Comparison of the Utility of Total Plasma Fatty Acids Versus those in Cholesteryl Ester, Phospholipid, and Triglyceride as Biomarkers of Fatty Acid Intake
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection
2.3. Dietary Assessment
2.4. Fatty Acid Analyses
2.4.1. Lipid Extraction and Fractionation
2.4.2. Lipid Methylation and Peak Identification
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albert, C.M.; Campos, H.; Stampfer, M.J.; Ridker, P.M.; Manson, J.E.; Willett, W.C.; Ma, J. Blood Levels of Long-Chain n–3 Fatty Acids and the Risk of Sudden Death. N. Engl. J. Med. 2002, 346, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Attaman, J.A.; Toth, T.L.; Furtado, J.; Campos, H.; Hauser, R.; Chavarro, J.E. Dietary fat and semen quality among men attending a fertility clinic. Hum. Reprod. 2012, 27, 1466–1474. [Google Scholar] [CrossRef]
- Campos, H.; Baylin, A.; Willett, W.C. α-Linolenic acid and risk of nonfatal acute myocardial infarction. Circulation 2008, 118, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Otto, M.C.D.O.; Wu, J.H.Y.; Baylin, A.; Vaidya, D.; Rich, S.S.; Tsai, M.Y.; Jacobs, D.R.; Mozaffarian, D. Circulating and Dietary Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000506. [Google Scholar]
- Del Gobbo, L.C.; Imamura, F.; Aslibekyan, S.; Marklund, M.; Virtanen, J.K.; Wennberg, M.; Yakoob, M.Y.; Chiuve, S.E.; Dela Cruz, L.; Frazier-Wood, A.C.; et al. Omega-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies. JAMA Int. Med. 2016, 176, 1155–1166. [Google Scholar] [CrossRef]
- Marklund, M.; Wu, J.H.; Imamura, F.; Del Gobbo, L.C.; Fretts, A.; De Goede, J.; Shi, P.; Tintle, N.; Wennberg, M.; Aslibekyan, S.; et al. Biomarkers of Dietary Omega-6 Fatty Acids and Incident Cardiovascular Disease and Mortality. Circulation 2019, 139, 2422–2436. [Google Scholar] [CrossRef]
- Grindel, A.; Staps, F.; Kuhnt, K. Cheek cell fatty acids reflect n-3 PUFA in blood fractions during linseed oil supplementation: A controlled human intervention study. Lipids Health Dis. 2013, 12, 173. [Google Scholar] [CrossRef]
- Malik, V.S.; Chiuve, S.E.; Campos, H.; Rimm, E.B.; Mozaffarian, D.; Hu, F.B.; Sun, Q. Circulating Very-Long Chain Saturated Fatty Acids and Incident Coronary Heart Disease in U.S. Men and Women. Circulation 2015, 132, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ma, J.; Campos, H.; Hankinson, S.E.; Hu, F.B. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 2007, 86, 74–81. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, J.; Campos, H.; Hu, F.B. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am. J. Clin. Nutr. 2007, 86, 929–937. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, J.; Campos, H.; Rexrode, K.M.; Albert, C.M.; Mozaffarian, D.; Hu, F.B. Blood Levels of Individual Long-chain n-3 Fatty Acids and Risk of Nonfatal Myocardial Infarction. Am. J. Clin. Nutr. 2008, 88, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, M.Y.; Shi, P.; Hu, F.B.; Campos, H.; Rexrode, K.M.; Orav, E.J.; Willett, W.C.; Mozaffarian, D. Circulating biomarkers of dairy fat and risk of incident stroke in U.S. men and women in 2 large prospective cohorts. Am. J. Clin. Nutr. 2014, 100, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, M.Y.; Shi, P.; Willett, W.C.; Rexrode, K.M.; Campos, H.; Orav, E.J.; Hu, F.B.; Mozaffarian, D. Circulating Biomarkers of Dairy Fat and Risk of Incident Diabetes Mellitus Among US Men and Women in Two Large Prospective Cohorts. Circulation 2016, 133, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Plourde, M.; Stark, K.D.; Jones, P.J.; Lin, Y.-H. Best practices for the design, laboratory analysis, and reporting of trials involving fatty acids. Am. J. Clin. Nutr. 2018, 108, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: Oxford, UK, 2013; Volume 40. [Google Scholar]
- Pranger, I.G.; Corpeleijn, E.; Muskiet, F.A.J.; Kema, I.P.; Singh-Povel, C.; Bakker, S.J.L. Circulating fatty acids as biomarkers of dairy fat intake: Data from the lifelines biobank and cohort study. Biomarkers 2019, 24, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Pranger, I.G.; Joustra, M.L.; Corpeleijn, E.; Muskiet, F.A.J.; Kema, I.P.; Oude Elferink, S.; Singh-Povel, C.; Bakker, S.J.L. Fatty acids as biomarkers of total dairy and dairy fat intakes: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 46–63. [Google Scholar] [CrossRef]
- Garcia-Aloy, M.; Hulshof, P.J.M.; Estruel-Amades, S.; Osté, M.C.J.; Lankinen, M.; Geleijnse, J.M.; De Goede, J.; Ulaszewska, M.; Mattivi, F.; Bakker, S.J.L.; et al. Biomarkers of food intake for nuts and vegetable oils: An extensive literature search. Genes Nutr. 2019, 14, 7. [Google Scholar] [CrossRef]
- Chung, H.; Nettleton, J.A.; Lemaitre, R.N.; Barr, R.G.; Tsai, M.Y.; Tracy, R.P.; Siscovick, D.S. Frequency and Type of Seafood Consumed Influence Plasma (n-3) Fatty Acid Concentrations. J. Nutr. 2008, 138, 2422–2427. [Google Scholar] [CrossRef]
- Raatz, S.K.; Rosenberger, T.A.; Johnson, L.K.; Wolters, W.W.; Burr, G.S.; Picklo, M.J. Dose-dependent consumption of farmed Atlantic salmon (Salmo salar) increases plasma phospholipid n-3 fatty acids differentially. J. Acad. Nutr. Diet. 2013, 113, 282–287. [Google Scholar] [CrossRef]
- Baylin, A.; Kim, M.K.; Donovan-Palmer, A.; Siles, X.; Dougherty, L.; Tocco, P.; Campos, H. Fasting Whole Blood as a Biomarker of Essential Fatty Acid Intake in Epidemiologic Studies: Comparison with Adipose Tissue and Plasma. Am. J. Epidemiol. 2005, 162, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Dayton, S.; Hashimoto, S.; Dixon, W.; Pearce, M.L. Composition of lipids in human serum and adipose tissue during prolonged feeding of a diet high in unsaturated fat. J. Lipid Res. 1966, 7, 103–111. [Google Scholar] [PubMed]
- Katan, M.B.; Deslypere, J.P.; Van Birgelen, A.P.; Penders, M.; Zegwaard, M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: An 18-month controlled study. J. Lipid Res. 1997, 38, 2012–2022. [Google Scholar] [PubMed]
- Baylin, A.; Campos, H. The use of fatty acid biomarkers to reflect dietary intake. Curr. Opin. Lipidol. 2006, 17, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kabagambe, E.K.; Baylin, A.; Allan, D.A.; Siles, X.; Spiegelman, D.; Campos, H. Application of the Method of Triads to Evaluate the Performance of Food Frequency Questionnaires and Biomarkers as Indicators of Long-term Dietary Intake. Am. J. Epidemiol. 2001, 154, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Campos, H.; Siles, X. Siesta and the risk of coronary heart disease: Results from a population-based, case-control study in Costa Rica. Int. J. Epidemiol. 2000, 29, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Beynen, A.C.; Katan, M.B. Rapid sampling and long-term storage of subcutaneous adipose-tissue biopsies for determination of fatty acid composition. Am. J. Clin. Nutr. 1985, 42, 317–322. [Google Scholar] [CrossRef]
- Baylin, A.; Kabagambe, E.K.; Siles, X.; Campos, H. Adipose tissue biomarkers of fatty acid intake. Am. J. Clin. Nutr. 2002, 76, 750–757. [Google Scholar] [CrossRef]
- Kabagambe, E.K.; Baylin, A.; Ascherio, A.; Campos, H. The type of oil used for cooking is associated with the risk of nonfatal acute myocardial infarction in Costa Rica. J. Nutr. 2005, 135, 2674–2679. [Google Scholar] [CrossRef][Green Version]
- Agren, J.J.; Julkunen, A.; Penttilä, I. Rapid separation of serum lipids for fatty acid analysis by a single aminopropyl column. J. Lipid Res. 1992, 33, 1871–1876. [Google Scholar]
- Burdge, G.C.; Wright, P.; Jones, A.E.; Wootton, S.A. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. Br. J. Nutr. 2000, 84, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Firl, N.; Kienberger, H.; Hauser, T.; Rychlik, M. Determination of the fatty acid profile of neutral lipids, free fatty acids and phospholipids in human plasma. Clin. Chem. Lab. Med. 2013, 51, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Louis-Ferdinand, R.T.; Therriault, D.G.; Blatt, W.F.; Mager, M. Application of thin-layer chromatography to the quantitation of plasma neutral lipids and free fatty acids. Clin. Chem. 1967, 13, 773–787. [Google Scholar] [PubMed]
- Mangold, H.K.; Malins, D.C. Fractionation of fats, oils, and waxes on thin layers of silicic acid. J. Am. Oil Chem. Soc. 1960, 37, 383–385. [Google Scholar] [CrossRef]
- Schlierf, G.; Wood, P. Quantitative determination of plasma free fatty acids and triglycerides by thin-layer chromatography. J. Lipid Res. 1965, 6, 317–319. [Google Scholar] [PubMed]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef] [PubMed]
- Zock, P.L.; Gerritsen, J.; Katan, M.B. Partial conservation of the sn-2 position of dietary triglycerides in fasting plasma lipids in humans. Eur. J. Clin. Investig. 1996, 26, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zock, P.L.; Mensink, R.P.; Harryvan, J.; De Vries, J.H.M.; Katan, M.B. Fatty Acids in Serum Cholesteryl Esters as Quantitative Biomarkers of Dietary Intake in Humans. Am. J. Epidemiol. 1997, 145, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Lillington, J.M.; Trafford, D.; Makin, H. A rapid and simple method for the esterification of fatty acids and steroid carboxylic acids prior to gas-liquid chromatography. Clin. Chim. Acta 1981, 111, 91–98. [Google Scholar] [CrossRef]
- Van Birgelen, A.; Deslypere, J.P.; Fenders, M.; Katan, M.B.; Van Staveren, W.A. Biological Markers of Dietary Intake, with Emphasis on Fatty Acids. Ann. Nutr. Metab. 1991, 35, 249–252. [Google Scholar]
- Arab, L.; Akbar, J. Biomarkers and the measurement of fatty acids. Public Health Nutr. 2002, 5, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Bjermo, H.; Kullberg, J.; Johansson, L.; Michaelsson, K.; Ahlstrom, H.; Lind, L.; Riserus, U. Fatty acid composition in serum cholesterol esters and phospholipids is linked to visceral and subcutaneous adipose tissue content in elderly individuals: A cross-sectional study. Lipids Health Dis. 2017, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Eyles, H.C.; McLachlan, K.J.; Bell, M.L.; Green, T.J.; Skeaff, C.M. Plasma and erythrocyte fatty acids reflect intakes of saturated and n-6 PUFA within a similar time frame. J. Nutr. 2014, 144, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.; Farquhar, J.W.; Ahrens, E.H.; Peterson, M.L.; Stoffel, W. Studies of adipose tissue in man. A microtechnic for sampling and analysis. Am. J. Clin. Nutr. 1960, 8, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, M.; Uusitupa, M.; Schwab, U. Genes and Dietary Fatty Acids in Regulation of Fatty Acid Composition of Plasma and Erythrocyte Membranes. Nutrients 2018, 10, 1785. [Google Scholar] [CrossRef] [PubMed]
- Hellmuth, C.; Demmelmair, H.; Schmitt, I.; Peissner, W.; Blüher, M.; Koletzko, B. Association between Plasma Nonesterified Fatty Acids Species and Adipose Tissue Fatty Acid Composition. PLoS ONE 2013, 8, e74927. [Google Scholar] [CrossRef] [PubMed]
- Yli-Jama, P.; Haugen, T.S.; Rebnord, H.M.; Ringstad, J.; Pedersen, J.I. Selective mobilisation of fatty acids from human adipose tissue. Eur. J. Int. Med. 2001, 12, 107–115. [Google Scholar] [CrossRef]
- Walker, C.G.; Browning, L.M.; Stecher, L.; West, A.L.; Madden, J.; Jebb, S.A.; Calder, P.C. Fatty acid profile of plasma NEFA does not reflect adipose tissue fatty acid profile. Br. J. Nutr. 2015, 114, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.M.; Trimbo, S.L.; Mascioli, E.A.; Blackburn, G.L. Human plasma fatty acid variations and how they are related to dietary intake. Am. J. Clin. Nutr. 1991, 53, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Asciutti-Moura, L.S.; Guilland, J.C.; Fuchs, F.; Richard, D.; Klepping, J. Fatty acid composition of serum lipids and its relation to diet in an elderly institutionalized population. Am. J. Clin. Nutr. 1988, 48, 980–987. [Google Scholar] [CrossRef] [PubMed]
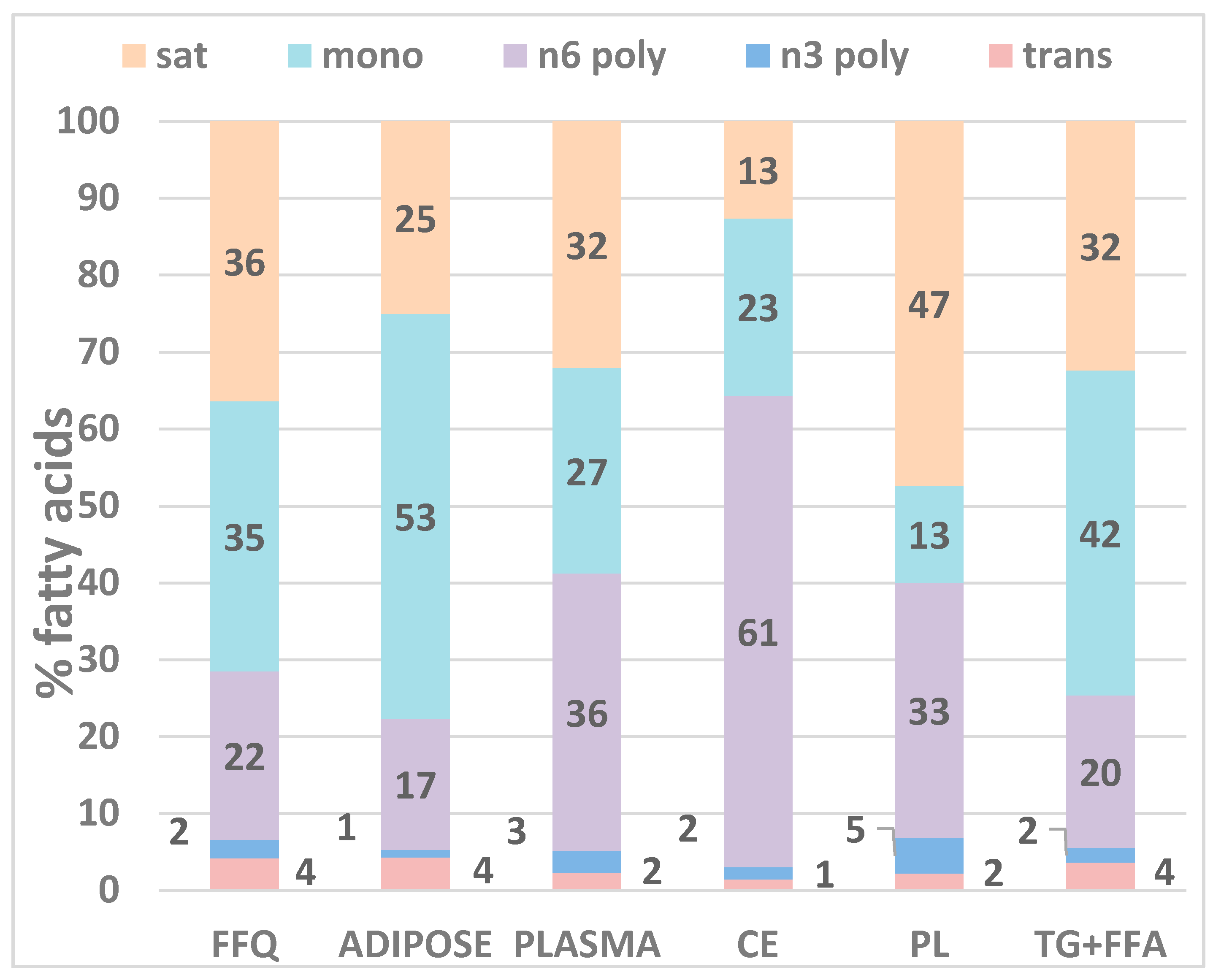
| Carbon Name | Common Name | Total | Plasma Fractions | ||||
|---|---|---|---|---|---|---|---|
| FFQ | Adipose | Plasma | CE | PL | TG + FFA | ||
| saturated | 36.5 (35.5, 37.5) | 25.0 (24.5, 25.5) | 32.1 (31.7, 32.5) | 12.6 (12.3, 12.9) | 47.4 (46.8, 47.9) | 32.4 (31.8, 32.9) | |
| 13:0 | 0.02 (0.01, 0.02) | 0.01 (0.00, 0.01) | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.01) | 0.00 | |
| 14:0 | Myristic Acid | 2.88 (2.70, 3.05) | 1.14 (1.07, 1.20) | 0.60 (0.56, 0.63) | 0.23 (0.21, 0.25) | 0.14 (0.13, 0.15) | 0.87 (0.82, 0.93) |
| 15:0 | 0.31 (0.30, 0.33) | 0.22 (0.21, 0.23) | 0.16 (0.15, 0.17) | 0.10 (0.09, 0.10) | 0.12 (0.11, 0.13) | 0.19 (0.18, 0.20) | |
| 16:0 | Palmitic Acid | 23.7 (22.9, 24.4) | 20.6 (20.2, 21.0) | 22.0 (21.7, 22.4) | 10.2 (10.0, 10.4) | 25.0 (24.4, 25.5) | 24.4 (23.9, 24.8) |
| 17:0 | Margaric Acid | 0.33 (0.32, 0.35) | 0.20 (0.20, 0.21) | 0.31 (0.30, 0.32) | 0.16 (0.15, 0.18) | 0.44 (0.42, 0.45) | 0.40 (0.39, 0.42) |
| 18:0 | Stearic Acid | 7.84 (7.64, 8.04) | 2.65 (2.53, 2.78) | 7.56 (7.43, 7.68) | 1.72 (1.57, 1.87) | 17.4 (17.0, 17.7) | 6.20 (5.98, 6.42) |
| 19:0 | 0.08 (0.08, 0.09) | 0.09 (0.09, 0.10) | 0.09 (0.08, 0.09) | 0.09 (0.07, 0.10) | 0.14 (0.13, 0.16) | 0.11 (0.11, 0.12) | |
| 20:0 | Arachidic Acid | 0.21 (0.21, 0.22) | 0.09 (0.08, 0.10) | 0.20 (0.19, 0.20) | 0.08 (0.07, 0.10) | 0.54 (0.52, 0.56) | 0.12 (0.11, 0.13) |
| 22:0 | Behenic Acid | 0.10 (0.09, 0.11) | 0.00 | 0.47 (0.45, 0.49) | 0.03 (0.02, 0.03) | 1.50 (1.44, 1.57) | 0.04 (0.04, 0.05) |
| 23:0 | 0.00 | 0.00 | 0.23 (0.22, 0.24) | 0.00 (0.00, 0.01) | 0.77 (0.73, 0.81) | 0.02 (0.02, 0.02) | |
| 24:0 | Lignoceric Acid | 0.00 | 0.02 (0.02, 0.03) | 0.42 (0.40, 0.44) | 0.01 (0.00, 0.01) | 1.43 (1.34, 1.52) | 0.06 (0.05, 0.07) |
| monounsaturated | 34.9 (33.8, 36.0) | 52.6 (52.0, 53.1) | 26.7 (26.2, 27.2) | 23.0 (22.4, 23.6) | 12.6 (12.3, 13.0) | 42.2 (41.7, 42.8) | |
| 14:1n-5c | Myristoleic Acid | 0.21 (0.20, 0.22) | 0.18 (0.17, 0.20) | 0.03 (0.03, 0.04) | 0.03 (0.03, 0.04) | 0.03 (0.02, 0.03) | 0.06 (0.05, 0.06) |
| 15:1n-5c | 0.00 | 0.00 | 0.01 (0.01, 0.01) | 0.04 (0.03, 0.06) | 0.06 (0.04, 0.08) | 0.02 (0.02, 0.03) | |
| 16:1n-7c | Palmitoleic Acid | 1.41 (1.36, 1.47) | 6.75 (6.45, 7.04) | 2.42 (2.29, 2.55) | 2.67 (2.49, 2.85) | 0.47 (0.43, 0.50) | 3.34 (3.19, 3.50) |
| 18:1n-9c | Oleic Acid | 31.7 (30.7, 32.8) | 42.9 (42.5, 43.3) | 21.6 (21.2, 22.0) | 18.9 (18.4, 19.4) | 8.70 (8.41, 8.99) | 35.7 (35.2, 36.2) |
| 18:1n-7c | 1.36 (1.32, 1.41) | 2.29 (2.22, 2.35) | 1.82 (1.77, 1.86) | 1.29 (1.26, 1.33) | 1.37 (1.33, 1.41) | 2.66 (2.59, 2.73) | |
| 20:1n-9c | Gondoic Acid | 0.17 (0.16, 0.18) | 0.43 (0.42, 0.45) | 0.17 (0.17, 0.18) | 0.02 (0.02, 0.02) | 0.18 (0.17, 0.18) | 0.33 (0.32, 0.34) |
| 24:1n-9c | Nervonic Acid | 0.01 (0.00, 0.01) | 0.00 | 0.61 (0.58, 0.64) | 0.06 (0.06, 0.07) | 1.82 (1.75, 1.90) | 0.13 (0.09, 0.17) |
| omega-3 polyunsaturated | 2.41 (2.29, 2.52) | 0.98 (0.95, 1.01) | 2.76 (2.65, 2.87) | 1.58 (1.50, 1.66) | 4.64 (4.44, 4.84) | 1.89 (1.79, 1.98) | |
| 18:3n-3c | Alpha-linolenic Acid (ALA) | 1.95 (1.85, 2.05) | 0.63 (0.61, 0.66) | 0.49 (0.47, 0.52) | 0.43 (0.41, 0.44) | 0.16 (0.15, 0.17) | 0.84 (0.80, 0.89) |
| 20:5n-3c | EPA | 0.15 (0.13, 0.18) | 0.02 (0.02, 0.03) | 0.38 (0.35, 0.41) | 0.53 (0.48, 0.58) | 0.50 (0.46, 0.54) | 0.17 (0.15, 0.19) |
| 22:5n-3c | DPA | 0.08 (0.07, 0.08) | 0.19 (0.18, 0.20) | 0.42 (0.40, 0.44) | 0.04 (0.03, 0.04) | 0.83 (0.79, 0.87) | 0.32 (0.29, 0.34) |
| 22:6n-3c | DHA | 0.23 (0.20, 0.26) | 0.14 (0.13, 0.15) | 1.47 (1.40, 1.54) | 0.58 (0.55, 0.61) | 3.16 (3.01, 3.31) | 0.56 (0.52, 0.60) |
| omega-6 polyunsaturated | 21.9 (20.9, 23.0) | 17.1 (16.6, 17.6) | 36.1 (35.4, 36.8) | 61.3 (60.6, 62.1) | 33.1 (32.6, 33.7) | 19.8 (19.1, 20.5) | |
| 18:2n-6c | Linoleic Acid (LA) | 21.5 (20.5, 22.5) | 15.8 (15.3, 16.2) | 27.7 (27.1, 28.3) | 52.0 (51.3, 52.7) | 19.1 (18.6, 19.6) | 17.4 (16.7, 18.0) |
| 18:3n-6c | Gamma-linolenic Acid (GLA) | 0.04 (0.03, 0.04) | 0.05 (0.05, 0.05) | 0.38 (0.36, 0.40) | 0.87 (0.82, 0.92) | 0.11 (0.09, 0.12) | 0.30 (0.28, 0.32) |
| 20:2n-6c | 0.05 (0.04, 0.05) | 0.24 (0.23, 0.25) | 0.27 (0.26, 0.28) | 0.04 (0.04, 0.05) | 0.40 (0.39, 0.42) | 0.30 (0.29, 0.31) | |
| 20:3n-6c | Dihomogammalinolenic Acid (DHGLA) | 0.04 (0.04, 0.04) | 0.33 (0.32, 0.35) | 1.75 (1.70, 1.81) | 1.03 (0.99, 1.07) | 3.74 (3.61, 3.87) | 0.40 (0.38, 0.42) |
| 20:4n-6c | Arachidonic Acid (AA) | 0.26 (0.25, 0.28) | 0.48 (0.46, 0.50) | 5.76 (5.52, 6.01) | 7.31 (6.96, 7.65) | 9.26 (8.92, 9.61) | 1.22 (1.16, 1.28) |
| 22:2n-6c | 0.00 | 0.01 (0.01, 0.01) | 0.02 (0.02, 0.02) | 0.02 (0.02, 0.02) | 0.05 (0.04, 0.05) | 0.02 (0.01, 0.02) | |
| 22:4n-6c | Adrenic Acid | 0.04 (0.03, 0.04) | 0.21 (0.20, 0.22) | 0.25 (0.24, 0.26) | 0.03 (0.03, 0.03) | 0.48 (0.47, 0.50) | 0.22 (0.21, 0.23) |
| trans fatty acids | 4.19 (3.94, 4.43) | 4.33 (4.21, 4.45) | 2.35 (2.28, 2.42) | 1.45 (1.41, 1.50) | 2.21 (2.14, 2.28) | 3.66 (3.55, 3.78) | |
| 14:1n-5t | Myristelaidic Acid | 0.00 | 0.00 | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.01) | 0.05 (0.04, 0.06) | 0.00 |
| 16:1n-7t | Palmitelaidic Acid | 0.19 (0.18, 0.21) | 0.17 (0.16, 0.17) | 0.12 (0.12, 0.13) | 0.07 (0.06, 0.07) | 0.15 (0.14, 0.16) | 0.11 (0.11, 0.12) |
| 18:1-trans | 2.38 (2.26, 2.51) | 1.96 (1.87, 2.05) | 1.02 (0.96, 1.07) | 0.33 (0.32, 0.35) | 1.02 (0.98, 1.07) | 1.84 (1.75, 1.94) | |
| 18:2-trans | 1.24 (1.12, 1.35) | 1.10 (1.06, 1.14) | 0.55 (0.54, 0.57) | 0.56 (0.53, 0.58) | 0.50 (0.47, 0.52) | 0.80 (0.77, 0.82) | |
| 18:2n-7c | c9, t11 conjugated linoleic acid (CLA) | 0.33 (0.31, 0.36) | 0.54 (0.42, 0.66) | 0.25 (0.24, 0.26) | 0.20 (0.19, 0.22) | 0.16 (0.15, 0.17) | 0.39 (0.37, 0.40) |
| 20:1n-9t | 0.04 (0.03, 0.05) | 0.02 (0.01, 0.02) | 0.00 | 0.00 | 0.01 (0.01, 0.02) | 0.00 | |
| Panel A | |||||
|---|---|---|---|---|---|
| Carbon Name | Common Name | Correlation with FFQ | |||
| Plasma | CE | PL | TG + FFA | ||
| saturated | 0.23 (0.09, 0.36) | 0.19 (0.04, 0.32) | 0.12 (−0.02, 0.26) | 0.27 (0.13, 0.40) | |
| 13:0 | −0.12 (−0.26, 0.02) | −0.02 (−0.17, 0.12) | 0.03 (−0.12, 0.17) | −0.05 (−0.19, 0.10) | |
| 14:0 | Myristic Acid | 0.07 (−0.07, 0.21) | −0.01 (−0.15, 0.13) | 0.03 (−0.11, 0.17) | 0.02 (−0.13, 0.16) |
| 15:0 | 0.23 (0.09, 0.36) | 0.16 (0.01, 0.29) | 0.15 (0.01, 0.29) | 0.20 (0.06, 0.33) | |
| 16:0 | Palmitic Acid | 0.30 (0.16, 0.42) | 0.26 (0.12, 0.39) | 0.24 (0.10, 0.37) | 0.31 (0.18, 0.44) |
| 17:0 | Margaric Acid | 0.06 (−0.08, 0.20) | 0.09 (−0.05, 0.23) | 0.02 (−0.12, 0.16) | 0.10 (−0.04, 0.24) |
| 18:0 | Stearic Acid | 0.05 (−0.09, 0.19) | 0.03 (−0.11, 0.17) | −0.01 (−0.15, 0.14) | 0.05 (−0.10, 0.19) |
| 19:0 | 0.10 (−0.05, 0.24) | 0.02 (−0.13, 0.16) | 0.03 (−0.11, 0.17) | 0.05 (−0.10, 0.19) | |
| 20:0 | Arachidic Acid | 0.08 (−0.06, 0.22) | 0.04 (−0.10, 0.18) | 0.02 (−0.12, 0.16) | 0.03 (−0.11, 0.17) |
| 22:0 | Behenic Acid | 0.04 (−0.10, 0.18) | 0.05 (−0.09, 0.19) | 0.12 (−0.02, 0.26) | 0.09 (−0.05, 0.23) |
| 23:0 | 0.11 (−0.04, 0.24) | −0.14 (−0.28, 0.00) | 0.09 (−0.05, 0.23) | 0.07 (−0.07, 0.21) | |
| 24:0 | Lignoceric Acid | . | . | . | . |
| monounsaturated | 0.14 (0.00, 0.28) | 0.10 (−0.05, 0.24) | 0.17 (0.03, 0.30) | 0.23 (0.09, 0.36) | |
| 14:1n-5c | Myristoleic Acid | 0.13 (−0.02, 0.26) | −0.06 (−0.20, 0.08) | −0.01 (−0.15, 0.13) | 0.07 (−0.07, 0.21) |
| 15:1n-5c | . | . | . | . | |
| 16:1n-7c | Palmitoleic Acid | 0.05 (−0.09, 0.19) | 0.05 (−0.1, 0.19) | 0.03 (−0.12, 0.17) | 0.02 (−0.13, 0.16) |
| 18:1n-9c | Oleic Acid | 0.19 (0.05, 0.32) | 0.12 (−0.02, 0.26) | 0.19 (0.05, 0.32) | 0.28 (0.15, 0.41) |
| 18:1n-7c | −0.06 (−0.20, 0.09) | −0.09 (−0.23, 0.05) | −0.01 (−0.15, 0.14) | −0.06 (−0.20, 0.08) | |
| 20:1n-9c | Gondoic Acid | 0.10 (−0.05, 0.23) | −0.09 (−0.23, 0.06) | 0.00 (−0.14, 0.14) | 0.08 (−0.06, 0.22) |
| 24:1n-9c | Nervonic Acid | −0.00 (−0.14, 0.14) | −0.00 (−0.14, 0.14) | 0.00 (−0.14, 0.14) | −0.08 (−0.22, 0.06) |
| omega-3 polyunsaturated | 0.27 (0.13, 0.39) | 0.28 (0.15, 0.41) | 0.22 (0.09, 0.35) | 0.34 (0.21, 0.46) | |
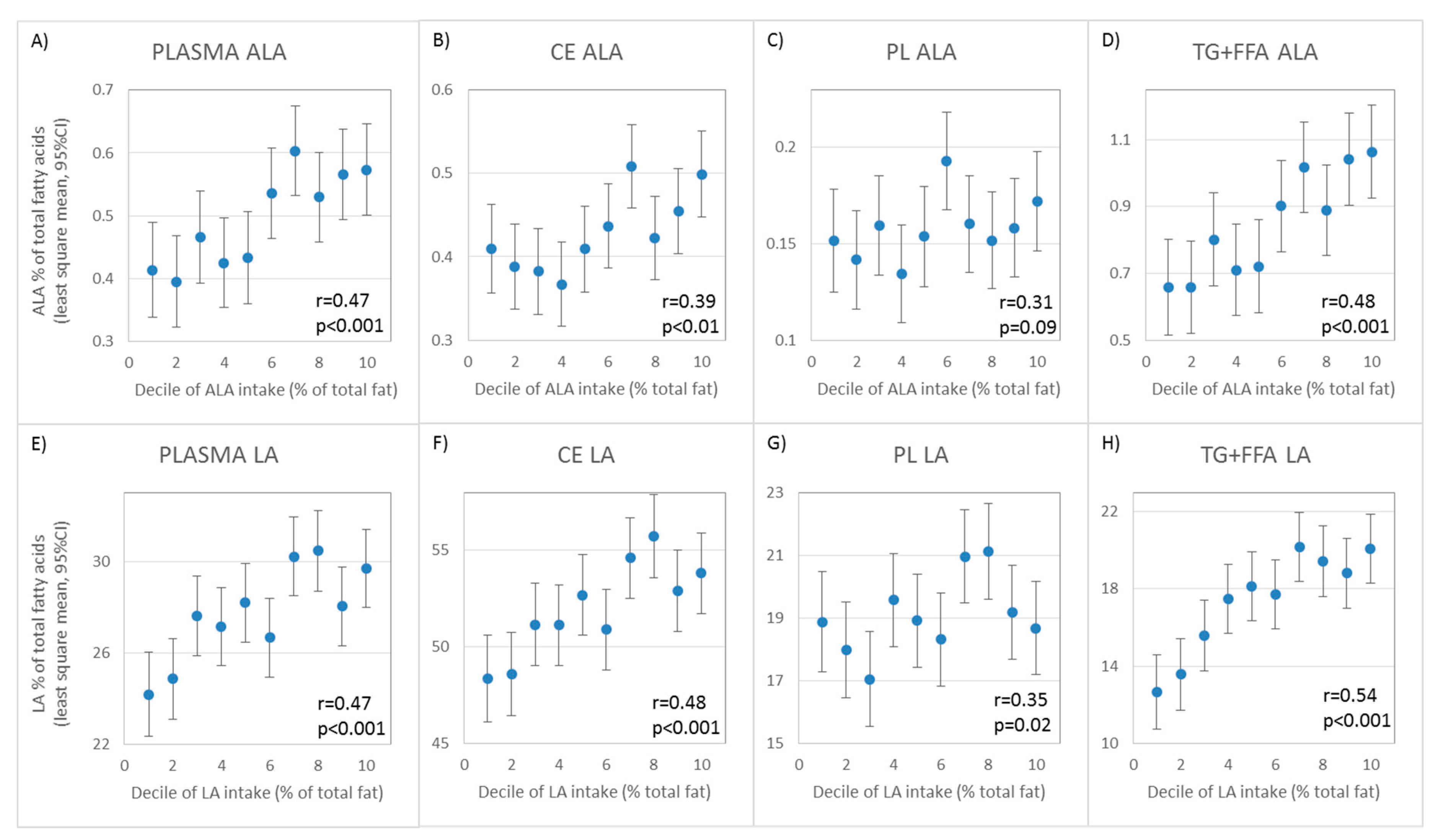
| 18:3n-3c | Alpha-linolenic Acid (ALA) | 0.41 (0.29, 0.52) | 0.31 (0.18, 0.44) | 0.15 (0.01, 0.28) † | 0.45 (0.33, 0.56) |
| 20:5n-3c | EPA | 0.24 (0.11, 0.37) | 0.26 (0.13, 0.39) | 0.28 (0.15, 0.41) | 0.14 (−0.00, 0.27) |
| 22:5n-3c | DPA | 0.10 (−0.04, 0.24) | −0.02 (−0.16, 0.13) | 0.13 (−0.01, 0.27) | 0.12 (−0.03, 0.25) |
| 22:6n-3c | DHA | 0.21 (0.08, 0.35) | 0.21 (0.07, 0.34) | 0.24 (0.10, 0.37) | 0.19 (0.05, 0.33) |
| omega-6 polyunsaturated | 0.36 (0.23, 0.47) | 0.40 (0.35, 0.57) | 0.24 (0.10, 0.37) | 0.47 (0.35, 0.57) | |
| 18:2n-6c | Linoleic Acid (LA) | 0.36 (0.23, 0.48) | 0.35 (0.22, 0.47) | 0.17 (0.03, 0.30) | 0.49 (0.37, 0.59) |
| 18:3n-6c | Gamma-linolenic Acid (GLA) | 0.07 (−0.07, 0.21) | 0.03 (−0.11, 0.17) | 0.05 (−0.09, 0.19) | 0.09 (−0.05, 0.23) |
| 20:2n-6c | −0.11 (−0.25, 0.03) | −0.01 (−0.16, 0.13) | −0.07 (−0.21, 0.07) | −0.05 (−0.19, 0.09) | |
| 20:3n-6c | Dihomogammalinolenic Acid (DHGLA) | −0.14 (−0.27, 0.01) | −0.13 (−0.27, 0.01) | −0.12 (−0.26, 0.02) | −0.12 (−0.26, 0.02) |
| 20:4n-6c | Arachidonic Acid (AA) | 0.14 (−0.01, 0.27) | 0.15 (0.01, 0.29) | 0.20 (0.06, 0.33) | 0.13 (−0.02, 0.26) |
| 22:2n-6c | . | . | . | . | |
| 22:4n-6c | Adrenic Acid | 0.02 (−0.12, 0.16) | 0.02 (−0.12, 0.16) | 0.02 (−0.12, 0.16) | 0.03 (−0.11, 0.17) |
| trans fatty acids | 0.28 (0.14, 0.40) | 0.36 (0.22, 0.47) | 0.22 (0.08, 0.35) | 0.21 (0.07, 0.34) | |
| 14:1n-5t | Myristelaidic Acid | . | . | . | . |
| 16:1n-7t | Palmitelaidic Acid | 0.15 (0.01, 0.29) | 0.14 (0.00, 0.28) | 0.17 (0.03, 0.31) | 0.20 (0.06, 0.33) |
| 18:1-trans | 0.20 (0.06, 0.34) | 0.23 (0.09, 0.36) | 0.18 (0.04, 0.31) | 0.16 (0.01, 0.29) | |
| 18:2-trans | 0.45 (0.32, 0.55) | 0.34 (0.21, 0.46) | 0.31 (0.17, 0.43) | 0.43 (0.30, 0.53) | |
| 18:2n-7c | c9, t11 conjugated linoleic acid (CLA) | −0.01 (−0.15, 0.14) | −0.01 (−0.16, 0.13) | 0.04 (−0.11, 0.18) | 0.08 (−0.06, 0.22) |
| 20:1n-9t | 0.29 (0.16, 0.42) | 0.25 (0.11, 0.38) | 0.20 (0.11, 0.38) | 0.21 (0.07, 0.34) | |
| Panel B | |||||
| Carbon Name | Common Name | Correlation with Adipose | |||
| Plasma | CE | PL | TG + FFA | ||
| saturated | 0.34 (0.21, 0.46) | 0.12 (−0.02, 0.26) | 0.17 (0.02, 0.30) | 0.38 (0.25, 0.50) | |
| 13:0 | −0.14 (−0.27, 0.00) | 0.11 (−0.03, 0.25) | −0.03 (−0.17, 0.11) | −0.02 (−0.16, 0.13) | |
| 14:0 | Myristic Acid | 0.17 (0.03, 0.30) | 0.24 (0.10, 0.37) | 0.06 (−0.08, 0.20) | 0.09 (−0.05, 0.23) |
| 15:0 | 0.41 (0.28, 0.52) | 0.35 (0.22, 0.47) | 0.22 (0.08, 0.35) | 0.39 (0.26, 0.50) | |
| 16:0 | Palmitic Acid | 0.40 (0.27, 0.51) | 0.18 (0.04, 0.31) | 0.26 (0.13, 0.39) | 0.46 (0.34, 0.57) |
| 17:0 | Margaric Acid | 0.40 (0.28, 0.51) | 0.13 (−0.02, 0.26) † | 0.28 (0.14, 0.41) | 0.32 (0.18, 0.44) |
| 18:0 | Stearic Acid | 0.09 (−0.05, 0.23) | 0.01 (−0.13, 0.15) | −0.07 (−0.21, 0.08) | 0.22 (0.08, 0.35) |
| 19:0 | 0.30 (0.16, 0.42) | 0.16 (0.02, 0.30) | 0.07 (−0.08, 0.21) | 0.24 (0.10, 0.37) | |
| 20:0 | Arachidic Acid | 0.02 (−0.13, 0.16) | 0.16 (0.01, 0.29) | −0.01 (−0.16, 0.13) | 0.29 (0.15, 0.41) |
| 22:0 | Behenic Acid | −0.05 (−0.19, 0.10) | −0.06 (−0.20, 0.09) | 0.06 (−0.09, 0.20) | −0.06 (−0.20, 0.08) |
| 23:0 | −0.08 (−0.22, 0.06) | 0.11 (−0.03, 0.25) | 0.16 (0.02, 0.29) | 0.10 (−0.05, 0.23) | |
| 24:0 | Lignoceric Acid | . | . | . | . |
| monounsaturated | 0.65 (0.56, 0.72) | 0.51 (0.39, 0.61) | 0.37 (0.24, 0.49) † | 0.52 (0.41, 0.62) | |
| 14:1n-5c | Myristoleic Acid | 0.14 (−0.01, 0.27) | 0.11 (−0.03, 0.25) | 0.18 (0.04, 0.31) | 0.12 (−0.02, 0.26) |
| 15:1n-5c | 0.10 (−0.05, 0.23) | 0.02 (−0.12, 0.16) | 0.04 (−0.10, 0.18) | 0.09 (−0.05, 0.23) | |
| 16:1n-7c | Palmitoleic Acid | 0.38 (0.25, 0.49) | 0.34 (0.20, 0.46) | 0.29 (0.15, 0.41) | 0.43 (0.31, 0.54) |
| 18:1n-9c | Oleic Acid | 0.42 (0.30, 0.53) | 0.43 (0.31, 0.54) | 0.45 (0.33, 0.55) | 0.48 (0.36, 0.58) |
| 18:1n-7c | 0.40 (0.28, 0.51) | 0.30 (0.17, 0.43) | 0.25 (0.11, 0.38) | 0.41 (0.28, 0.52) | |
| 20:1n-9c | Gondoic Acid | 0.23 (0.09, 0.36) | −0.10 (−0.24, 0.04) † | 0.10 (−0.04, 0.24) | 0.22 (0.07, 0.35) |
| 24:1n-9c | Nervonic Acid | 0.09 (−0.05, 0.23) | 0.02 (−0.12, 0.16) | 0.02 (−0.13, 0.16) | 0.04 (−0.10, 0.18) |
| omega-3 polyunsaturated | 0.41 (0.28, 0.52) | 0.33 (0.19, 0.45) | 0.29 (0.15, 0.42) | 0.54 (0.42, 0.63) | |
| 18:3n-3c | Alpha-linolenic Acid (ALA) | 0.55 (0.44, 0.64) | 0.34 (0.21, 0.46) | 0.26 (0.12, 0.39) † | 0.68 (0.60, 0.75) |
| 20:5n-3c | EPA | 0.28 (0.15, 0.41) | 0.40 (0.27, 0.51) | 0.24 (0.10, 0.37) | 0.31 (0.18, 0.44) |
| 22:5n-3c | DPA | 0.29 (0.15, 0.41) | 0.11 (−0.03, 0.25) | 0.27 (0.13, 0.40) | 0.34 (0.21, 0.46) |
| 22:6n-3c | DHA | 0.38 (0.25, 0.49) | 0.29 (0.16, 0.42) | 0.40 (0.27, 0.51) | 0.47 (0.35, 0.57) |
| omega-6 polyunsaturated | 0.57 (0.46, 0.65) | 0.56 (0.46, 0.65) | 0.27 (0.13, 0.40) † | 0.67 (0.58, 0.74) | |
| 18:2n-6c | Linoleic Acid (LA) | 0.62 (0.53, 0.70) | 0.57 (0.46, 0.66) | 0.27 (0.14, 0.40) † | 0.70 (0.61, 0.76) |
| 18:3n-6c | Gamma-linolenic Acid (GLA) | 0.23 (0.09, 0.36) | 0.20 (0.06, 0.33) | 0.18 (0.04, 0.31) | 0.28 (0.14, 0.40) |
| 20:2n-6c | 0.35 (0.22, 0.47) | −0.02 (−0.17, 0.12) † | 0.28 (0.15, 0.41) | 0.30 (0.16, 0.42) | |
| 20:3n-6c | Dihomogammalinolenic Acid (DHGLA) | 0.26 (0.12, 0.38) | 0.29 (0.15, 0.41) | 0.30 (0.17, 0.43) | 0.19 (0.05, 0.32) |
| 20:4n-6c | Arachidonic Acid (AA) | 0.37 (0.24, 0.49) | 0.37 (0.24, 0.49) | 0.42 (0.30, 0.53) | 0.31 (0.18, 0.43) |
| 22:2n-6c | −0.08 (−0.22, 0.06) | 0.06 (−0.08, 0.20) | 0.01 (−0.13, 0.15) | −0.02 (−0.16, 0.13) | |
| 22:4n-6c | Adrenic Acid | 0.22 (0.08, 0.35) | −0.03 (−0.17, 0.11) | 0.17 (0.03, 0.30) | 0.25 (0.11, 0.37) |
| trans fatty acids | 0.54 (0.43, 0.63) | 0.34 (0.21, 0.46) | 0.40 (0.28, 0.52) | 0.54 (0.43, 0.63) | |
| 14:1n-5t | Myristelaidic Acid | 0.01 (−0.14, 0.15) | 0.12 (−0.03, 0.25) | −0.09 (−0.23, 0.06) | 0.03 (−0.11, 0.18) |
| 16:1n-7t | Palmitelaidic Acid | 0.39 (0.26, 0.50) | 0.17 (0.03, 0.31) | 0.29 (0.15, 0.41) | 0.25 (0.11, 0.38) |
| 18:1-trans | 0.45 (0.33, 0.56) | 0.37 (0.24, 0.49) | 0.38 (0.25, 0.49) | 0.47 (0.35, 0.57) | |
| 18:2-trans | 0.52 (0.40, 0.61) | 0.27 (0.13, 0.39) † | 0.21 (0.07, 0.34) † | 0.61 (0.51, 0.69) | |
| 18:2n-7c | c9, t11 conjugated linoleic acid (CLA) | 0.01 (−0.14, 0.15) | 0.02 (−0.12, 0.16) | −0.06 (−0.20, 0.08) | −0.06 (−0.20, 0.08) |
| 20:1n-9t | 0.36 (0.23, 0.47) | 0.27 (0.13, 0.40) | 0.19 (0.05, 0.32) | 0.43 (0.31, 0.54) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furtado, J.D.; Beqari, J.; Campos, H. Comparison of the Utility of Total Plasma Fatty Acids Versus those in Cholesteryl Ester, Phospholipid, and Triglyceride as Biomarkers of Fatty Acid Intake. Nutrients 2019, 11, 2081. https://doi.org/10.3390/nu11092081
Furtado JD, Beqari J, Campos H. Comparison of the Utility of Total Plasma Fatty Acids Versus those in Cholesteryl Ester, Phospholipid, and Triglyceride as Biomarkers of Fatty Acid Intake. Nutrients. 2019; 11(9):2081. https://doi.org/10.3390/nu11092081
Chicago/Turabian StyleFurtado, Jeremy D., Jorind Beqari, and Hannia Campos. 2019. "Comparison of the Utility of Total Plasma Fatty Acids Versus those in Cholesteryl Ester, Phospholipid, and Triglyceride as Biomarkers of Fatty Acid Intake" Nutrients 11, no. 9: 2081. https://doi.org/10.3390/nu11092081
APA StyleFurtado, J. D., Beqari, J., & Campos, H. (2019). Comparison of the Utility of Total Plasma Fatty Acids Versus those in Cholesteryl Ester, Phospholipid, and Triglyceride as Biomarkers of Fatty Acid Intake. Nutrients, 11(9), 2081. https://doi.org/10.3390/nu11092081






