Does the Metabolically Healthy Obese Phenotype Protect Adults with Class III Obesity from Biochemical Alterations Related to Bone Metabolism?
Abstract
1. Introduction
2. Materials and Methods
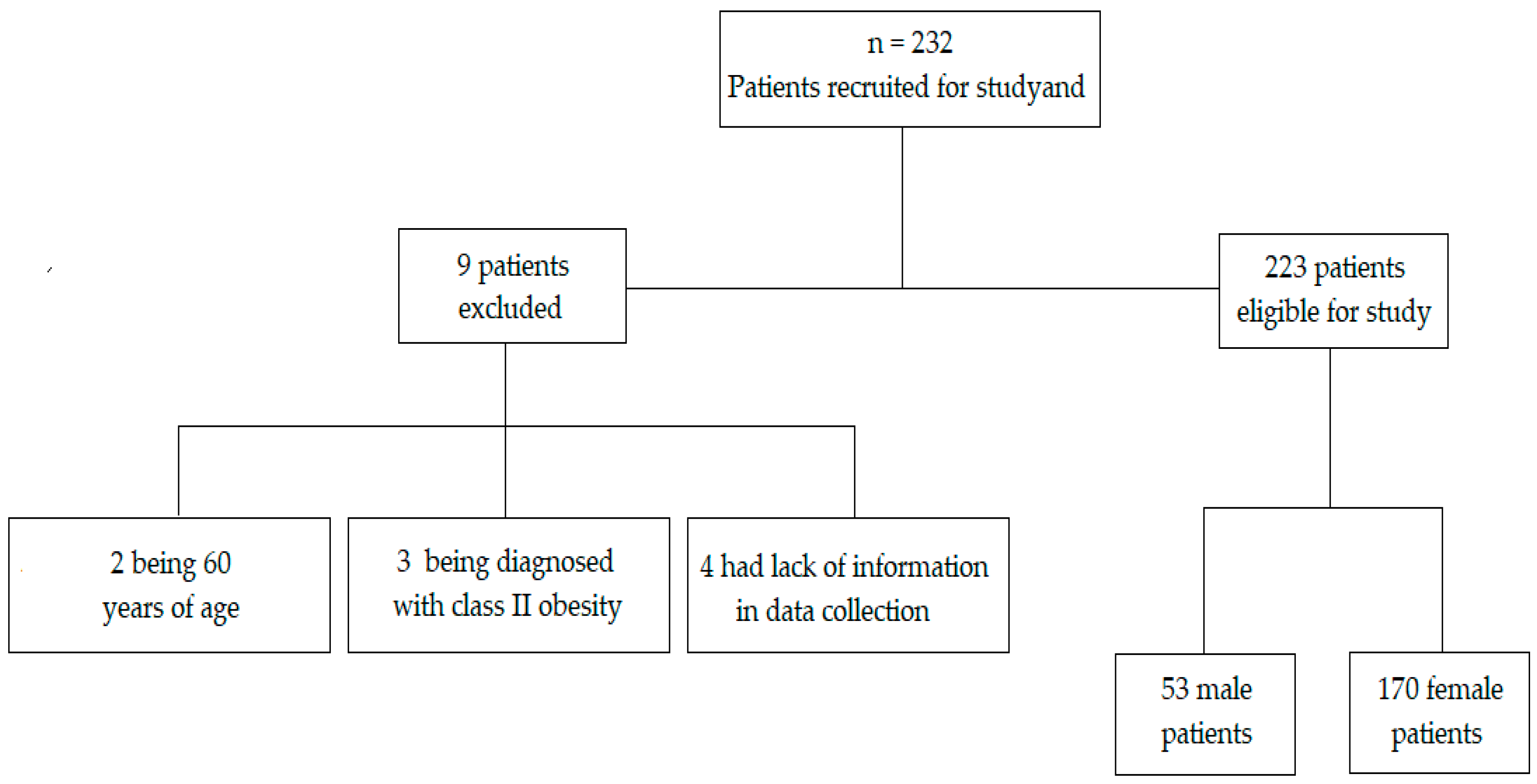
2.1. Participants
2.2. Study Design
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. General Characterization of the Studied Population
3.2. Characterization of the Population According to the MHO and MUHO Phenotypes
3.3. Association and Odds Ratio (OR) of the Markers of Bone Metabolism, According to the MHO and MUHO Phenotypes
4. Discussion
4.1. General Characterization of the Population, According to the MHO and MUHO Phenotypes
4.2. Characterization of Biochemical Markers of Bone Metabolism, According to the MHO and MUHO Phenotypes
4.3. Association and Odds Ratio of Markers and Micronutrients of Bone Metabolism, According to the MHO and MUHO Phenotypes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Fact Sheet: Obesity and Overweight, n. 311. 2015. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/index.html (accessed on 6 June 2016).
- Demirağ, M.D.; Ozkan, S.; Haznedaroğlu, Ş.; Kilinç, E.A.; Aksakal, F.N.B.; Aycan, S.; Göker, B. Associations between obesity and the radiographic phenotype in knee osteoarthritis. Turk. J. Med. Sci. 2017, 47, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.É.; Williams, D.; Lee, M.J. Osteoporosis, obesity, and sarcopenia on abdominal CT: A review of epidemiology, diagnostic criteria, and management strategies for the reporting radiologist. Abdom. Radiol. 2017, 42, 2376–2386. [Google Scholar] [CrossRef] [PubMed]
- Fazel, Y.; Koenig, A.B.; Sayiner, M.; Goodman, Z.D.; Younossi, Z.M. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 2016, 65, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Haffner-Luntzer, M.; Prystaz, K.; Scheidt, A.V.; Busse, B.; Schinke, T.; Amling, M.; Ignatius, A. Calcium and vitamin-D deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice. Sci. Rep. 2017, 7, 7223. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Prado, C.M.; Ilich, J.Z.; Purcell, S.; Siervo, M.; Folsom, A.; Panton, L. Osteosarcopenic obesity: The role of bone, muscle, and fat on health. J. Cachexia Sarcopenia Muscle 2014, 5, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kohora, K.; Ochi, M.; Tabara, Y.; Nagai, T.; Igase, M.; Miki, T. Leptin in sarcopenic visceral obesity: Possible link between adipocytes and myocytes. PLoS ONE 2011, 6, e24633. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Li, C.I.; Li, T.C.; Li, T.C.; Liu, C.S.; Lin, C.H.; Lin, W.Y.; Lin, C.C. Association of Sarcopenic Obesity with Higher Serum High-Sensitivity C-Reactive Protein Levels in Chinese Older Males—A Community-Based Study (Taichung Community Health Study-Elderly, TCHS-E). PLoS ONE 2015, 10, e0132908. [Google Scholar]
- Levine, M.E.; Crimmins, E.M. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity 2012, 20, 2101–2106. [Google Scholar] [CrossRef]
- Gutiérrez-Grobe, Y.; Juárez-Hernández, E.; Sánchez-Jiménez, B.; Uribe-Ramos, M.; Ramos-Ostos, M.; Uribe, M.; Chávez-Tapia, N. Less liver fibrosis in metabolically healthy compared with metabolically unhealthy obese patients with non-alcoholic fatty liver disease. Diabetes Metab. 2017, 43, 332–337. [Google Scholar] [CrossRef]
- Abenavoli, L.; Peta, V. Role of adipokines and cytokines in non-alcoholic fatty liver disease. Rev. Recent Clin. Trials 2014, 9, 134–140. [Google Scholar] [CrossRef]
- Pujia, A.; Gazzaruso, C.; Ferro, Y.; Mazza, E.; Maurotti, S.; Russo, C.; Lazzaro, V.; Romeo, S.; Montalcini, T. Individuals with Metabolically Healthy Overweight/Obesity Have Higher Fat Utilization than Metabolically Unhealthy Individuals. Nutrients 2016, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Jeon, W.S.; Han, K.J.; Lee, S.Y.; Kim, N.H.; Chae, H.B.; Jang, C.M.; Yoo, K.M.; Park, H.J.; Lee, M.K.; et al. Comparison of Serum Adipocytokine Levels according to Metabolic Health and Obesity Status. Endocrinol. Metab. 2015, 30, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Bairey Merz, C.N.; Brewer, H.B.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith, S.C., Jr.; Stone, N.J.; Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004, 110, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Dullaart, R.P.F.; Schreuder, H.B.; Faber, K.N. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Díez-Rodríguez, R.; Ballesteros, P.M.D.; Calleja, F.A.; Calleja, A.S.; Cano, R.I.; Linares, T.P.; Jorquera, P.F.; Olcoz, G.J.L. Vitamin D levels and bone turnover markers are not related to non-alcoholic fatty liver disease in severely obese patients. Nutr. Hosp. 2014, 30, 1256–1262. [Google Scholar] [PubMed]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- World Health Organization (WHO). The World Health Report 2001—Mental Health: New Understanding, New Hope. 2001. Available online: http://www.who.int/whr/2001/en/ (accessed on 29 June 2016).
- Lohman, T.; Caballero, B.; Davis, S. Pathways: A school-based program for the primary prevention of obesity in American Indian children. J. Nutr. Biochem. 1998, 9, 535–543. [Google Scholar]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Pratt, D.S.; Kaplan, M.M. Avaliação da função hepática. In Harrison Principios de Medicina Interna, 15th ed.; Braunwald, E., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Jameson, L.R., Eds.; Mc Graw Hill: Rio de Janeiro, Brazil, 2002; pp. 1813–1816. [Google Scholar]
- SBC (Sociedade Brasileira de Cardiologia). VII Diretriz Brasileira de Hipertensão Arterial 2016. Available online: http://publicacoes.cardiol.br/2014/diretrizes/2016/05_HIPERTENSAO_ARTERIAL.pdf (accessed on 28 May 2016).
- Durward, C.M.; Hartman, T.J.; Nickols-Richardson, S.M. All-cause mortality risk of metabolically healthy obese individuals in NHANES III. J. Obes. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., III; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A Statement for Healthcare Professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Kao, P.C. Parathyroid hormone assay. Mayo Clin. Proc. 1982, 57, 596–597. [Google Scholar] [PubMed]
- Bramer, J.A.; Abudu, A.A.; Tillman, R.M.; Carter, S.R.; Sumathi, V.P.; Grimer, R.J. Pre and post chemotherapy alkaline phosphatase levels as prognostic indicators in adults with localized osteosarcoma. Eur. J. Cancer 2005, 41, 2846–2852. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R.; Green, R.; Rosenblatt, D.S.; Watkins, D. Update on cobalamin, folate, and homocysteine. Hematol. Am. Soc. Hematol. Educ. Progr. 2003, 2003, 62–81. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, H.E.C.; Vieth, R.; Cole, D.E.; Scillitani, A.; Modoni, S.; Frusciante, V.; Ritrovato, G.; Chiodini, I.; Minisola, S.; Carnevale, V. Sun exposure questionnaire predicts circulating 25-hydroxyvitamin D concentrations in Caucasian hospital workers in southern Italy. J. Steroid Biochem. Mol. Biol. 2010, 121, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.A.; Ertingshausen, G. Direct method for determining inorganic phosphate in serum with the “Centrifi Chem”. Clin. Chem. 1972, 18, 263–265. [Google Scholar] [PubMed]
- Lowenstein, F.W.; Stanton, M.F. Serum magnesium levels in the United States, 1971–1974. J. Am. Coll. Nutr. 1986, 5, 399–414. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The World Health Report, Fighting Disease, Fostering Development 1996. Available online: www.who.int/whr/1996/en/ (accessed on 6 June 2016).
- Chang, Y.; Jung, H.S.; Cho, J.; Zhang, Y.; Yun, K.E.; Lazo, M.; Pastor-Barriuso, R.; Ahn, J.; Kim, C.W.; Rampal, S.; et al. Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2016, 111, 1133–1140. [Google Scholar] [CrossRef]
- Latifi, S.M.; Karandish, M.; Shahbazian, H.; Cheraghian, B.; Moradi, M. Prevalence of Metabolically Health Y Obesity (MHO) and its relation with incidence of metabolic syndrome, hypertension and type 2 Diabetes amongst individuals aged over 20 years in Ahvaz: A 5 Year cohort Study (2009–2014). Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S1037–S1040. [Google Scholar] [CrossRef]
- Huh, J.H.; Kim, K.J.; Kim, S.U.; Han, S.H.; Han, K.H.; Cha, B.S.; Chung, C.H.; Lee, B.W. Obesity is more closely related with hepatic steatosis and fibrosis measured by transient elastography than metabolic health status. Metabolism 2017, 66, 23–31. [Google Scholar] [CrossRef]
- Yoon, J.W.; Jung, C.H.; Kim, M.K.; Park, H.E.; Park, K.S.; Jang, H.C.; Moon, M.K.; Choi, S.Y.; Koo, B.K. Influence of the definition of “metabolically healthy obesity” on the progression of coronary artery calcification. PLoS ONE 2017, 12, e0178741. [Google Scholar] [CrossRef] [PubMed]
- Gonçalvez, C.G.; Glade, M.J.; Meguid, M.M. Metabolically healthy obese individuals: Key protective factors. Nutrition 2016, 32, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Gasevic, D.; Carlsson, A.C.; Lesser, I.A.; Mancini, G.J.; Lear, S.A. The association between "hypertriglyceridemic waist" and sub-clinical atherosclerosis in a multiethnic population: A cross-sectional study. Lipids Health Dis. 2014, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Camhi, S.M.; Katzmarzyk, P.T. Differences in Body Composition between Metabolically Healthy Obese and Metabolically Abnormal Obese Adults. Int. J. Obes. 2014, 38, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Goday, A.; Calvo, E.; Vázquez, L.A.; Caveda, E.; Margallo, T.; Catalina-Romero, C.; Reviriego, J. Prevalence and clinical characteristics of metabolically healthy obese individuals and other obese/non-obese metabolic phenotypes in a working population: Results from the Icaria study. BMC Public Health 2016, 16, 11971209. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-C.; Hayashi, T.; Fujimoto, W.Y.; Kahn, S.E.; Leonetti, D.L.; McNeely, M.J.; Boyko, E.J. Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotype. Int. J. Obes. 2015, 39, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M. Metabolically healthy obesity: Definitions, determinants and clinical implications. Rev. Endocr. Metab. Disord. 2013, 14, 219–227. [Google Scholar] [CrossRef]
- Cordeiro, A.; Pereira, A.; Saboya, C.J.; Ramalho, A. Relationship between Nonalcoholic Fatty Liver Disease and Vitamin D Nutritional Status in Extreme Obesity. Can. J. Gastroenterol. Hepatol. 2017, 2017, 9456897. [Google Scholar] [CrossRef]
- Diniz, M.F.H.; Beleigoli, A.M.R.; Ribeiro, A.L.P.; Vidigal, P.G.; Bensenor, I.M.; Lotufo, P.; Duncan, B.B.; Schmidt, M.I.; Barreto, S.M. Factors associated with metabolically healthy status in obesity, overweight, and normal weight at baseline of ELSA-Brasil. Medicine 2016, 95, 27. [Google Scholar] [CrossRef]
- Stefan, N.; Haring, H.U.; Hu, F.B.; Schulze, M.B. Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013, 1, 152–162. [Google Scholar] [CrossRef]
- Kini, U.; Nandeesh, B.N. Physiology of Bone Formation, Remodeling, and Metabolism; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Bordalo, L.A.; Mourão, D.M.; Bressan, J. Deficiências nutricionais após a cirurgia bariátrica. Por que ocorrem? Acta Med. Port. 2011, 24, 1021–1028. [Google Scholar] [PubMed]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell. Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, G.L.; Larazetti-Castro, M. Marcadores Bioquímicos da Remodelação Óssea na Prática Clínica. Arq. Bras. Endocrinol. Metabol. 2002, 46, 72–78. [Google Scholar] [CrossRef]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Kim, H.; Yoshida, H.; Shimada, H.; Suzuki, T. Serum 25-hydroxyvitamin D level risk of falls in Japanese community-dwelling elderly women: A 1-year follow-up study. Osteoporos. Int. 2005, 26, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Severson, T.J.; Bessur, S.; Bonkovsky, H.L. Genetic factors that affect nonalcoholic fatty liver disease: A systematic clinical review. World J. Gastroenterol. 2016, 22, 6742–6756. [Google Scholar] [CrossRef] [PubMed]
- Magain, A.C.; Richard, T.; De Becker, B.; Rorive, S.; Benhamed, M.; Vanhaeverbeek, M. Complications non-chirurgicales de la chirurgie bariatrique: À propos de quatre case trevue de la littérature. Rev. Méd. Brux. 2016, 37, 26–34. [Google Scholar]
- Prince, R.L.; Glendenning, P. What is the therapeutic target level of 25-hydroxyvitamin D in osteoporosis and how accurately can we measure it? Intern. Med. J. 2012, 42, 1069–1072. [Google Scholar]
- Himbert, C.; Ose, J.; Delphan, M.; Ulrich, C.M. A systematic review of the interrelation between diet and surgery-induced weight loss and vitamin D status. Nutr. Res. 2017, 38, 13–26. [Google Scholar] [CrossRef]
- Wolf, E.; Utech, M.; Stehle, P.; Büsing, M.; Stoffel-Wagner, B.; Ellinger, S. Preoperative micronutrient status in morbidly obese patients before undergoing bariatric surgery: Results of a cross-sectional study. Surg. Obes. Relat. Dis. 2015, 11, 1157–1163. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Lim, K.I.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Choi, H.; Baik, S.H.; et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: The Korean Sarcopenic Obesity Study. Clin. Endocrinol. 2013, 78, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Bellia, A.; Marinoni, G.; D’Adamo, M.; Guglielmi, V.; Lombardo, M.; Donadel, G.; Gentileschi, P.; Lauro, D.; Federici, M.; Lauro, R.; et al. Parathyroid Hormone and Insulin Resistance in Distinct Phenotypes of Severe Obesity: A Cross-Sectional Analysis in Middle-Aged Men and Premenopausal Women. J. Clin. Endocrinol. Metab. 2012, 97, 4724–4732. [Google Scholar] [CrossRef] [PubMed]
- Kelly, O.J.; Gilman, J.C.; Kim, Y.; Ilich, J.Z. Micronutrient intake in the etiology, prevention and treatment of osteosarcopenic obesity. Curr. Aging Sci. 2016, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D deficiency is a surrogate marker for visceral Fat content, metabolic syndrome, Type 2 Diabetes, and Future Metabolic Complications. J. Diabetes Metab. Disord. Control 2016, 3, 00059. [Google Scholar] [CrossRef][Green Version]
- Aung, K. Review: In postmenopausal women and older men, vitamin D plus calcium reduces some fractures. Ann. Intern. Med. 2014, 161, JC5. [Google Scholar] [CrossRef]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054–1060. [Google Scholar] [CrossRef]
- Craney, A. Effectiveness and safety of vitamin D in relation to bone health. Evid. Rep. Technol. Assess. 2007, 158, 1–235. [Google Scholar]
- Hirani, V.; Cimming, R.G.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Hsu, B.; Handelsman, D.J.; Waite, L.M.; Seibel, M.J. Longitudinal Associations Between Vitamin D Metabolites and Sarcopenia in Older Australian men: The Concord Health and Aging in Men Project. J. Gerontol. Ser. A Biol. Sci. Med. 2017, 73, 131–138. [Google Scholar] [CrossRef]
- Verheyen, N.; Fahrleitner-Pammer, A.; Belyavskiy, E.; Gruebler, M.R.; Dimai, H.P.; Amrein, K.; Ablasser, K.; Martensen, J.; Catena, C.; Pieske-Kraigher, E.; et al. Relationship between bone turnover and left ventricular function in primary hyperparathyroidism: The EPATH trial. PLoS ONE 2017, 12, 0173799. [Google Scholar] [CrossRef]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
| General Characteristics | MHO (n = 73) Mean ± SD | MUHO (n = 150) Mean ± SD | p-Value | |
|---|---|---|---|---|
| Clinical Variables | ||||
| Systolic H (mmHg) | 123.3 ± 23.4 | 140.0 ± 30.4 | 0.001 * | |
| Diastolic H (mmHg) | 76.6 ± 18.0 | 90.2 ± 24.4 | 0.001 * | |
| SH (%) | Yes | 35.6 (n = 26) | 87.3 (n = 131) | 0.001 ** |
| No | 64.4 (n = 47) | 12.7 (n = 19) | ||
| NAFLD (%) | Yes | 83.6 (n = 61) | 94.0 (n = 141) | 0.025 ** |
| No | 16.4 (n = 12) | 6.0 (n = 9) | ||
| Anthropometric Variables | ||||
| Weight (Kg) | 118.4 ± 16.3 | 118.0 ± 19.8 | 0.876 | |
| Height (meters) | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.723 | |
| BMI (Kg/m2) | 42.9 ± 4.4 | 42.5 ± 4.8 | 0.465 | |
| AC (cm) | 118.1 ± 11.7 | 120.5 ± 13.8 | 0.186 | |
| VAI | 4.2 ± 3.5 | 7.9 ± 8.7 | 0.001 * | |
| Biochemical Variables | ||||
| Glucose (mg/dL) | 91.3 ± 15.9 | 106.8 ± 30.4 | 0.001 * | |
| Insulin (mg/dL) | 16.3 ± 8.9 | 19.7 ± 11.6 | 0.018 * | |
| HOMA-IR | 3.7 ± 2.3 | 5.4 ± 4.2 | 0.002 * | |
| Total Cholesterol (mg/dL) | 197.6 ± 36.1 | 199.0 ± 57.1 | 0.826 | |
| LDL-c (mg/dL) | 121.1 ± 31.6 | 120.3 ± 35.5 | 0.867 | |
| HDL-c (mg/dL) | 52.2 ± 11.6 | 42.4 ± 8.9 | <0.001 * | |
| Triglycerides (mg/dL) | 123.0 ± 140.9 | 190.2 ± 239.3 | 0.009 * | |
| CRP (mg/dL) | 0.8 ± 0.7 | 0.9 ± 0.9 | 0.427 | |
| Variables | MHO (n = 73) | MUHO (n = 150) | p-Value |
|---|---|---|---|
| Mean/SD | Mean/SD | ||
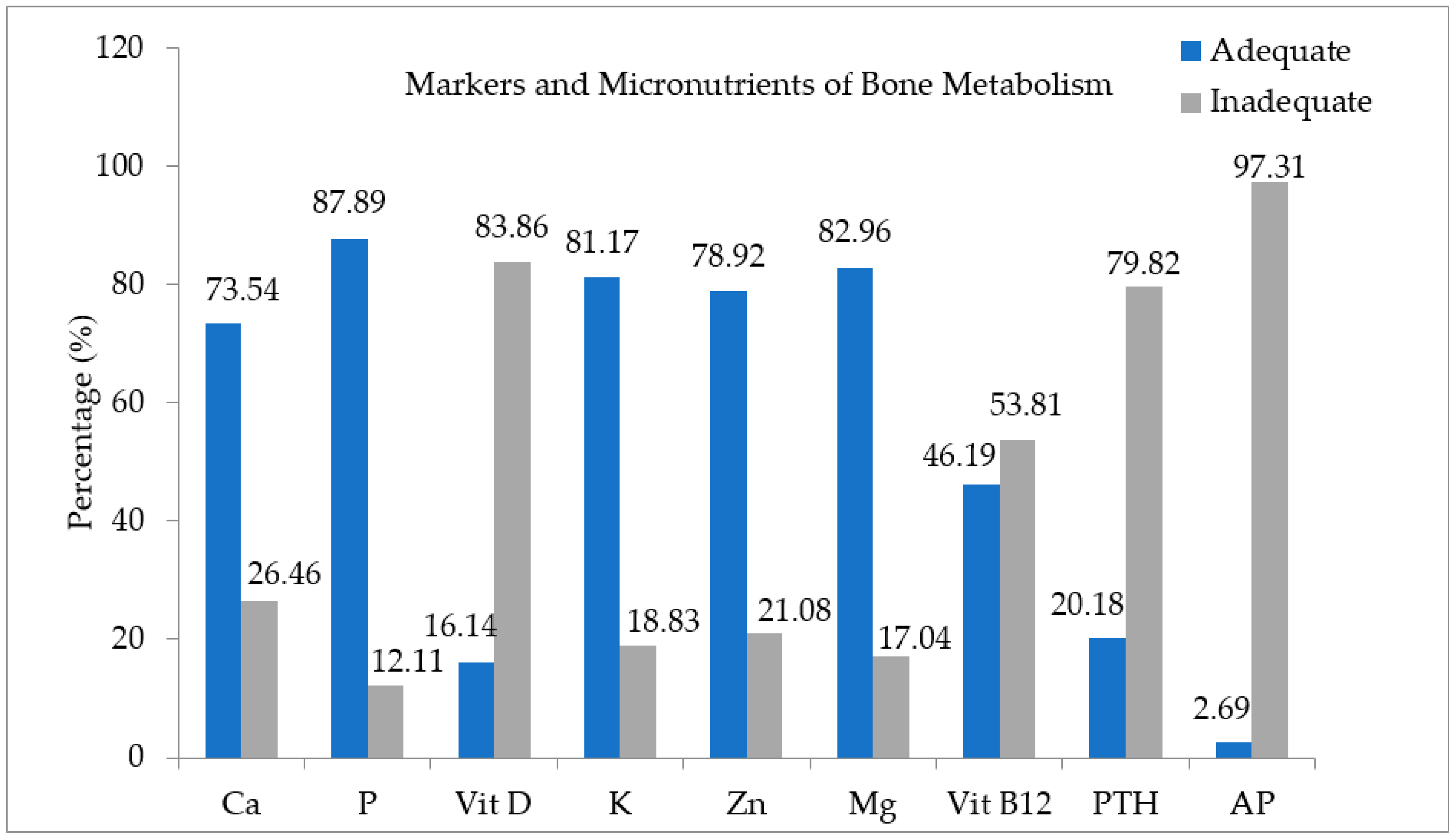
| Calcium (mg/dL) | 3.9 ± 1.4 | 3.9 ± 1.8 | 0.987 |
| Phosphorus (mg/dL) | 3.5 ± 0.5 | 3.6 ± 0.6 | 0.799 |
| 25(OH)D (ng/mL) | 22.3 ± 7.7 | 22.6 ± 8.3 | 0.747 |
| Potassium (mg/dL) | 5.2 ± 6.9 | 4.6 ± 4.8 | 0.527 |
| Zinc (mg/dL) | 99.9 ± 32.3 | 99.9 ± 27.2 | 0.994 |
| Magnesium (mg/dL) | 2.0 ± 0.1 | 2.0 ± 0.2 | 0.168 |
| Vitamin B12 (pg/mL) | 370.5 ± 200.5 | 344.1 ± 183.4 | 0.345 |
| PTH (pg/mL) | 42.0 ± 18.1 | 42.7 ± 16.0 | 0.779 |
| AP (U/L) | 70.5 ± 27.5 | 80.1 ± 27.5 | 0.016 * |
| Variables | Status Nutritional | MHO (n = 73) | MUHO (n = 150) | p-Value |
|---|---|---|---|---|
| Calcium (%) | Adequate | 79.5 (n = 58) | 70.7 (n = 106) | 0.108 |
| Inadequate | 20.5 (n = 15) | 29.3 (n = 44) | ||
| Phosphorus (%) | Adequate | 89.0 (n = 65) | 87.3 (n = 131) | 0.449 |
| Inadequate | 11.0 (n = 8) | 12.7 (n = 19) | ||
| 25(OH)D (%) | Deficient | 38.4 (n = 28) | 39.3 (n = 59) | 0.720 |
| Insufficient | 47.9 (n = 35) | 43.3 (n = 65) | ||
| Sufficient | 13.7 (n = 10) | 17.4 (n = 26) | ||
| Potassium (%) | Adequate | 84.9 (n = 62) | 79.3 (n = 119) | 0.207 |
| Inadequate | 15.1 (n = 11) | 20.7 (n = 31) | ||
| Zinc (%) | Adequate | 75.3 (n = 55) | 80.7 (n = 121) | 0.228 |
| Inadequate | 24.7 (n = 18) | 19.3 (n = 29) | ||
| Magnesium (%) | Adequate | 89.0 (n = 65) | 80.0 (n = 120) | 0.064 |
| Inadequate | 11.0 (n = 8) | 20.0 (n = 30) | ||
| Vitamin B12 (%) | Adequate | 42.5 (n = 31) | 48.0 (n = 72) | 0.263 |
| Inadequate | 57.5 (n = 42) | 52.0 (n = 78) | ||
| PTH (%) | Yes | 12.3 (n = 9) | 24.0 (n = 36) | 0.029 * |
| No | 87.7 (n = 64) | 76.0 (n = 114) | ||
| AP (%) | Altered | 2.7 (n = 2) | 2.7 (n = 4) | 0.640 |
| Unaltered | 97.3 (n = 71) | 97.3 (n = 146) |
| Variables | MHO (n = 73) | MUHO (n = 150) |
|---|---|---|
| AOR | AOR | |
| Calcium (mg/dL) | 3.1 | 4.1 * |
| (2.2–6.0) | (2.3–6.6) | |
| Phosphorus (mg/dL) | 2.0 | 3.0 * |
| (0.8–4.6) | (1.9–5.7) | |
| 25(OH)D (ng/mL) | 2.8 | 5.0 * |
| (1.9–3.9) | (2.9–6.7) | |
| Potassium (mg/dL) | 1.1 | 1.7 |
| (0.4–2.8) | (0.6–3.0) | |
| Zinc (mg/dL) | 1.9 | 1.8 |
| (0.9–2.9) | (0.8–3.9) | |
| Magnesium (mg/dL) | 1.0 | 3.5 |
| (0.9–1.9) | (2.0–4.7) | |
| Vitamin B12 (pg/mL) | 0.8 | 2.5 |
| (1.1–3.4) | (1.5–5.4) | |
| PTH (pg/mL) | 5.1 | 5.4 * |
| (4.1–6.4) | (4.4–6.7) | |
| AP (U/L) | 2.3 | 2.0 |
| (0.8–4.6) | (0.6–4.6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques Loureiro, L.; Lessa, S.; Mendes, R.; Pereira, S.; Saboya, C.J.; Ramalho, A. Does the Metabolically Healthy Obese Phenotype Protect Adults with Class III Obesity from Biochemical Alterations Related to Bone Metabolism? Nutrients 2019, 11, 2125. https://doi.org/10.3390/nu11092125
Marques Loureiro L, Lessa S, Mendes R, Pereira S, Saboya CJ, Ramalho A. Does the Metabolically Healthy Obese Phenotype Protect Adults with Class III Obesity from Biochemical Alterations Related to Bone Metabolism? Nutrients. 2019; 11(9):2125. https://doi.org/10.3390/nu11092125
Chicago/Turabian StyleMarques Loureiro, Ligiane, Suzane Lessa, Rodrigo Mendes, Sílvia Pereira, Carlos José Saboya, and Andrea Ramalho. 2019. "Does the Metabolically Healthy Obese Phenotype Protect Adults with Class III Obesity from Biochemical Alterations Related to Bone Metabolism?" Nutrients 11, no. 9: 2125. https://doi.org/10.3390/nu11092125
APA StyleMarques Loureiro, L., Lessa, S., Mendes, R., Pereira, S., Saboya, C. J., & Ramalho, A. (2019). Does the Metabolically Healthy Obese Phenotype Protect Adults with Class III Obesity from Biochemical Alterations Related to Bone Metabolism? Nutrients, 11(9), 2125. https://doi.org/10.3390/nu11092125





