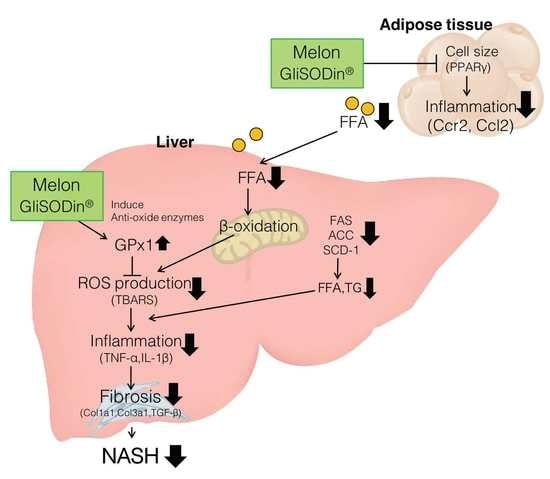
Melon GliSODin® Prevents Diet-Induced NASH Onset by Reducing Fat Synthesis and Improving Liver Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Melon Extract
2.2. Histology and Staining Analysis
2.3. Lipid Parameters: Blood Chemistry and Liver Tissue Analysis
2.4. mRNA Expression Analysis via Quantitative RT-PCR
2.5. 2-Thiobarbituric Acid Reactive Substances (TBARS) Measurement
2.6. Statistical Analysis
3. Results
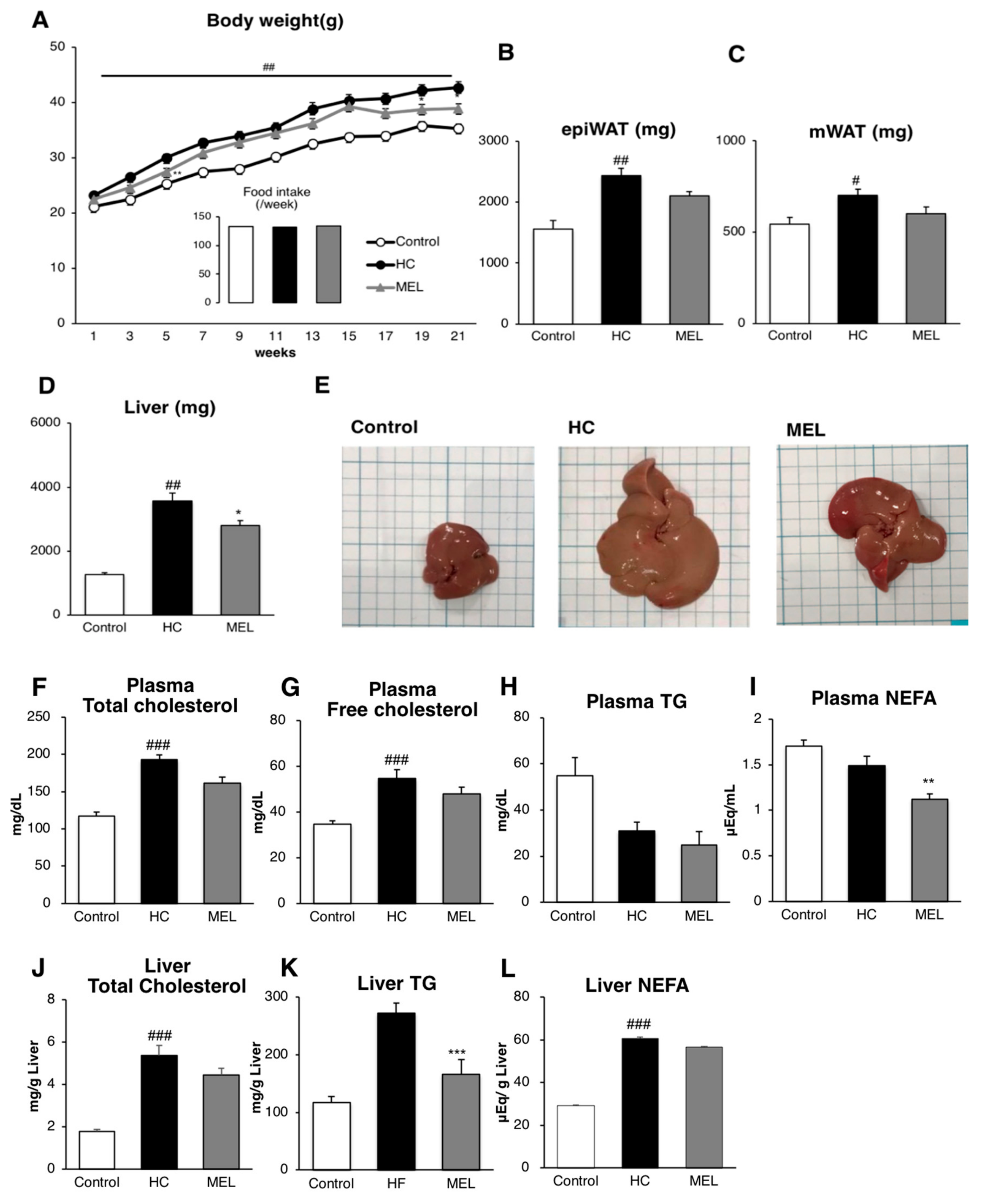
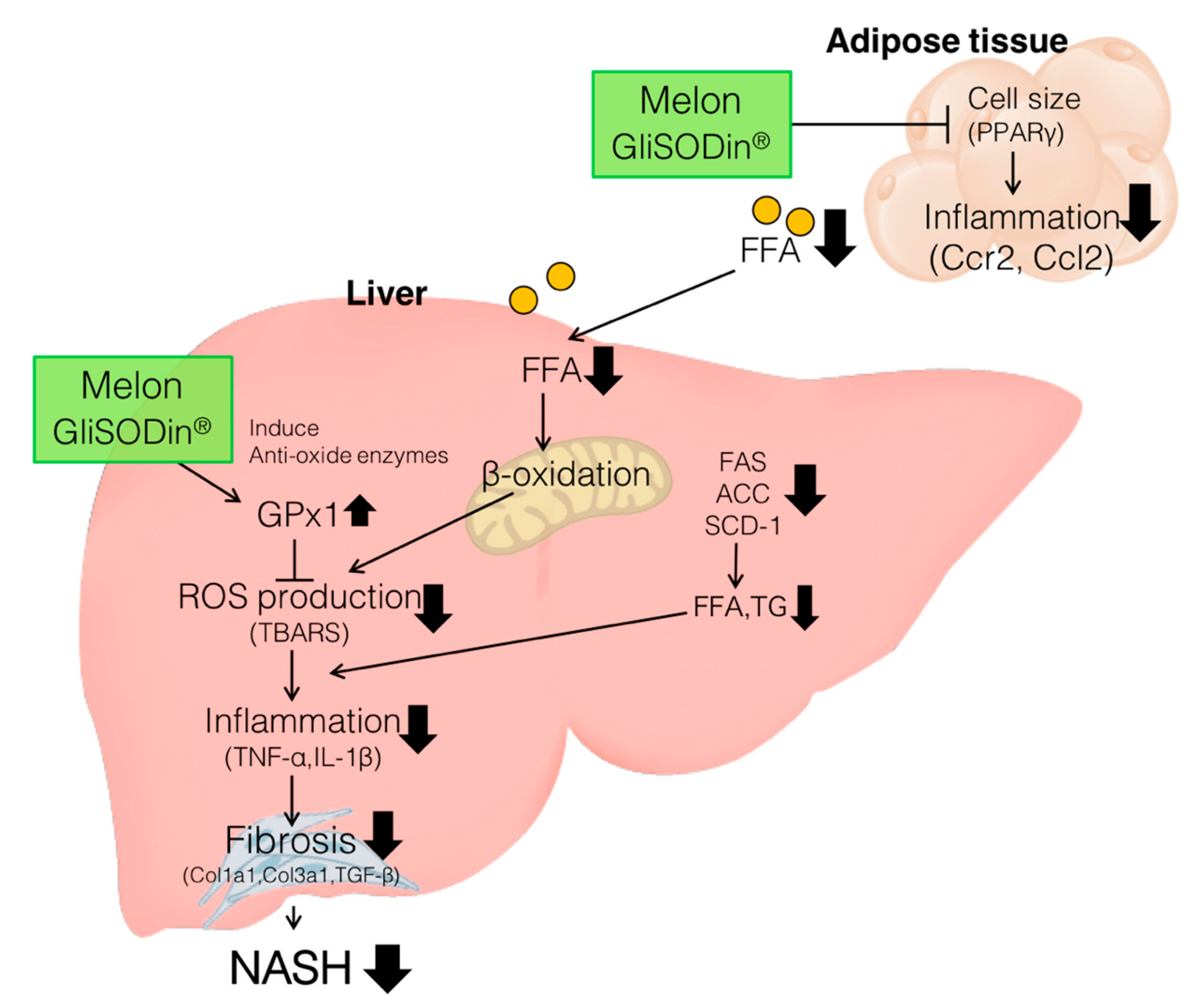
3.1. Melon GliSODin® Prevents Lipid Accumulation in the Mouse Model of Diet-Induced NASH
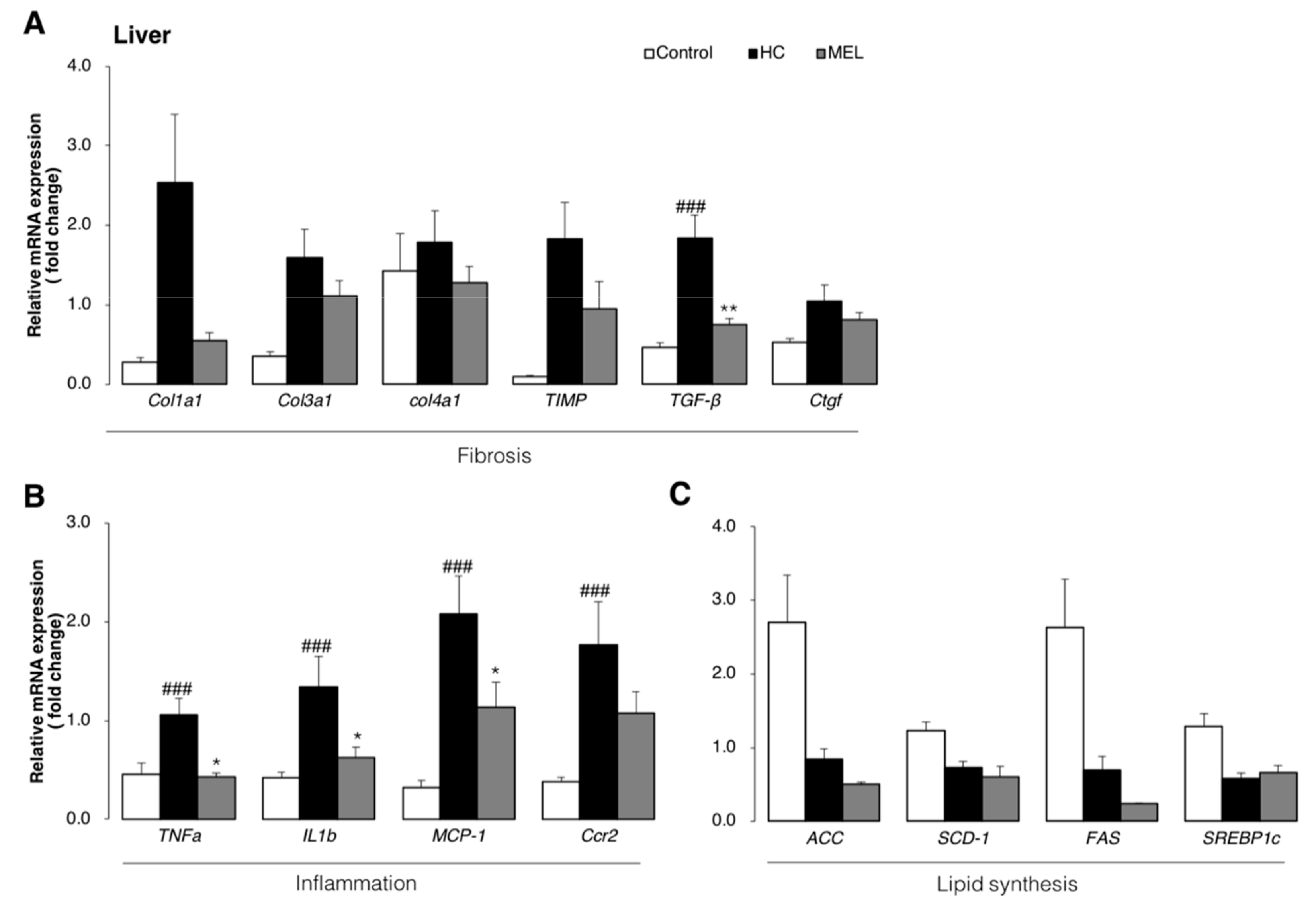
3.2. Melon GliSODin® Prevents Liver Fibrosis in A Mouse Model Of Hc-Induced NASH
3.3. Melon GliSODin® Attenuated Liver Inflammation and Fibrosis at the Molecular Level
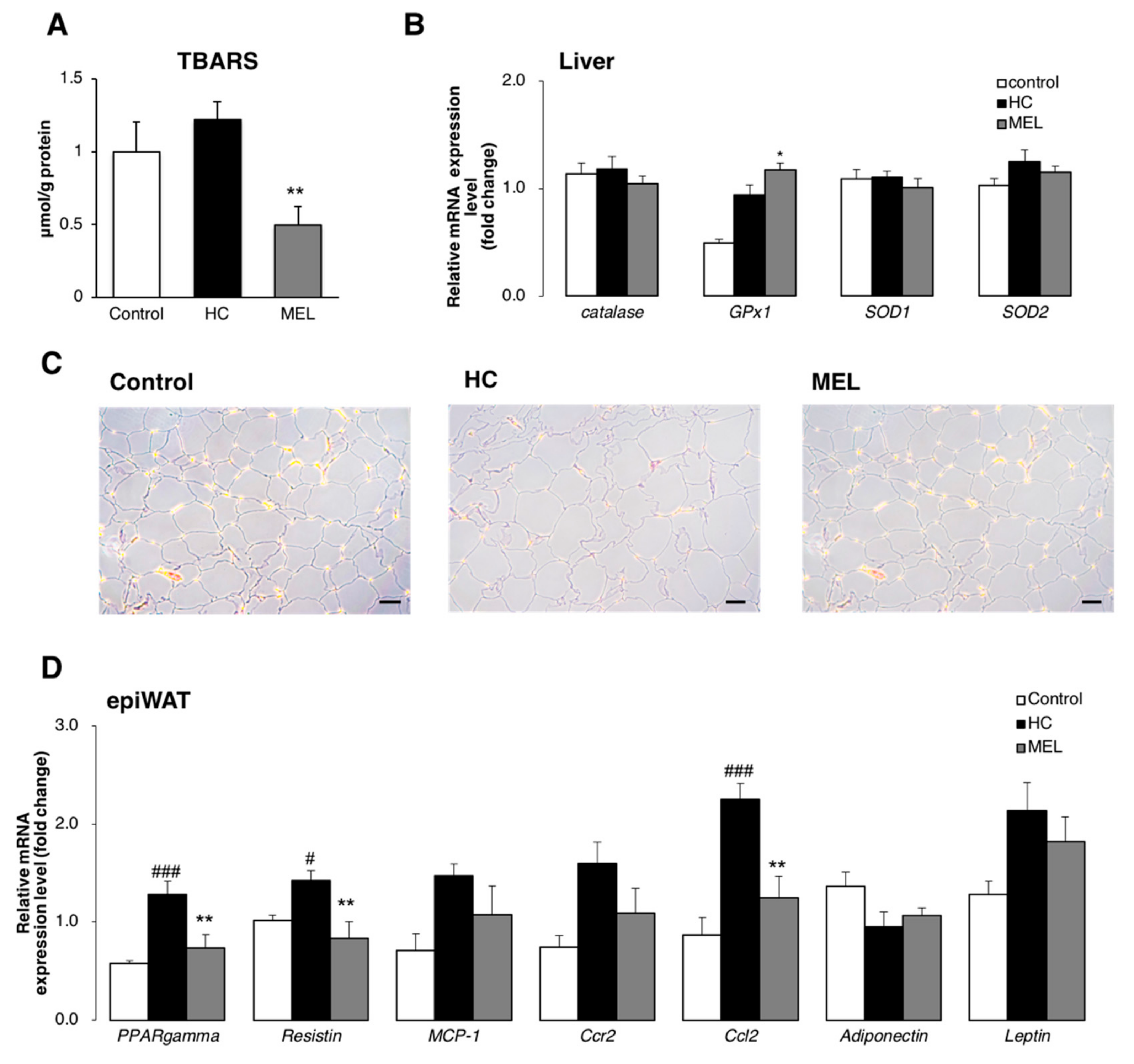
3.4. Melon GliSODin® Boosts Antioxidation Defence Systems in the Hc Diet Loaded Liver.
3.5. Melon GliSODin® Reduces the Size of Adipocytes and Suppresses the Flux of Free Fatty Acids (FFA) in the Liver
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. Biomed. Res. Int. 2019, 2019, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F.W. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin Resistance in Nonalcoholic Fatty Liver Disease. Curr. Pharm. Des. 2010, 16, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef] [PubMed]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Joanny Menvielle-Bourg, F. Superoxide Dismutase (SOD), a Powerful Antioxidant, is now available Orally. Phytothérapie 2005, 3, 1–4. [Google Scholar]
- Ore, A.; Akinloye, O.A. Oxidative Stress and Antioxidant Biomarkers in Clinical and Experimental Models of Non-Alcoholic Fatty Liver Disease. Medicina (Kaunas). 2019, 55, 26. [Google Scholar] [CrossRef]
- Vouldoukis, I.; Conti, M.; Krauss, P.; Kamaté, C.; Blazquez, S.; Tefit, M.; Mazier, D.; Calenda, A.; Dugas, B. Supplementation with gliadin-combined plant superoxide dismutase extract promotes antioxidant defences and protects against oxidative stress. Phyther. Res. 2004, 18, 957–962. [Google Scholar] [CrossRef]
- Vouldoukis, I.; Lacan, D.; Kamate, C.; Coste, P.; Calenda, A.; Mazier, D.; Conti, M.; Dugas, B. Antioxidant and anti-inflammatory properties of a Cucumis melo LC. extract rich in superoxide dismutase activity. J. Ethnopharmacol. 2004, 94, 67–75. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-beta and fibrosis. World J. Gastroenterol. 2007, 13, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Karimi Galougahi, K.; Liu, C.-C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Kaser, S. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut 2005, 54, 117–121. [Google Scholar] [CrossRef]
- Matsunami, T.; Sato, Y.; Ariga, S.; Sato, T.; Shimomura, T.; Kashimura, H.; Hasegawa, Y.; Yukawa, M. Regulation of synthesis and oxidation of fatty acids by adiponectin receptors (AdipoR1/R2) and insulin receptor substrate isoforms (IRS-1/-2) of the liver in a nonalcoholic steatohepatitis animal model. Metabolism. 2011, 60, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Poirier, Y.; Antonenkov, V.D.; Glumoff, T.; Hiltunen, J.K. Peroxisomal β-oxidation—A metabolic pathway with multiple functions. Biochim. Biophys. Acta - Mol. Cell Res. 2006, 1763, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic. Biol. Med. 2008, 45, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Invest. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Berg, A.H.; Scherer, P.E. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef]
- Nakamura, S.; Takamura, T.; Matsuzawa-Nagata, N.; Takayama, H.; Misu, H.; Noda, H.; Nabemoto, S.; Kurita, S.; Ota, T.; Ando, H.; et al. Palmitate Induces Insulin Resistance in H4IIEC3 Hepatocytes through Reactive Oxygen Species Produced by Mitochondria. J. Biol. Chem. 2009, 284, 14809–14818. [Google Scholar] [CrossRef]
- Begriche, K.; Igoudjil, A.; Pessayre, D.; Fromenty, B. Mitochondrial dysfunction in NASH: Causes, consequences and possible means to prevent it. Mitochondrion 2006, 6, 1–28. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| 18S | TTCTGGCCAACGGTCTAGACAAC | CCAGTGGTCTTGGTGTGCTGA |
| Acc | ACCCACTCCACTGTTTGTGA | CCTTGGAATTCAGGAGAGGA |
| Catalase | CCAGCGACCAGATGAAGCAG | CCACTCTCTCAGGAATCCGC |
| Ccl2 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT |
| Ccr2 | AGCACATGTGGTGAATCCAA | TGCCATCATAAAGGAGCCA |
| Ccr2 | AGCACATGTGGTGAATCCAA | TGCCATCATAAAGGAGCCA |
| Col1a1 | CCTCAGGGTATTGCTGGACAAC | TTGATCCAGAAGGACCTTGTTTG |
| Col3a1 | TTGATGTGCAGCTGGCATTC | GCCACTGGCCTGATCCATAT |
| Col4a1 | CACATTTTCCACAGCCAGAG | GTCTGGCTTCTGCTGCTCTT |
| Ctgf | ACCCGAGTTACCAATGACAATACC | CCGCAGAACTTAGCCCTGTATG |
| FAS | TCTGCCAGTGAGTTGAGGAC | CTGCAGAGAAGCGAGCATAC |
| GPx1 | AGTCCACCGTGTATGCCTTCT | GAGACGCGACATTCTCAATGA |
| IL1b | CTGAACTCAACTGTGAAATGCCA | AAAGGTTTGGAAGCAGCCCT |
| MCP-1 | CCACTCACCTGCTGCTACTCAT | TGGTGATCCTCTTGTAGCTCTCC |
| PPARgamma | TGGCCACCTCTTTGCTCTGCTC | AGGCCGAGAAGGAGAAGCTGTTG |
| Resistin | CCCTCCTTTTCCTTTTCTTCCTTG | AGACTGCTGTGCCTTCTGGG |
| Scd1 | CTCCTGCTGATGTGCTTCAT | AAGGTGCTAACGAACAGGCT |
| SOD1 | TGAGGTCCTGCACTGGTAC | CAAGCGGTGAACCAGTTGTG |
| SOD2 | TTAACGCGCAGATCATGCA | GGTGGCGTTGAGATTGTTCA |
| Srebp1c | CGTGAGCTACCTGGACTGAA | CGGGACAGCTTAGCCTCTAC |
| TGF-β | TTGCTTCAGCTCCACAGAGA | TGGTTGTAGAGGGCAAGGAC |
| TIMP | AGGTGGTCTCGTTGATTTCT | GTAAGGCCTGTAGCTGTGCC |
| TNFa | CTGGGACAGTGACCTGGACT | GCACCTCAGGGAAGAGTCTG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, A.; Kitamura, N.; Yokoyama, Y.; Uchida, S.; Kumadaki, K.; Tsubota, K.; Watanabe, M. Melon GliSODin® Prevents Diet-Induced NASH Onset by Reducing Fat Synthesis and Improving Liver Function. Nutrients 2019, 11, 1779. https://doi.org/10.3390/nu11081779
Nakamura A, Kitamura N, Yokoyama Y, Uchida S, Kumadaki K, Tsubota K, Watanabe M. Melon GliSODin® Prevents Diet-Induced NASH Onset by Reducing Fat Synthesis and Improving Liver Function. Nutrients. 2019; 11(8):1779. https://doi.org/10.3390/nu11081779
Chicago/Turabian StyleNakamura, Anna, Naho Kitamura, Yoko Yokoyama, Sena Uchida, Kayo Kumadaki, Kazuo Tsubota, and Mitsuhiro Watanabe. 2019. "Melon GliSODin® Prevents Diet-Induced NASH Onset by Reducing Fat Synthesis and Improving Liver Function" Nutrients 11, no. 8: 1779. https://doi.org/10.3390/nu11081779
APA StyleNakamura, A., Kitamura, N., Yokoyama, Y., Uchida, S., Kumadaki, K., Tsubota, K., & Watanabe, M. (2019). Melon GliSODin® Prevents Diet-Induced NASH Onset by Reducing Fat Synthesis and Improving Liver Function. Nutrients, 11(8), 1779. https://doi.org/10.3390/nu11081779