Health Benefits of β-Hydroxy-β-Methylbutyrate (HMB) Supplementation in Addition to Physical Exercise in Older Adults: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
- Participants: Studies of participants aged 50 or older. We made no restrictions of participants’ gender, health and socio-economic status, ethnicity or geographical area. We emphasized searching for studies including people with frailty, sarcopenia, cachexia, or muscle weakness.
- Intervention: Any intervention combining physical exercise in addition to HMB oral supplementation. We considered every exercise activity requiring increased energy output without taking into account frequency or intensity. We considered any HMB dosage, supplementation form (powders, pills, nutritional drink) and nature (free acid or enriched).
- Comparators: Participants not provided with HMB supplementation (controls or placebo).
- Outcomes: Clinical outcomes on physical and cognitive health, including (but not restricted to) changes in physical function, muscular strength, body composition, cognitive impairment, and quality of life.
- Study designs: Randomized controlled trials (RCT) were included in order to determine if the HMB oral supplementation (investigational treatment) was more effective than a control or placebo group when provided during a physical exercise program. The comprehensive search of RCT was set to identify gaps in the current evidence.
2.3. Data Collection
2.4. Quality Assessment and Risk of Bias
2.5. Data Analysis
3. Results
3.1. Characteristics of Studies
3.2. Quality of Studies and Risk of Bias
3.3. Studies’ Outcomes and Results
3.4. Meta-Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Travers, J.; Romero-Ortuno, R.; Bailey, J.; Cooney, M.T. Delaying and reversing frailty: A systematic review of primary care interventions. Br. J. Gen. Pract. 2019, 69, e61–e69. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, N.R.; Izquierdo, M.; Higginson, I.J.; Harridge, S.D.R. Exercise Deficiency Diseases of Ageing: The Primacy of Exercise and Muscle Strengthening as First-Line Therapeutic Agents to Combat Frailty. J. Am. Med. Dir. Assoc. 2018, 19, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Sáez de Asteasu, M.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Casas-Herrero, Á.; Izquierdo, M. Role of physical exercise on cognitive function in healthy older adults: A systematic review of randomized clinical trials. Ageing Res. Rev. 2017, 37, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Velilla, N.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Sáez de Asteasu, M.L.; Lucia, A.; Galbete, A.; García-Baztán, A.; Alonso-Renedo, J.; González-Glaría, B.; Gonzalo-Lázaro, M.; et al. Effect of Exercise Intervention on Functional Decline in Very Elderly Patients During Acute Hospitalization. JAMA Intern. Med. 2019, 179, 28. [Google Scholar] [CrossRef] [PubMed]
- Tarazona-Santabalbina, F.J.; Gómez-Cabrera, M.C.; Pérez-Ros, P.; Martínez-Arnau, F.M.; Cabo, H.; Tsaparas, K.; Salvador-Pascual, A.; Rodriguez-Mañas, L.; Viña, J. A Multicomponent Exercise Intervention that Reverses Frailty and Improves Cognition, Emotion, and Social Networking in the Community-Dwelling Frail Elderly: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2016, 17, 426–433. [Google Scholar] [CrossRef] [PubMed]
- García-Molina, R.; Ruíz-Grao, M.C.; Noguerón-García, A.; Martínez-Reig, M.; Esbrí-Víctor, M.; Izquierdo, M.; Abizanda, P. Benefits of a multicomponent Falls Unit-based exercise program in older adults with falls in real life. Exp. Gerontol. 2018, 110, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodriguez-Mañas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age 2014, 36, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Sáez de Asteasu, M.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Casas-Herrero, Á.; Cadore, E.L.; Galbete, A.; Izquierdo, M. Assessing the impact of physical exercise on cognitive function in older medical patients during acute hospitalization: Secondary analysis of a randomized trial. PLoS Med. 2019, 16, e1002852. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Rodriguez-Mañas, L.; Casas-Herrero, A.; Martinez-Velilla, N.; Cadore, E.L.; Sinclair, A.J. Is It Ethical Not to Precribe Physical Activity for the Elderly Frail? J. Am. Med. Dir. Assoc. 2016, 17, 779–781. [Google Scholar] [CrossRef] [PubMed]
- De Souto Barreto, P.; Demougeot, L.; Vellas, B.; Rolland, Y. How much exercise are older adults living in nursing homes doing in daily life? A cross-sectional study. J. Sports Sci. 2015, 33, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Valencia, M.; Izquierdo, M.; Lacalle-Fabo, E.; Marín-Epelde, I.; Ramón-Espinoza, M.F.; Domene-Domene, T.; Casas-Herrero, Á.; Galbete, A.; Martínez-Velilla, N. Relationship between frailty, polypharmacy, and underprescription in older adults living in nursing homes. Eur. J. Clin. Pharmacol. 2018, 74, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Casas-Herrero, A.; Cadore, E.L.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Martínez-Ramirez, A.; Gómez, M.; Rodriguez-Mañas, L.; Marcellán, T.; de Gordoa, A.R.; et al. Functional Capacity, Muscle Fat Infiltration, Power Output, and Cognitive Impairment in Institutionalized Frail Oldest Old. Rejuvenation Res. 2013, 16, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Sáez de Asteasu, M.L.; Izquierdo, M. Multicomponent exercise and the hallmarks of frailty: Considerations on cognitive impairment and acute hospitalization. Exp. Gerontol. 2019, 122, 10–14. [Google Scholar] [CrossRef]
- Hörder, H.; Johansson, L.; Guo, X.; Grimby, G.; Kern, S.; Östling, S.; Skoog, I. Midlife cardiovascular fitness and dementia: A 44-year longitudinal population study in women. Neurology 2018, 90, e1298–e1305. [Google Scholar] [CrossRef] [PubMed]
- Kossioni, A.E. The Association of Poor Oral Health Parameters with Malnutrition in Older Adults: A Review Considering the Potential Implications for Cognitive Impairment. Nutrients 2018, 10, 1709. [Google Scholar] [CrossRef] [PubMed]
- Crichton, M.; Craven, D.; Mackay, H.; Marx, W.; de van der Schueren, M.; Marshall, S. A systematic review, meta-analysis and meta-regression of the prevalence of protein-energy malnutrition: Associations with geographical region and sex. Age Ageing 2018, 48, 38–48. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’Angelo, E.; Sisto, A.; Marzetti, E. Protein intake and muscle health in old age: From biological plausibility to clinical evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef]
- Doorduijn, A.S.; Visser, M.; van de Rest, O.; Kester, M.I.; de Leeuw, F.A.; Boesveldt, S.; Fieldhouse, J.L.P.; van den Heuvel, E.G.H.M.; Teunissen, C.E.; Scheltens, P.; et al. Associations of AD Biomarkers and Cognitive Performance with Nutritional Status: The NUDAD Project. Nutrients 2019, 11, 1161. [Google Scholar] [CrossRef]
- Meijers, J.M.M.; Halfens, R.J.G.; Wilson, L.; Schols, J.M.G.A. Estimating the costs associated with malnutrition in Dutch nursing homes. Clin. Nutr. 2012, 31, 65–68. [Google Scholar] [CrossRef]
- Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef]
- Nissen, S.L.; Abumrad, N.N. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J. Nutr. Biochem. 1997, 8, 300–311. [Google Scholar] [CrossRef]
- Holecek, M.; Muthny, T.; Kovarik, M.; Sispera, L. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem. Toxicol. 2009, 47, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xia, Y.; Jiang, J.; Du, H.; Guo, X.; Liu, X.; Li, C.; Huang, G.; Niu, K. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2015, 61, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Bear, D.E.; Langan, A.; Dimidi, E.; Wandrag, L.; Harridge, S.D.R.; Hart, N.; Connolly, B.; Whelan, K. Beta-hydroxy-beta-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 109, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Munroe, M.; Mahmassani, Z.S.; Dvoretskiy, S.; Reid, J.J.; Miller, B.F.; Hamilton, K.; Rhodes, J.S.; Boppart, M.D. Cognitive function is preserved in aged mice following long-term β-hydroxy-β-methylbutyrate supplementation. Nutr. Neurosci. 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hankosky, E.R.; Sherrill, L.K.; Ruvola, L.A.; Haake, R.M.; Kim, T.; Hammerslag, L.R.; Kougias, D.G.; Juraska, J.M.; Gulley, J.M. Effects of β-hydroxy-β-methyl butyrate on working memory and cognitive flexibility in an animal model of aging. Nutr. Neurosci. 2017, 20, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kougias, D.G.; Das, T.; Perez, A.B.; Pereira, S.L. A role for nutritional intervention in addressing the aging neuromuscular junction. Nutr. Res. 2018, 53, 1–14. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Picca, A.; Marzetti, E. Beta-hydroxy-beta-methylbutyrate and sarcopenia: From biological plausibility to clinical evidence. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 37–43. [Google Scholar] [CrossRef]
- Shreeram, S.; Ramesh, S.; Puthan, J.K.; Balakrishnan, G.; Subramanian, R.; Reddy, M.T.; Pereira, S.L. Age associated decline in the conversion of leucine to β-hydroxy-β-methylbutyrate in rats. Exp. Gerontol. 2016, 80, 6–11. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Health Care; University of York: York, UK, 2009; ISBN 9781900640473. [Google Scholar]
- Higgins, J.P.T.; Sterne, J.A.C.; Savovic, J.; Page, M.J.; Hróbjartsson, A.; Boutron, I.; Reeves, B.; Eldridge, S. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst. Rev. 2016, 10, 29–31. [Google Scholar] [CrossRef]
- Tipton, E. Small sample adjustments for robust variance estimation with meta-regression. Psychol. Methods 2015, 20, 375. [Google Scholar] [CrossRef]
- Hedges, L.V.; Tipton, E.; Johnson, M.C. Robust variance estimation in meta-regression with dependent effect size estimates. Res. Synth. Methods 2010, 1, 39–65. [Google Scholar] [CrossRef]
- Olveira, G.; Olveira, C.; Doña, E.; Palenque, F.J.; Porras, N.; Dorado, A.; Godoy, A.M.; Rubio-Martínez, E.; Rojo-Martínez, G.; Martín-Valero, R. Oral supplement enriched in HMB combined with pulmonary rehabilitation improves body composition and health related quality of life in patients with bronchiectasis (Prospective, Randomised Study). Clin. Nutr. 2016, 35, 1015–1022. [Google Scholar] [CrossRef]
- Berton, L.; Bano, G.; Carraro, S.; Veronese, N.; Pizzato, S.; Bolzetta, F.; De Rui, M.; Valmorbida, E.; De Ronch, I.; Perissinotto, E.; et al. Effect of Oral Beta-hydroxy-Beta-methylbutyrate (HMB) Supplementation on Physical Performance in Healthy Old Women Over 65 Years: An Open Label Randomized Controlled Trial. PLoS ONE 2015, 10, e0141757. [Google Scholar] [CrossRef]
- Din, U.S.U.; Brook, M.S.; Selby, A.; Quinlan, J.; Boereboom, C.; Abdullah, H.; Franchi, M.; Narici, M.V.; Phillips, B.E.; Williams, J.W.; et al. A double-blind placebo controlled trial into the impacts of HMB supplementation and exercise on free-living muscle protein synthesis, muscle mass and function, in older adults. Clin. Nutr. 2018. [Google Scholar] [CrossRef]
- Standley, R.A.; Distefano, G.; Pereira, S.L.; Tian, M.; Kelly, O.J.; Coen, P.M.; Deutz, N.E.P.; Wolfe, R.R.; Goodpaster, B.H. Effects of β-hydroxy-β-methylbutyrate on skeletal muscle mitochondrial content and dynamics, and lipids after 10 days of bed rest in older adults. J. Appl. Physiol. 2017, 123, 1092–1100. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Pereira, S.L.; Hays, N.P.; Oliver, J.S.; Edens, N.K.; Evans, C.M.; Wolfe, R.R. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin. Nutr. 2013, 32, 704–712. [Google Scholar] [CrossRef]
- Vukovich, M.D.; Stubbs, N.B.; Bohlken, R.M. Body composition in 70-year-old adults responds to dietary beta-hydroxy-beta-methylbutyrate similarly to that of young adults. J. Nutr. 2001, 131, 2049–2052. [Google Scholar] [CrossRef]
- Stout, J.R.; Fukuda, D.H.; Kendall, K.L.; Smith-Ryan, A.E.; Moon, J.R.; Hoffman, J.R. Beta-hydroxy-beta-methylbutyrate (HMB) supplementation and resistance exercise significantly reduce abdominal adiposity in healthy elderly men. Exp. Gerontol. 2015, 64, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.R.; Smith-Ryan, A.E.; Fukuda, D.H.; Kendall, K.L.; Moon, J.R.; Hoffman, J.R.; Wilson, J.M.; Oliver, J.S.; Mustad, V.A. Effect of calcium β-hydroxy-β-methylbutyrate (CaHMB) with and without resistance training in men and women 65+ yrs: A randomized, double-blind pilot trial. Exp. Gerontol. 2013, 48, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Malafarina, V.; Uriz-Otano, F.; Malafarina, C.; Martinez, J.A.; Zulet, M.A. Effectiveness of nutritional supplementation on sarcopenia and recovery in hip fracture patients. A multi-centre randomized trial. Maturitas 2017, 101, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.; Sharp, R.L.; Panton, L.; Vukovich, M.; Trappe, S.; Fuller, J.C. Beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J. Nutr. 2000, 130, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The Effect of beta-hydroxy-beta-methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review. J. Nutr. Heal. Aging 2019, 23, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Paris, A.; Camprubi-Robles, M.; Lopez-Pedrosa, J.M.; Pereira, S.L.; Rueda, R.; Ballesteros-Pomar, M.D.; Garcia Almeida, J.M.; Cruz-Jentoft, A.J. Role of Oral Nutritional Supplements Enriched with B-hydroxy-B-Methylbutyrate in Maintaining Muscle Function and Improving Clinical Outcomes in Various Clinical Settings. J. Nutr. Heal. Aging 2018, 22, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults. Position Statement from the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Lopez, P.; Pinto, R.S.; Radaelli, R.; Rech, A.; Grazioli, R.; Izquierdo, M.; Cadore, E.L. Benefits of resistance training in physically frail elderly: A systematic review. Aging Clin. Exp. Res. 2018, 30, 889–899. [Google Scholar] [CrossRef]
- Ekinci, O.; Yanik, S.; Terzioglu Bebitoglu, B.; Yilmaz Akyuz, E.; Dokuyucu, A.; Erdem, S. Effect of Calcium beta-hydroxy-beta-methylbutyrate (CaHMB), Vitamin D, and Protein Supplementation on Postoperative Immobilization in Malnourished Older Adult Patients with Hip Fracture: A Randomized Controlled Study. Nutr. Clin. Pract. 2016, 31, 829–835. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nagatsuma, Y.; Fukuda, Y.; Hirao, M.; Nishikawa, K.; Miyamoto, A.; Ikeda, M.; Nakamori, S.; Sekimoto, M.; Fujitani, K.; et al. Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer 2017, 20, 913–918. [Google Scholar] [CrossRef]
- Eley, H.L.; Russell, S.T.; Baxter, J.H.; Mukerji, P.; Tisdale, M.J. Signaling pathways initiated by β-hydroxy-β-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am. J. Physiol. Metab. 2007, 293, E923–E931. [Google Scholar] [CrossRef] [PubMed]
- Eley, H.L.; Russell, S.T.; Tisdale, M.J. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by β-hydroxy-β-methylbutyrate. Am. J. Physiol. Metab. 2008, 295, E1409–E1416. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, D.; Marco, E.; Ronquillo-Moreno, N.; Miralles, R.; Mojal, S.; Vázquez-Ibar, O.; Escalada, F.; Muniesa, J.M. The PSSMAR study. Postacute sarcopenia: Supplementation with β-hydroxy-β-methylbutyrate after resistance training: Study protocol of a randomized, double-blind controlled trial. Maturitas 2016, 94, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Courel-Ibáñez, J.; Pallarés, J.G. Effects of β-hydroxy-β-methylbutyrate (HMB) supplementation in addition to multicomponent exercise in adults older than 70 years living in nursing homes, a cluster randomized placebo-controlled trial: The HEAL study protocol. BMC Geriatr. 2019, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Osuka, Y.; Kojima, N.; Wakaba, K.; Miyauchi, D.; Tanaka, K.; Kim, H. Effects of resistance training and/or beta-hydroxy-beta-methylbutyrate supplementation on muscle mass, muscle strength and physical performance in older women with reduced muscle mass: Protocol for a randomised, double-blind, placebo-controlled trial. BMJ Open 2019, 9, e025723. [Google Scholar] [CrossRef] [PubMed]
- Santos-Fandila, A.; Zafra-Gómez, A.; Barranco, A.; Navalón, A.; Rueda, R.; Ramírez, M. Quantitative determination of β-hydroxy-β-methylbutyrate and leucine in culture media and microdialysates from rat brain by UHPLC-tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2863–2872. [Google Scholar] [CrossRef] [PubMed]
- Kougias, D.G.; Hankosky, E.R.; Gulley, J.M.; Juraska, J.M. Beta-hydroxy-beta-methylbutyrate (HMB) ameliorates age-related deficits in water maze performance, especially in male rats. Physiol. Behav. 2017, 170, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Kougias, D.G.; Nolan, S.O.; Koss, W.A.; Kim, T.; Hankosky, E.R.; Gulley, J.M.; Juraska, J.M. Beta-hydroxy-beta-methylbutyrate ameliorates aging effects in the dendritic tree of pyramidal neurons in the medial prefrontal cortex of both male and female rats. Neurobiol. Aging 2016, 40, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Salto, R.; Vílchez, J.D.; Girón, M.D.; Cabrera, E.; Campos, N.; Manzano, M.; Rueda, R.; López-Pedrosa, J.M. β-hydroxy-β-methylbutyrate (HMB) Promotes Neurite Outgrowth in Neuro2a Cells. PLoS ONE 2015, 10, e0135614. [Google Scholar] [CrossRef] [PubMed]
| Section/Topic | Item | Checklist Item | Page |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 2 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 2 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 4 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 3 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 3 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 4 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 4 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 5 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 5 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 5 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 5 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 5 |
| RESULTS | |||
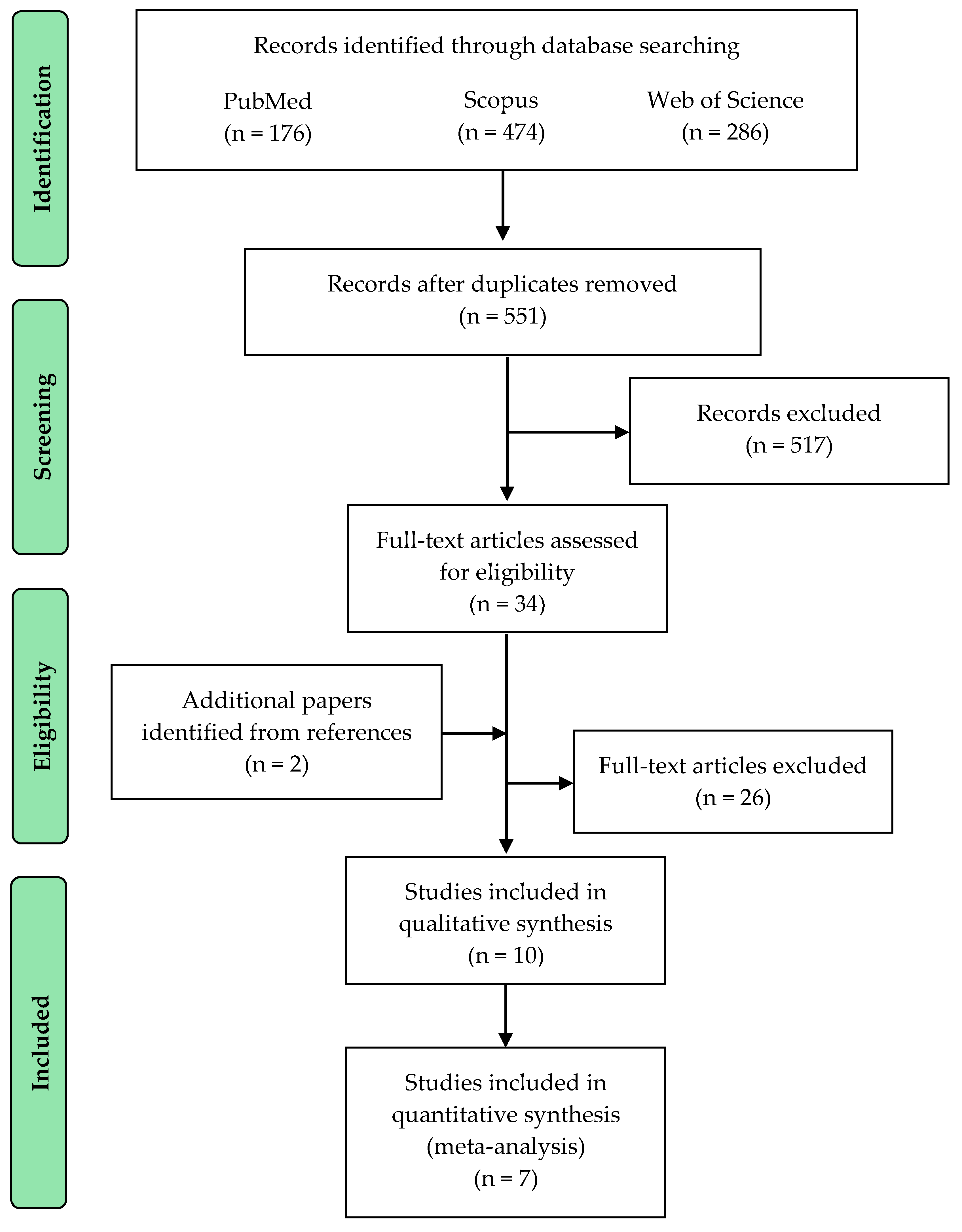
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 6 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 7 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 6 |
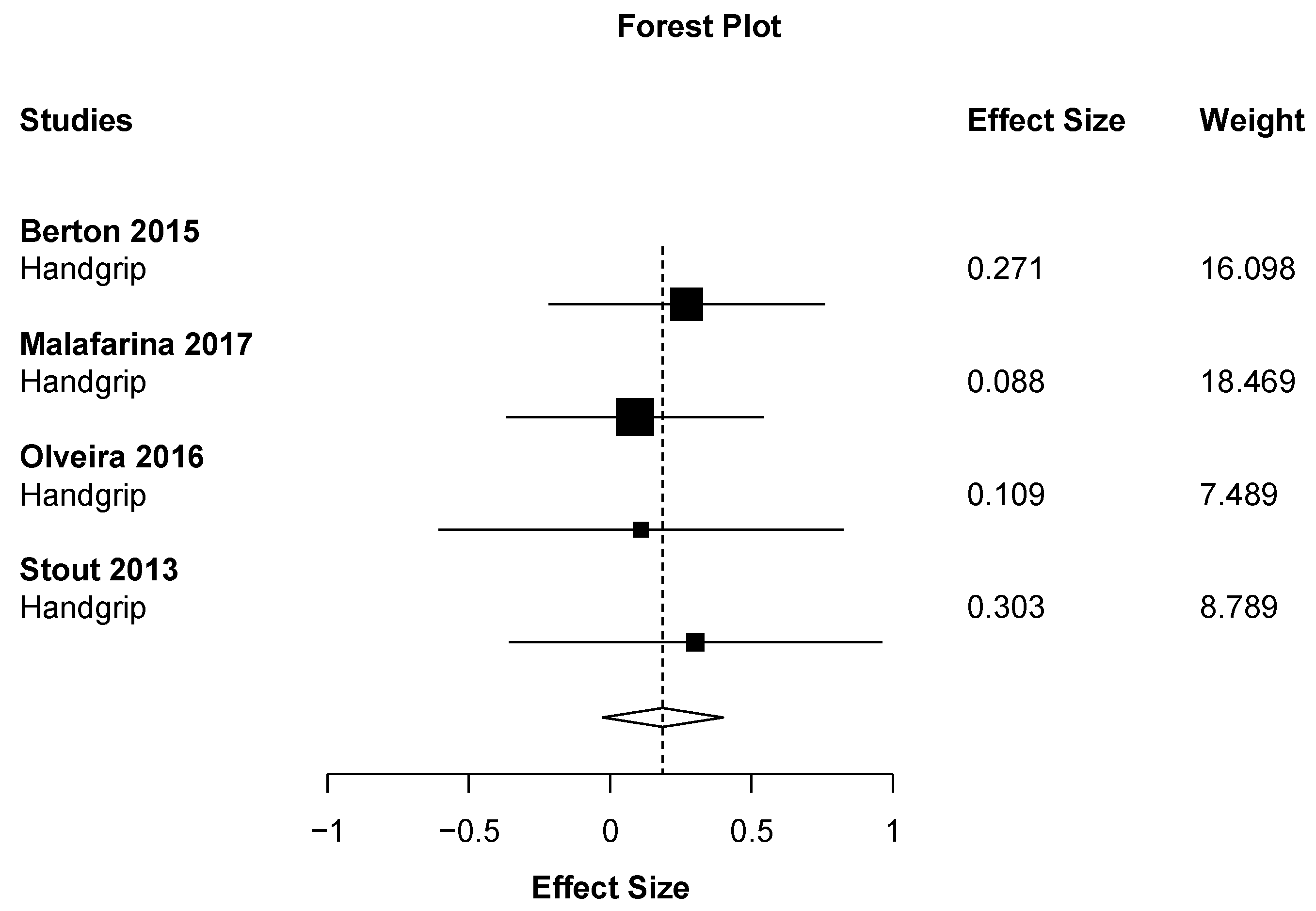
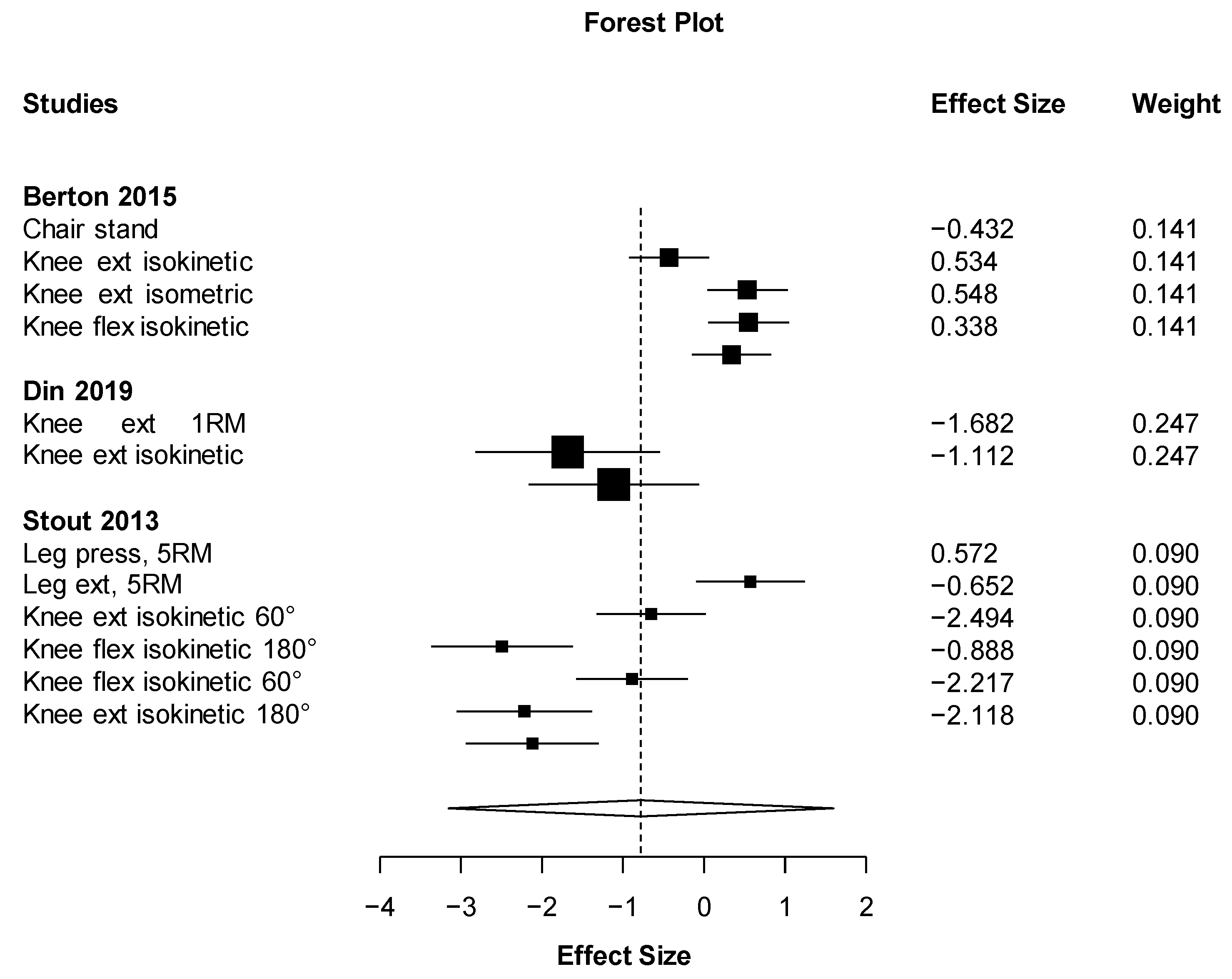
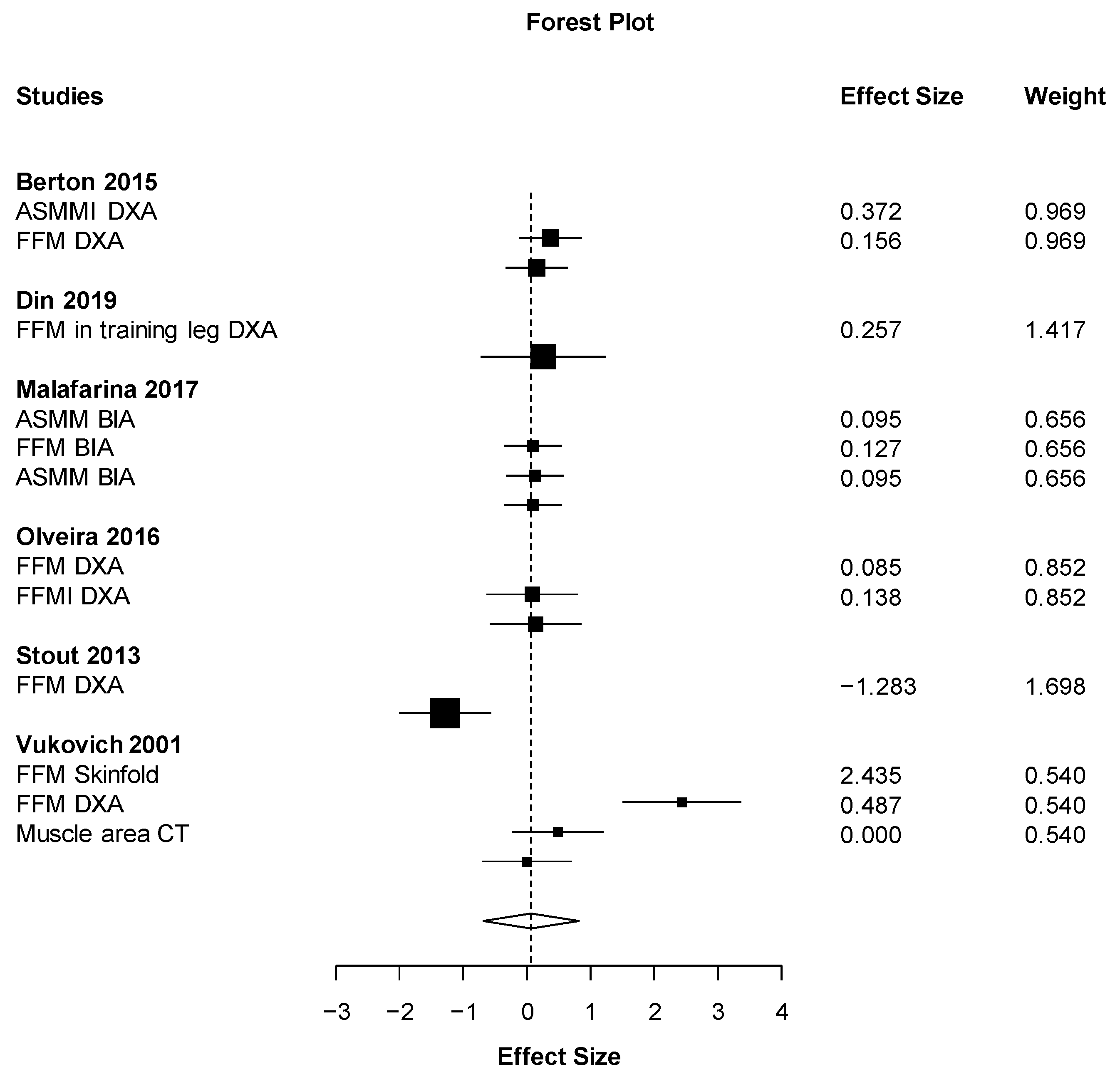
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 5 |
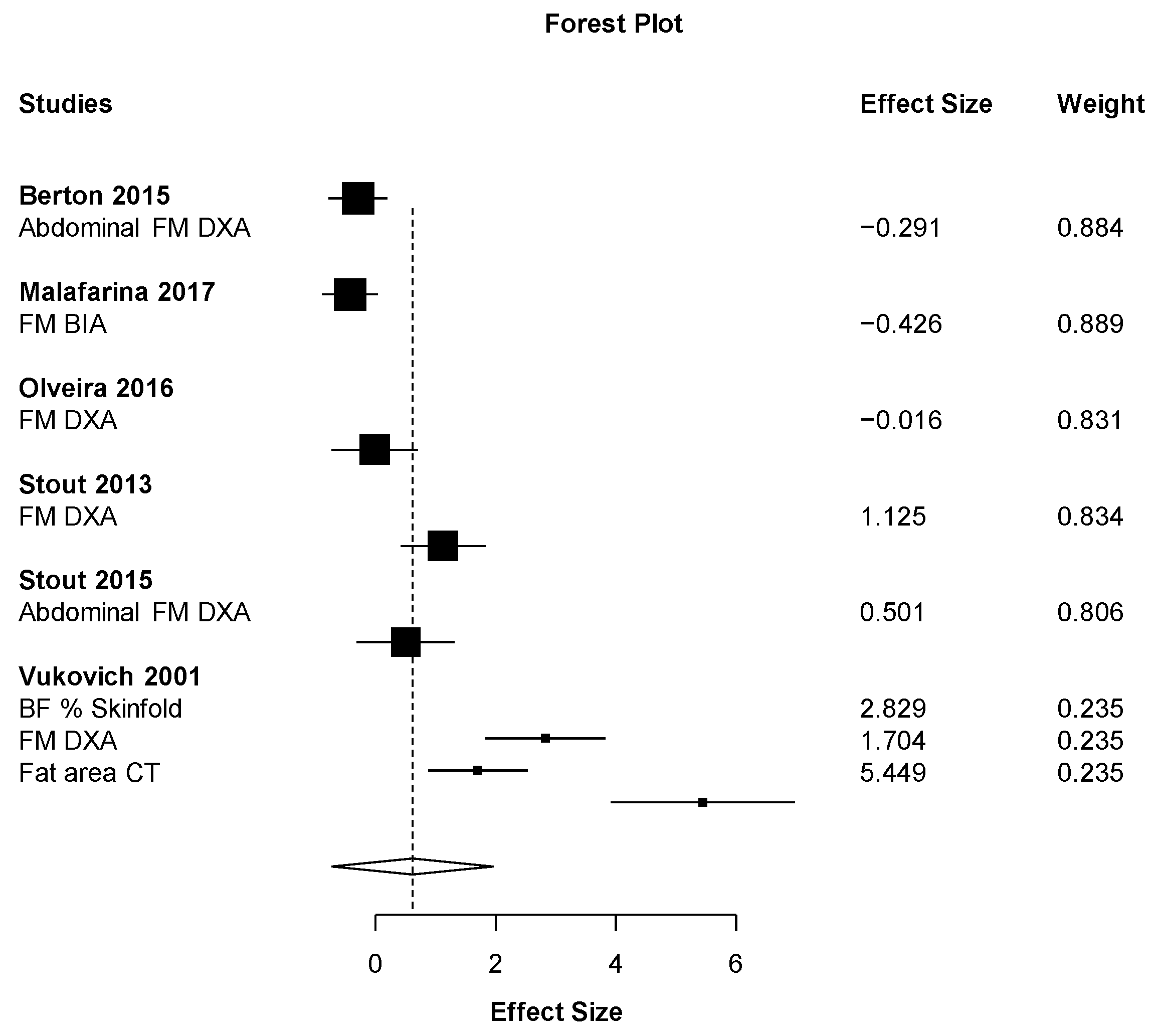
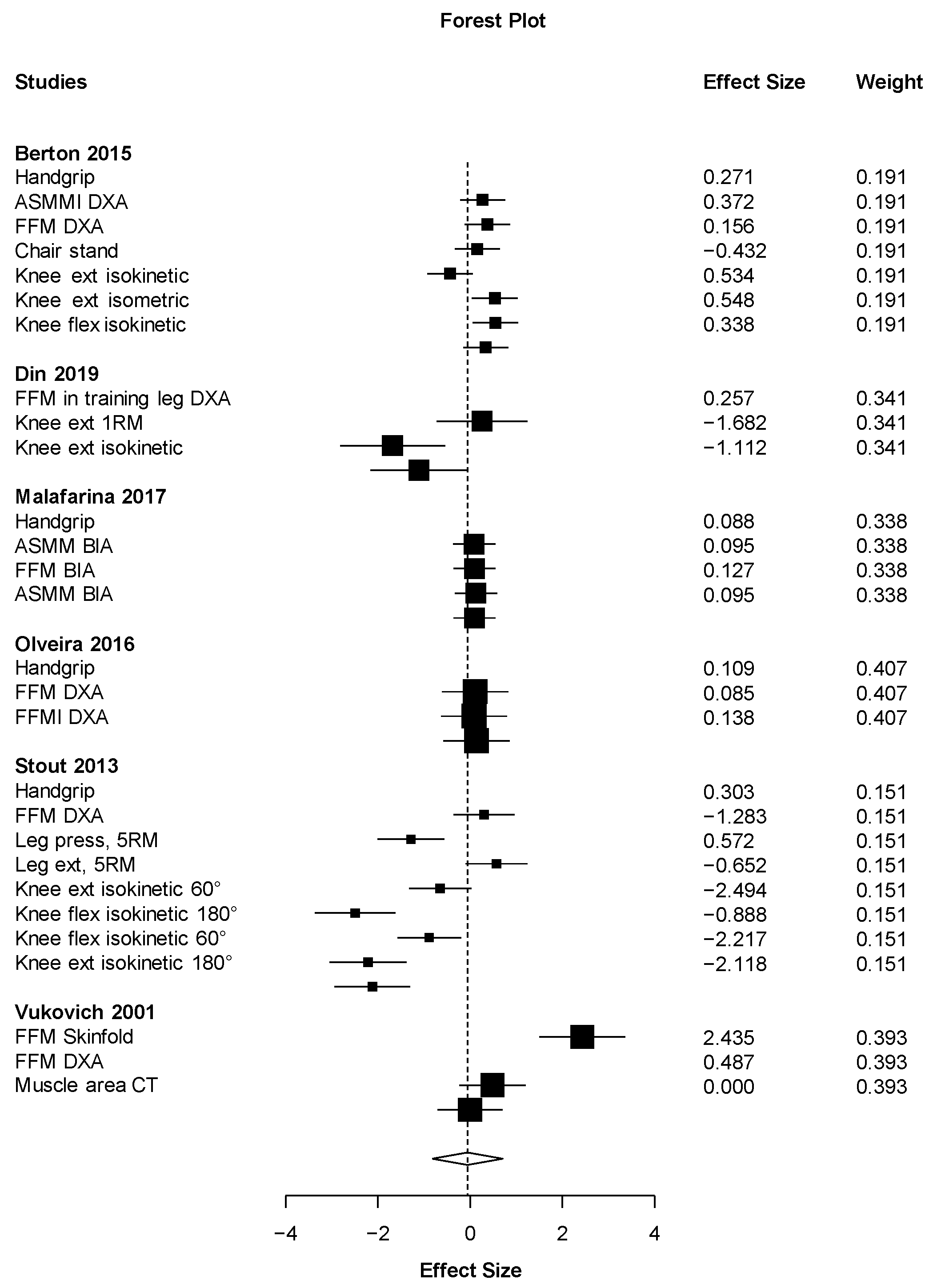
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 9 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 7 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression (see item 16)). | 9 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 13 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 14 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 15 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 15 |
| Database | Keywords | Records |
|---|---|---|
| PubMed | Search (((((HMB)[Title/Abstract] OR beta-hydroxy-beta-methylbutyrate)[Title/Abstract] OR β-hydroxy-β-methylbutyrate[Title/Abstract]))) AND (((((elder *) OR elderly)) OR (((exercise *) OR intervention *) OR training *)) OR ((((sarcopen *) OR frail *) OR cachexia) OR “muscle weakness”)) | 176 |
| Scopus | ((TITLE-ABS-KEY (β-hydroxy-β-methylbutyrate) OR TITLE-ABS-KEY (hmb) OR TITLE-ABS-KEY (beta-hydroxy-beta-methylbutyrate) OR TITLE-ABS-KEY (b-hydroxy-b-methylbutyrate))) AND (((TITLE-ABS-KEY (elder *) OR TITLE-ABS-KEY (“old * adult *”))) OR ((TITLE-ABS-KEY (sarcopen *) OR TITLE-ABS-KEY (frail *) OR TITLE-ABS-KEY (cachexia) OR TITLE-ABS-KEY (“muscle weakness”))) OR ((TITLE-ABS-KEY (exercise *) OR TITLE-ABS-KEY (intervention *) OR TITLE-ABS-KEY (training *)))) | 474 |
| Web of Science | TOPIC: (β-Hydroxy-β-Methylbutyrate) OR TOPIC: (hmb) OR TOPIC: (beta-hydroxy-beta-methylbutyrate) OR TOPIC: (b-hydroxy-b-methylbutyrate) AND ((TOPIC: (elder *) OR TOPIC: (“old * adult *”)) OR (TOPIC: (sarcopen *) OR TOPIC: (frail *) OR (TOPIC: (cachexia) OR TOPIC: (“muscle weakness”)) OR (TOPIC: (exercise *) OR TOPIC: (intervention *) OR TOPIC: (training *))) | 286 |
| Study | Length | Age | Sample | Participants | Supplementation | Compliance | SAEs | Control | Exercise |
|---|---|---|---|---|---|---|---|---|---|
| Berton (2015) | 8 weeks | 69.5 (5.3) | EG = 32 CG = 34 | Healthy women | 1.5 g/d Ca-HMB in Ensure Plus Advance enriched with 25(OH)D 227 IU/100 mL | HMB: 96 ± 6% Exercise: N.R. | Abdominal pain, constipation (n = 2) and itching (n = 1) | Standard diet | 2 × a week, mild fitness program at public gyms. Aerobic exercises to improve speed of muscle contraction, and a small part dedicated to resistance exercises, essentially to improve handgrip strength |
| Din (2019) | 6 weeks | 68.5 (1.1) a | EG = 8 CG = 8 | Healthy men | 1.0 g/d HMB-FA in BetaTOR® | HMB: 99% Exercise: N.R. | N.R. | Placebo | 3 × a week, supervised unilateral progressive resistance training. Leg extension of the dominant leg (6 sets, 8 rep, 75% 1-RM, adjusted each 10 days) |
| Malafarina (2017) | 42.3 ± 20.9 days | 85.4 (6.3) | EG = 49 CG = 43 | Patients with a hip fracture 73.8% women | 3.0 g/d Ca-HMB in Ensure Plus Advance enriched with 25(OH)D 227 IU/100 mL | HMB: >80% Exercise: N.R. | N.R. | Standard diet | 5 × a week, 50-min supervised rehabilitation therapy. Exercises to strengthen the lower limbs, balance exercises, and walking re-training in individual or group |
| Olveira (2015) | 12 weeks | 56.1 (1.3) | EG = 15 CG = 15 | Patients with non-cystic fibrosis bronchiectasis 60% women | 1.5 g/d Ca-HMB in Ensure Plus Advance enriched with 25(OH)D 227 IU/100 mL | HMB: N.R. Exercise: 100% | N.R. | Standard diet | 2 × a week, 60-min supervised exercise program at a hospital and 1 × 30-min unsupervised session. Cycle ergometer and treadmill (30 min, 75–80% VO2 max), upper and lower limb strength (8 min, 1 set, 8–10 rep), breathing retraining (15 min), and stretching and relaxation (7 min) |
| Stout (2013) † | 24 weeks | 73.0 (1.0) a | EG = 16 CG = 20 | Healthy older adults 54.2% women | 3.0 g/d Ca-HMB + 8 g/d carbohydrate | HMB: >67% Exercise: >60% | N.R. | Placebo | 3 × a week, supervised resistance training. Bench press, leg press, leg extension (1–3 sets, 8–12 rep, 80% 1RM, adjusted), lat pulldown hack squat (1–3 sets, 8–12 rep, 2–5 min rest) |
| Stout (2015) † | 12 weeks | 72.1 (5.7) | EG = 12 CG = 12 | Healthy men | 3.0 g/d Ca-HMB + 8g/d carbohydrate | HMB: >67% Exercise: >60% | N.R. | Placebo | 3 × a week, supervised resistance training. Bench press, leg press, leg extension (1–3 sets, 80% 1RM, adjusted), lat pulldown hack squat (1–3 sets, 8–12 rep, 2–5 min rest) |
| Vukovich (2001) | 8 weeks | 70 (1.0) | EG = 14 CG = 17 | Healthy older adults 54.6% women | 3.0 g/d Ca-HMB | HMB: 100% Exercise: 100% | No adverse reaction or medical complication | Placebo | 2 × a week, supervised resistance training and 3 × walking (40 min self-paced) and stretching (10 min). Overhead press, bench press, l at pulldown, elbow extension and flexion, leg flexion/extension, and leg press (2 sets, 8–12 reps. 70% 1RM, adjusted each 2 weeks) |
| After bed rest | |||||||||
| Deutz (2013) * Standley (2017) * | 8 weeks | 67.4 (1.4) a | EG = 11 CG = 8 | Healthy older adults 78.9% women | 3.0 g/d Ca-HMB | HMB >67% Exercise >60% | No serious adverse events | Placebo | 3 × a week, 1-h resistance training rehabilitation after a 10-day bed rest. 1-h circuit training for combined hip and knee extensors and flexors, light upper body exercises (3 sets, 8–10 rep, 80% 1RM) and self-paced walking |
| Results were not showed separately for old people | |||||||||
| Nissen (2000) | 8 weeks | 63–81 b | EG = 18 CG = 18 | Healthy older adults | 3.0 g/d Ca-HMB | N.R. | Less diarrhea and less loss of appetite | Placebo | 3 × a week, supervised resistance training. Alternated exercising of either the upper or lower body during each exercise session |
| 8 weeks | 62–79 b | EG = 16 CG = 18 | Healthy older adults | 3.0 g/d Ca-HMB | N.R. | Less diarrhea and less loss of appetite | Placebo | 2 × a week resistance training + 3 × 60-min walking and stretching | |
| Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall Bias | |
|---|---|---|---|---|---|---|
| Berton (2016) |  |  |  |  |  |  |
| Deutz (2013) |  |  |  |  |  |  |
| Din (2019) |  |  |  |  |  |  |
| Malafarina (2017) |  |  |  |  |  |  |
| Olveira (2015) |  |  |  |  |  |  |
| Standley (2017) |  |  |  |  |  |  |
| Stout (2013) |  |  |  |  |  |  |
| Stout (2015) |  |  |  |  |  |  |
| Vukovich (2001) |  |  |  |  |  |  |
| Nissen (2000) |  |  |  |  |  |  |
 Low risk of bias;
Low risk of bias;  Unclear risk of bias;
Unclear risk of bias;  High risk of bias.
High risk of bias.| Outcome | Measure | Overall effect * | Study |
|---|---|---|---|
| Physical performance | SPPB | No effect | Berton (2015) |
| No effect a | Deutz (2013) | ||
| 6-min walking test | Positive | Berton (2015) | |
| Gait speed | No effect | Malafarina (2017) | |
| Get-up-and-go | No effect | Stout (2013) | |
| No effect a | Deutz (2013) | ||
| Muscular strength | Isokinetic knee flexion | Positive | Berton (2015) |
| No effect | Stout (2013) | ||
| Isokinetic knee extension | Positive | Berton (2015) | |
| No effect | Stout (2013) | ||
| No effect | Din (2019) | ||
| No effect a | Deutz (2013) | ||
| Isometric knee extension | Positive | Berton (2015) | |
| Handgrip strength | No effect | Berton (2015) | |
| No effect | Malafarina (2017) | ||
| No effect | Stout (2013) | ||
| Handgrip strength endurance | Positive | Berton (2015) | |
| Handgrip work index | No effect | Malafarina (2017) | |
| Knee extension, 1RM | No effect | Din (2019) | |
| Bench press, 5RM | No effect | Stout (2013) | |
| Leg press, 5RM | No effect | Stout (2013) | |
| Leg extensor, 5RM | No effect | Stout (2013) | |
| Body composition | Fat free mass (DXA) | No effect | Berton (2015) |
| Positiveb | Stout (2013) | ||
| No effect | Vukovich (2001) | ||
| No effect a | Deutz (2013) | ||
| Fat free mass (BIA) | Positive | Malafarina (2017) | |
| Fat free mass (Skin fold thickness) | No effect | Vukovich (2001) | |
| ASMMI | No effect | Berton (2015) | |
| Muscle mass (BIA) | Positive | Malafarina (2017) | |
| Appendicular lean mass (BIA) | Positive | Malafarina (2017) | |
| Skeletal muscle mass (BIA) | No effect | Malafarina (2017) | |
| ASMM (BIA) | Positive | Malafarina (2017) | |
| Fatty mass (DXA) | No effect | Stout (2013) | |
| Fatty mass (BIA) | No effect | Malafarina (2017) | |
| Fatty mass % (Skin fold thickness) | Positive | Vukovich (2001) | |
| Fatty mass % (DXA) | No effect | Vukovich (2001) | |
| Leg lean mass (DXA) | No effect | Stout (2013) | |
| No effect a | Deutz (2013) | ||
| Arm lean mass (DXA) | Positiveb | Stout (2013) | |
| Abdominal fat mass (DXA) | No effect | Berton (2015) | |
| Positive | Stout (2015) | ||
| Radial muscle density (CT) | Positive | Berton (2015) | |
| Radial muscle area (CT) | No effect | Berton (2015) | |
| Radial fat area (CT) | No effect | Berton (2015) | |
| Radial fat/muscle ratio (CT) | Positive | Berton (2015) | |
| Tibial muscle density (CT) | Positive | Berton (2015) | |
| Tibial muscle area (CT) | No effect | Berton (2015) | |
| Tibial fat area (CT) | No effect | Berton (2015) | |
| Tibial fat/muscle ratio (CT) | No effect | Berton (2015) | |
| Fat area (CT) | Positive | Vukovich (2001) | |
| Muscle area (CT) | No effect | Vukovich (2001) | |
| Cross-sectional area (VLB) | No effect a | Standley (2017) | |
| Thigh lean mass (DXA) | No effect | Din (2019) | |
| Vastus lateralis thickness (DXA) | No effect | Din (2019) | |
| Others | Muscle quality (Isokinetic knee extension 60°) | No effect | Stout (2013) |
| Muscle quality (Isokinetic knee extension 180°) | No effect | Stout (2013) | |
| Muscle quality (Handgrip strength) | No effect | Stout (2013) | |
| Proteins expression (histology) | Positivea | Standley (2017) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courel-Ibáñez, J.; Vetrovsky, T.; Dadova, K.; Pallarés, J.G.; Steffl, M. Health Benefits of β-Hydroxy-β-Methylbutyrate (HMB) Supplementation in Addition to Physical Exercise in Older Adults: A Systematic Review with Meta-Analysis. Nutrients 2019, 11, 2082. https://doi.org/10.3390/nu11092082
Courel-Ibáñez J, Vetrovsky T, Dadova K, Pallarés JG, Steffl M. Health Benefits of β-Hydroxy-β-Methylbutyrate (HMB) Supplementation in Addition to Physical Exercise in Older Adults: A Systematic Review with Meta-Analysis. Nutrients. 2019; 11(9):2082. https://doi.org/10.3390/nu11092082
Chicago/Turabian StyleCourel-Ibáñez, Javier, Tomas Vetrovsky, Klara Dadova, Jesús G. Pallarés, and Michal Steffl. 2019. "Health Benefits of β-Hydroxy-β-Methylbutyrate (HMB) Supplementation in Addition to Physical Exercise in Older Adults: A Systematic Review with Meta-Analysis" Nutrients 11, no. 9: 2082. https://doi.org/10.3390/nu11092082
APA StyleCourel-Ibáñez, J., Vetrovsky, T., Dadova, K., Pallarés, J. G., & Steffl, M. (2019). Health Benefits of β-Hydroxy-β-Methylbutyrate (HMB) Supplementation in Addition to Physical Exercise in Older Adults: A Systematic Review with Meta-Analysis. Nutrients, 11(9), 2082. https://doi.org/10.3390/nu11092082







