez, P.; Uceda, M.; Lopez-Miranda, J.; et al. Gene expression changes in mononuclear cells in patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC Genom. 2010, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Rangel-Zuniga, O.A.; Haro, C.; Meza-Miranda, E.R.; Pena-Orihuela, P.; Meneses, M.E.; Marin, C.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Delgado-Lista, J.; et al. Olive oil phenolic compounds decrease the postprandial inflammatory response by reducing postprandial plasma lipopolysaccharide levels. Food Chem. 2014, 162, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Barwari, T.; Joshi, A.; Mayr, M. MicroRNAs in Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 68, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009, 4, 199–227. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.Y. Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol. 2012, 22, 125–132. [Google Scholar] [CrossRef]
- Allegra, A.; Alonci, A.; Campo, S.; Penna, G.; Petrungaro, A.; Gerace, D.; Musolino, C. Circulating microRNAs: New biomarkers in diagnosis, prognosis and treatment of cancer (review). Int. J. Oncol. 2012, 41, 1897–1912. [Google Scholar] [CrossRef]
- Afman, L.; Milenkovic, D.; Roche, H.M. Nutritional aspects of metabolic inflammation in relation to health--insights from transcriptomic biomarkers in PBMC of fatty acids and polyphenols. Mol. Nutr. Food Res. 2014, 58, 1708–1720. [Google Scholar] [CrossRef]
- Macfarlane, L.A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Raitoharju, E.; Lyytikainen, L.P.; Levula, M.; Oksala, N.; Mennander, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kahonen, M.; Karhunen, P.J.; et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis 2011, 219, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Jaeger, S.A.; Hirsch, H.A.; Bulyk, M.L.; Struhl, K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol. Cell 2010, 39, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Pirola, C.J.; Gianotti, T.F.; Castaño, G.O.; Mallardi, P.; San Martino, J.; Ledesma, M.M.G.L.; Flichman, D.; Mirshahi, F.; Sanyal, A.J.; Sookoian, S. Circulating microRNA signature in non-alcoholic fatty liver disease: From serum non-coding RNAs to liver histology and disease pathogenesis. Gut 2015, 64, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xu, Z.; Zhang, M.; Zuo, Y. MiR-19a promotes epithelial-mesenchymal transition through PI3K/AKT pathway in gastric cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 7286–7296. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, P.; Cascione, L.; Landi, L.; Carasi, S.; Lovat, F.; Tibaldi, C.; Ali, G.; D’Incecco, A.; Minuti, G.; Chella, A.; et al. microRNA classifiers are powerful diagnostic/prognostic tools in ALK-, EGFR-, and KRAS-driven lung cancers. Proc. Natl. Acad. Sci. USA 2015, 112, 14924–14929. [Google Scholar] [CrossRef]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011, 474, 649–653. [Google Scholar] [CrossRef]
- Zhu, S.; Pan, W.; Song, X.; Liu, Y.; Shao, X.; Tang, Y.; Liang, D.; He, D.; Wang, H.; Liu, W.; et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat. Med. 2012, 18, 1077–1086. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Kim, M.M.; Srikantan, S.; Mercken, E.M.; Brennan, S.E.; Wilson, G.M.; Cabo, R.; Gorospe, M. miR-519 suppresses tumor growth by reducing HuR levels. Cell Cycle 2010, 9, 1354–1359. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Srikantan, S.; Tominaga, K.; Kang, M.J.; Yaniv, Y.; Martindale, J.L.; Yang, X.; Park, S.S.; Becker, K.G.; Subramanian, M.; et al. Growth inhibition by miR-519 via multiple p21-inducing pathways. Mol. Cell Biol. 2012, 32, 2530–2548. [Google Scholar] [CrossRef]
- D’Amore, S.; Hardfeldt, J.; Cariello, M.; Graziano, G.; Copetti, M.; Di Tullio, G.; Piglionica, M.; Scialpi, N.; Sabba, C.; Palasciano, G.; et al. Identification of miR-9-5p as direct regulator of ABCA1 and HDL-driven reverse cholesterol transport in circulating CD14+ cells of patients with metabolic syndrome. Cardiovasc. Res. 2018, 114, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Ruiz-Canela, M.; Hruby, A.; Liang, L.; Trichopoulou, A.; Hu, F.B. Intervention Trials with the Mediterranean Diet in Cardiovascular Prevention: Understanding Potential Mechanisms through Metabolomic Profiling. J. Nutr. 2016, 146, 913S–919S. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Bronze, M.R.; Figueira, M.E.; Siwy, J.; Mischak, H.; Combet, E.; Mullen, W. Impact of a 6-wk olive oil supplementation in healthy adults on urinary proteomic biomarkers of coronary artery disease, chronic kidney disease, and diabetes (types 1 and 2): A randomized, parallel, controlled, double-blind study. Am. J. Clin. Nutr. 2015, 101, 44–54. [Google Scholar] [CrossRef] [PubMed]
| Trial Identifier | Trial Phase (Status) | Disease | Intervention |
|---|---|---|---|
| Obesity | |||
| NCT03101436 | Completed | Obesity | Dietary supplement: extra virgin olive oil and red wine |
| NCT03024359 | Recruiting | Obesity | Dietary supplement: extra virgin olive oil |
| NCT03441802 | Completed | Obesity | High quality extra virgin olive oil |
| NCT02463435 | Completed | Severe obesity | Behavioral: nutritional intervention Nutritional intervention plus olive oil Dietary supplement: olive oil |
| Diabetes and Hypertension | |||
| NCT03891927 | Not yet recruiting | Insulin resistance | Dietary supplement: extra virgin olive oil |
| NCT03447301 | Not yet recruiting | Type 2 diabetes mellitus | Dietary supplement: extra virgin olive oil (30 mL daily) |
| NCT02831803 | Completed | Hypertension | Dietary supplement: walnuts Dietary supplement: extra virgin olive oil |
| Cardiovascular diseases | |||
| NCT03528603 | Recruiting | Platelet aggregation Nutritional and metabolic disease Cardiovascular diseases | Oleocanthal-rich extra virgin olive oil Oleocanthal-low extra virgin olive oil |
| NCT03053843 | Recruiting | Atrial fibrillation Atrial arrhythmia | Dietary supplement: Mediterranean diet plus extra virgin olive oil |
| NCT03796780 | Recruiting | Cardiovascular risk factor | Dietary supplement: extra-virgin olive oil Dietary supplement: refined olive oil |
| NCT03105947 | Completed | Cardiovascular risk factor | Coconut oil Butter Olive oil |
| NCT03683134 | Completed | Cardiovascular Diseases Cardiovascular risk factor Obesity | Behavioral: Mediterranean diet Dietary supplement: olive oil and mixed nuts Behavioral: American Heart Association |
| NCT03005535 | Unknown | Atherosclerosis | Vitaminized corn oil Olive oil |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Santis, S.; Cariello, M.; Piccinin, E.; Sabbà, C.; Moschetta, A. Extra Virgin Olive Oil: Lesson from Nutrigenomics. Nutrients 2019, 11, 2085. https://doi.org/10.3390/nu11092085
De Santis S, Cariello M, Piccinin E, Sabbà C, Moschetta A. Extra Virgin Olive Oil: Lesson from Nutrigenomics. Nutrients. 2019; 11(9):2085. https://doi.org/10.3390/nu11092085
Chicago/Turabian StyleDe Santis, Stefania, Marica Cariello, Elena Piccinin, Carlo Sabbà, and Antonio Moschetta. 2019. "Extra Virgin Olive Oil: Lesson from Nutrigenomics" Nutrients 11, no. 9: 2085. https://doi.org/10.3390/nu11092085
APA StyleDe Santis, S., Cariello, M., Piccinin, E., Sabbà, C., & Moschetta, A. (2019). Extra Virgin Olive Oil: Lesson from Nutrigenomics. Nutrients, 11(9), 2085. https://doi.org/10.3390/nu11092085






