Maternal Fiber Dietary Intakes during Pregnancy and Infant Allergic Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Maternal Dietary Assessment
2.3. Infant Clinical Outcomes Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Population Characteristics
3.2. Maternal Dietary Intake During Late Pregnancy
3.3. Infant Allergic Disease Outcomes
3.4. Maternal Dietary Intakes and Infant Allergic Disease Outcomes
3.5. Adjusted Model Results of Maternal Dietary Fiber Intakes and Infant Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paaso, E.M.; Jaakkola, M.S.; Rantala, A.K.; Hugg, T.T.; Jaakkola, J.J. Allergic diseases and asthma in the family predict the persistence and onset-age of asthma: A prospective cohort study. Respir. Res. 2014, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Koplin, J.J.; Allen, K.J.; Gurrin, L.C.; Peters, R.L.; Lowe, A.J.; Tang, M.L.K.; Dharmage, S.C. The Impact of Family History of Allergy on Risk of Food Allergy: A Population-Based Study of Infants. Int. J. Environ. Res. Public Heal. 2013, 10, 5364–5377. [Google Scholar] [CrossRef] [PubMed]
- Dold, S.; Wjst, M.; Von Mutius, E.; Reitmeir, P.; Stiepel, E. Genetic risk for asthma, allergic rhinitis, and atopic dermatitis. Arch. Dis. Child. 1992, 67, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Halkjær, L.B.; Hinge, R.; Giwercman, C.; Palmer, C.; Silveira, L.; Strand, M. Risk analysis of early childhood eczema. J. Allergy Clin. Immunol. 2009, 123, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Koplin, J.J.; Wake, M.; Dharmage, S.C.; Matheson, M.; Tang, M.L.; Gurrin, L.C.; Dwyer, T.; Peters, R.L.; Prescott, S.; Ponsonby, A.-L.; et al. Cohort Profile: The HealthNuts Study: Population prevalence and environmental/genetic predictors of food allergy. Int. J. Epidemiol. 2015, 44, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, P.; Anand, S.S.; Becker, A.B.; Befus, A.D.; Brauer, M.; Brook, J.R.; Denburg, J.A.; HayGlass, K.T.; Kobor, M.S.; Kollmann, T.R.; et al. The Canadian Healthy Infant Longitudinal Development (CHILD) Study: Examining developmental origins of allergy and asthma: Table 1. Thorax 2015, 70, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Macaubas, C.; Holt, B.J.; Smallacombe, T.B.; Loh, R.; Sly, P.D.; Holt, P.G. Transplacental priming of the human immune system to environmental allergens: Universal skewing of initial T cell responses toward the Th2 cytokine profile. J. Immunol. 1998, 160, 4730–4737. [Google Scholar] [PubMed]
- Tulic, M.K.; Hodder, M.; Forsberg, A.; McCarthy, S.; Richman, T.; D’Vaz, N.; Biggelaar, A.H.V.D.; Thornton, C.A.; Prescott, S.L. Differences in innate immune function between allergic and nonallergic children: New insights into immune ontogeny. J. Allergy Clin. Immunol. 2011, 127, 470–478. [Google Scholar] [CrossRef]
- Prescott, S.L.; Macaubas, C.; Smallacombe, T.; Holt, B.J.; Sly, P.D.; Holt, P.G. Development of allergen-specific T-cell memory in atopic and normal children. Lancet 1999, 353, 196–200. [Google Scholar] [CrossRef]
- Gold, D.R.; Bloomberg, G.R.; Cruikshank, W.W.; Visness, C.M.; Schwarz, J.; Kattan, M.; O’Connor, G.T.; Wood, R.A.; Burger, M.S.; Wright, R.J.; et al. Parental Characteristics, Somatic Fetal Growth, and Season of Birth Influence Innate and Adaptive Cord Blood Cytokine Responses. J. Allergy Clin. Immunol. 2009, 124, 1078–1087. [Google Scholar] [CrossRef]
- Pfefferle, P.I.; Büchele, G.; Blümer, N.; Roponen, M.; Ege, M.J.; Krauss-Etschmann, S.; Genuneit, J.; Hyvärinen, A.; Hirvonen, M.-R.; Lauener, R.; et al. Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: The PASTURE Study. J. Allergy Clin. Immunol. 2010, 125, 108–115. [Google Scholar] [CrossRef]
- Wopereis, H.; Sim, K.; Shaw, A.; Warner, J.O.; Knol, J.; Kroll, J.S. Intestinal microbiota in infants at high risk for allergy: Effects of prebiotics and role in eczema development. J. Allergy Clin. Immunol. 2018, 141, 1334–1342. [Google Scholar] [CrossRef]
- Sausenthaler, S.; Koletzko, S.; Schaaf, B.; Lehmann, I.; Borte, M.; Herbarth, O.; Von Berg, A.; Wichmann, H.E.; Heinrich, J.; LISA Study Group. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 years of age. Am. J. Clin. Nutr. 2007, 85, 530–537. [Google Scholar]
- Viljoen, K.; Segurado, R.; O’Brien, J.; Mehegan, J.; Bury, G.; Daly, L.; Daly, S.; Doyle, O.; Fallon, U.B.; Hannon, F.B.; et al. Pregnancy diet and offspring asthma risk over a 10-year period: The Lifeways Cross Generation Cohort Study, Ireland. BMJ Open 2018, 8, e017013. [Google Scholar] [CrossRef]
- West, C.E.; Dunstan, J.; McCarthy, S.; Metcalfe, J.; D’Vaz, N.; Meldrum, S.; Oddy, W.H.; Tulic, M.K.; Prescott, S.L. Associations between Maternal Antioxidant Intakes in Pregnancy and Infant Allergic Outcomes. Nutrients 2012, 4, 1747–1758. [Google Scholar] [CrossRef]
- Dunstan, J.A.; West, C.; McCarthy, S.; Metcalfe, J.; Meldrum, S.; Oddy, W.H.; Tulic, M.K.; D’Vaz, N.; Prescott, S.L. The relationship between maternal folate status in pregnancy, cord blood folate levels, and allergic outcomes in early childhood. Allergy 2012, 67, 50–57. [Google Scholar] [CrossRef]
- Romieu, I.; Torrent, M.; Garcia-Esteban, R.; Ferrer, C.; Ribas-Fitó, N.; Antó, J.M.; Sunyer, J.; Garcia-Esteban, R.; Ribas-Fitó, N. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin. Exp. Allergy 2007, 37, 518–525. [Google Scholar] [CrossRef]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy 2010, 65, 758–765. [Google Scholar] [CrossRef]
- Chatzi, L.; Torrent, M.; Romieu, I.; Garcia-Esteban, R.; Ferrer, C.; Vioque, J.; Kogevinas, M.; Sunyer, J. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax 2008, 63, 507–513. [Google Scholar] [CrossRef]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef]
- Wopereis, H.; Oozeer, R.; Knipping, K.; Belzer, C.; Knol, J. The first thousand days - intestinal microbiology of early life: Establishing a symbiosis. Pediatr. Allergy Immunol. 2014, 25, 428–438. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- McRae, M.P. Dietary Fiber Is Beneficial for the Prevention of Cardiovascular Disease: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2017, 16, 289–299. [Google Scholar] [CrossRef]
- McRae, M.P. Dietary Fiber Intake and Type 2 Diabetes Mellitus: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2018, 17, 44–53. [Google Scholar] [CrossRef]
- Wong, C. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Roberfroid, M. Dietary fiber, inulin, and oligofructose: A review comparing their physiological effects. Crit. Rev. Food Sci. Nutr. 1993, 33, 103–148. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- McKenzie, C.; Tan, J.; Macia, L.; Mackay, C.R. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 2017, 278, 277–295. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Halnes, I.; Baines, K.J.; Berthon, B.S.; MacDonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Soluble Fibre Meal Challenge Reduces Airway Inflammation and Expression of GPR43 and GPR41 in Asthma. Nutrients 2017, 9, 57. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Wawrzyniak, P.; Akdis, C.A.; Finkelman, F.D.; Rothenberg, M.E. Advances and highlights in mechanisms of allergic disease in 2015. J. Allergy Clin. Immunol. 2016, 137, 1681–1696. [Google Scholar] [CrossRef]
- Brogdon, J.L.; Xu, Y.; Szabo, S.J.; An, S.; Buxton, F.; Cohen, D.; Huang, Q. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood 2007, 109, 1123–1130. [Google Scholar] [CrossRef]
- Shadid, R.; Haarman, M.; Knol, J.; Theis, W.; Beermann, C.; Rjosk-Dendorfer, D.; Schendel, D.J.; Koletzko, B.V.; Krauss-Etschmann, S. Effects of galactooligosaccharide and long-chain fructooligosaccharide supplementation during pregnancy on maternal and neonatal microbiota and immunity—A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2007, 86, 1426–1437. [Google Scholar] [CrossRef]
- Jinno, S.; Toshimitsu, T.; Nakamura, Y.; Kubota, T.; Igoshi, Y.; Ozawa, N.; Suzuki, S.; Nakano, T.; Morita, Y.; Arima, T.; et al. Maternal Prebiotic Ingestion Increased the Number of Fecal Bifidobacteria in Pregnant Women but Not in Their Neonates Aged One Month. Nutrients 2017, 9, 196. [Google Scholar] [CrossRef]
- Hodge, A.; Patterson, A.; Brown, W.; Ireland, P. The Anti Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust. N. Z. J. Public Health 2000, 24, 576–583. [Google Scholar] [CrossRef]
- Ireland, P.; Jolley, D.; Giles, G.; O’Dea, K.; Powles, J.; Rutishauser, I.; Wahlqvist, M.L.; Williams, J. Development of Melbourne FFQ: A food frequency for use in a Australian prospective study involving and ethnically diverse cohort. Asia Pac. J. Clin. Nutr. 1994, 3, 19–31. [Google Scholar]
- Australian Bureau of Statistics. Australian Health Survey: 2015. Available online: https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/4364.0.55.0072011-12 (accessed on 4 September 2018).
- Food Standards Australia New Zealand. 2010. Available online: http://www.foodstandards.gov.au/science/monitoringnutrients/pages/default.aspx (accessed on 28 August 2018).
- Cashel, K.; English, R.; Lewis, J. Composition of Food. Nutrition Section, Department of Community Services and Health; Australian Government Publishing Services: Canberra, Australia, 1989.
- Foodworks, version 9; Foodworks: Melbourne, Australia, 2018. Available online: http://xyris.com.au (accessed on 12 September 2018).
- FoodZone. FoodZone: 2014. Available online: http://www.foodzone.com.au (accessed on 12 September 2018).
- Biesiekierski, J.R.; Rosella, O.; Rose, R.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J. Hum. Nutr. Diet. 2011, 24, 154–176. [Google Scholar] [CrossRef]
- Muir, J.; Rosella, Q.; Liesl, K.; Barret, J.S.; Sheperd, S.J.; Gibson, R.P. Measurement of Short-Chain Carbohydrates in Common Australian Vegetables and Fruits by High-Performance Liquid Chromatography (HPLC). J. Agric. Food Chem. 2009, 57, 554–565. [Google Scholar] [CrossRef]
- Muir, J.; Sheperd, S.J.; Rosella, Q.; Rose, R.; Barret, J.S.; Gibson, R.P. Fructan and Free Fructose Content of Common Australian Vegetables and Fruit. J. Agric. Food Chem. 2007, 55, 6619–6627. [Google Scholar] [CrossRef]
- Van Loo, J.; Coussement, P.; De Leenheer, L.; Hoebregs, H.; Smits, G. On the presence of Inulin and Oligofructose as natural ingredients in the western diet. Crit. Rev. Food Sci. Nutr. 1995, 35, 525–552. [Google Scholar] [CrossRef]
- Gresham, E.; Collins, C.E.; Mishra, G.D.; Byles, J.E.; Hure, A.J. Diet quality before or during pregnancy and the relationship with pregnancy and birth outcomes: The Australian Longitudinal Study on Women’s Health. Public Health Nutr. 2016, 19, 2975–2983. [Google Scholar] [CrossRef]
- Meltzer, H.; Brantsæter, A.; Ydersbond, T.; Haugen, M.; Alexander, J.; Group TMDS. Methodological challenges when monitoring the diet of pregnant women in a large study: Experiences from the Norwegian Mother and Child Cohort Study (MoBa). Matern. Child Nutr. 2008, 4, 14–27. [Google Scholar] [CrossRef]
- Nurmatov, U.; Nwaru, B.I.; Devereux, G.; Sheikh, A.; Nwaru, B. Confounding and effect modification in studies of diet and childhood asthma and allergies. Allergy 2012, 67, 1041–1059. [Google Scholar] [CrossRef]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I. Research advances on structural characterization of resistant starch and its structure-physiological function relationship: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1059–1083. [Google Scholar] [CrossRef]
- Hogenkamp, A.; Thijssen, S.; Van Vlies, N.; Garssen, J. Supplementing pregnant mice with a specific mixture of nondigestible oligosaccharides reduces symptoms of allergic asthma in male offspring. J. Nutr. 2015, 145, 640–646. [Google Scholar] [CrossRef]
- Michael, C.F.; Waters, C.M.; LeMessurier, K.S.; Samarasinghe, A.E.; Song, C.Y.; Malik, K.U.; Lew, D.B. Airway Epithelial Repair by a Prebiotic Mannan Derived from Saccharomyces cerevisiae. J. Immunol. Res. 2017, 2017, 8903982. [Google Scholar] [CrossRef]
- Fujiwara, R.; Takemura, N.; Watanabe, J.; Sonoyama, K. Maternal consumption of fructo-oligosaccharide diminishes the severity of skin inflammation in offspring of NC/Nga mice. Br. J. Nutr. 2010, 103, 530–538. [Google Scholar] [CrossRef]
- Gough, H.; Grabenhenrich, L.; Reich, A.; Eckers, N.; Nitsche, O.; Schramm, D.; Beschorner, J.; Hoffmann, U.; Schuster, A.; Bauer, C.-P.; et al. Allergic multimorbidity of asthma, rhinitis and eczema over 20 years in the German birth cohort MAS. Pediatr. Allergy Immunol. 2015, 26, 431–437. [Google Scholar] [CrossRef]
- Maas, S.L.; Soehnlein, O.; Viola, J.R. Organ-Specific Mechanisms of Transendothelial Neutrophil Migration in the Lung, Liver, Kidney, and Aorta. Front. Immunol. 2018, 9, 2739. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestation and infections during the first two years of life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar] [CrossRef]
- Boyle, R.J.; Tang, M.K.; Chiang, W.C.; Chua, M.C.; Ismail, I.; Nauta, A.; Hourihane, J.; Smith, P.; Gold, M.; Ziegler, J.; et al. Prebiotic-supplemented partially hydrolysed cow’s milk formula for the prevention of eczema in high-risk infants: A randomized controlled trial. Allergy 2016, 71, 701–710. [Google Scholar] [CrossRef]
- Shibata, R.; Kimura, M.; Takahashi, H.; Mikami, K.; Aiba, Y.; Takeda, H.; Koga, Y. Clinical effects of kestose, a prebiotic oligosaccharide, on the treatment of atopic dermatitis in infants. Clin. Exp. Allergy 2009, 39, 1397–1403. [Google Scholar] [CrossRef]
- Moro, G.; Arslanoglu, S.; Stahl, B.; Jelinek, J.; Wahn, U.; Boehm, G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch. Dis. Child. 2006, 91, 814–819. [Google Scholar] [CrossRef]
- Grüber, C.; Van Stuijvenberg, M.; Mosca, F.; Moro, G.; Chirico, G.; Braegger, C.P.; Riedler, J.; Boehm, G.; Wahn, U.; MIPS 1 Working Group. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J. Allergy Clin. Immunol. 2010, 126, 791–797. [Google Scholar]
| Maternal and Infant Characteristics | |
|---|---|
| Maternal age (years) a | 32 (30–36) |
| Maternal allergic disease history b | 579 (90.6%) |
| Maternal ethnicity (Caucasian) b | 563 (91.1%) |
| Education level (further education post high school) b | 474 (74.6%) |
| Multivitamin consumption in pregnancy b | 525 (86.8%) |
| Pet ownership in pregnancy (cat, dog, or both) b | 382 (59.8%) |
| First born child b | 306 (47.9%) |
| Vaginal birth b | 416 (66.5%) |
| Child gender (male) b | 333 (52.1%) |
| Infant gestational age at birth (weeks) a | 39 (39–40) |
| Infant weight at birth (g) c | 3496 (±431) |
| Dietary Intake Variables | Median (IQR) |
|---|---|
| Energy (kJ/day) | 8082 (6612–9845) |
| Carbohydrate (g/day) | 207.7 (156.3–268.2) |
| Total dietary fiber (g/day) | 23.8 (19.0–29.0) |
| Soluble dietary fiber (g/day) | 5.6 (4.4–6.9) |
| Insoluble dietary fiber (g/day) | 6.7 (5.0–8.6) |
| Prebiotic fiber (g/day) | 1.4 (1.1–2.0) |
| Resistant starch (g/day) | 2.8 (2.1–3.5) |
| Total fiber from grains (g/day) | 9.2 (7.0–11.9) |
| Total fiber from fruit (g/day) | 3.5 (2.2–5.2) |
| Total fiber from vegetables (g/day) | 3.5 (2.5–4.8) |
| Total fiber from green vegetables (g/day) | 1.1 (0.7–1.6) |
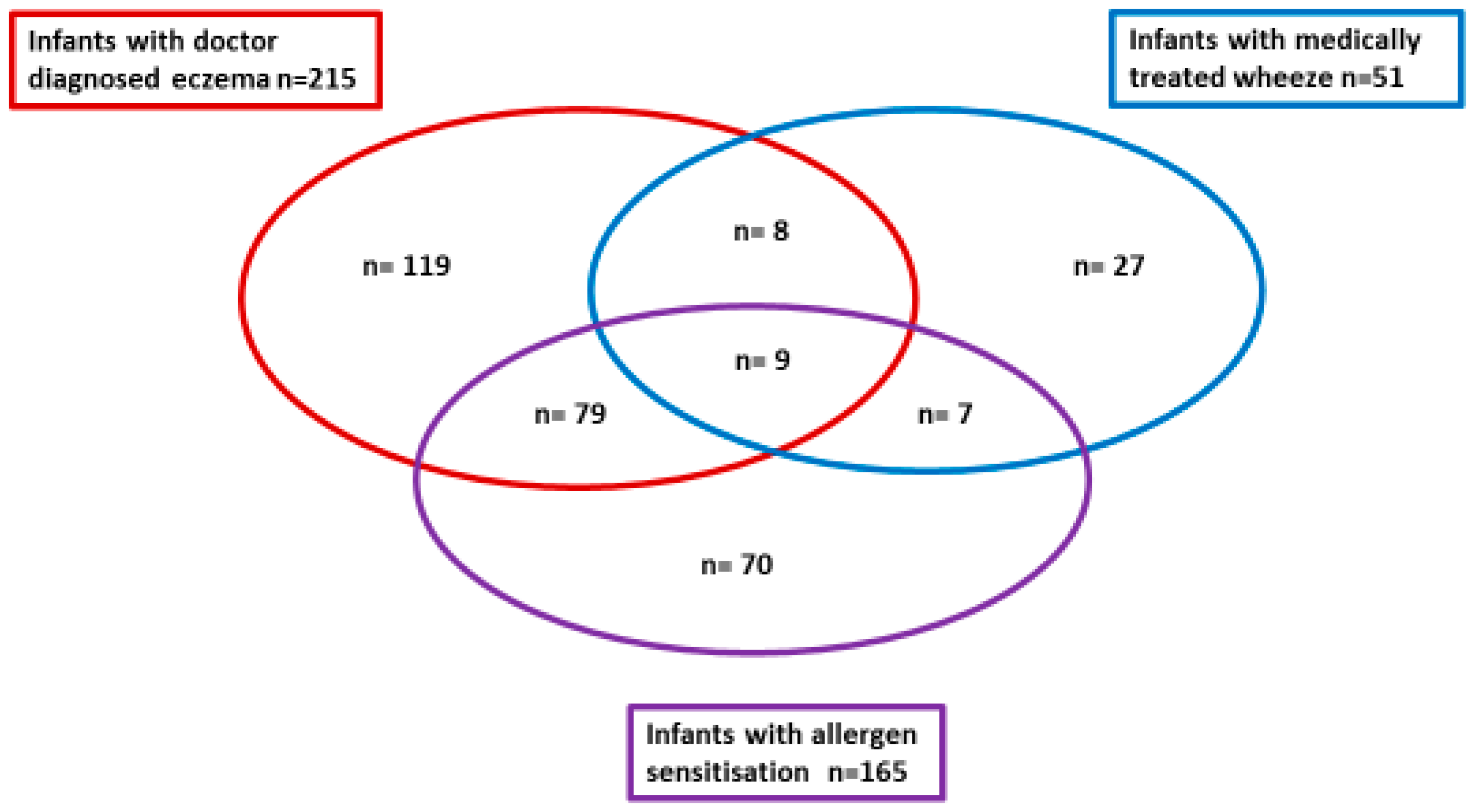
| Parent Reported Wheeze | Doctor Diagnosed Wheeze | Parent Reported Eczema | Doctor Diagnosed Eczema | Steroid Treated Eczema | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Maternal dietary intakes | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No |
| 166/638 (26%) | 472/638 (74%) | 51/629 (8%) | 578/629 (92%) | 288/639 (45%) | 351/639 (55%) | 215/635 (34%) | 420/635 (66%) | 166/630 (26%) | 464/630 (74%) | |
| Energy (kJ/day) | 7846 | 8135 | 7897 | 8093 | 8164 | 7900 | 8104 | 7974 | 8190 | 7926 |
| (6294–9836) | (6771–9869) | (5770–9858) | (6758–9864) | (6894–9861) | (6358–9835) | (6994–9861) | (6390–9840) | (6849–10211) | (6507–9731) | |
| p-value | 0.07 | 0.15 | 0.18 | 0.22 | 0.15 | |||||
| Carbohydrate | 200.43 | 208.99 | 193.63 | 209.43 | 215.32 | 202.24 | 216.34 | 203.27 | 215.39 | 205.41 |
| (g/day) | (151.49–254.37) | (159.62–272.33) | (143.04–257.06) | (156.82–270.76) | (168.25–273.97) | (150.84–261.87) | (173.39–274.20) | (149.37–262.90) | (174.41–274.74) | (150.11–262.19) |
| p-value | 0.34 | 0.28 | 0.05 | 0.01 | 0.03 | |||||
| Total fiber (g/day) | 23.04 | 24.14 | 22.75 | 24.01 | 24.58 | 22.68 | 25.01 | 23.1 | 25.01 | 23.27 |
| (17.71–27.65) | (19.32–29.56) | (17.50–25.78) | (19.14–29.36) | 20.25–29.03) | (17.96–29.04) | (20.29–28.68) | (18.19–29.20) | (20.23–28.63) | (18.28–29.07) | |
| p-value | 0.81 | 0.1 | 0.01 | 0.04 | 0.81 | |||||
| Soluble fiber | 5.23 | 5.73 | 5.01 | 5.66 | 5.65 | 5.54 | 5.71 | 5.5 | 5.71 | 5.52 |
| (g/day) | (4.29–6.73) | (4.59–7.09) | (4.20–6.70) | (4.44–6.99) | (4.43–6.94) | (4.38–6.94) | (4.47–7.08) | (4.37–6.89) | (4.45–7.12) | (4.38–6.81) |
| p-value | 0.02 | 0.08 | 0.8 | 0.01 | 0.03 | |||||
| Insoluble fiber | 6.35 | 6.77 | 6.68 | 6.71 | 6.36 | 6.76 | 6.85 | 6.65 | 6.71 | 6.67 |
| (g/day) | (4.98–8.43) | (5.07–8.73) | (5.20–9.37) | (5.02–8.59) | (4.92–8.44) | (5.23–8.98) | (5.09–8.61) | (5.00–8.63) | (5.04–8.62) | (5.00–8.61) |
| p-value | 0.15 | 0.65 | 0.12 | 0.75 | 0.9 | |||||
| Prebiotic fiber | 1.45 | 1.44 | 1.38 | 1.44 | 1.52 | 1.39 | 1.52 | 1.42 | 1.51 | 1.43 |
| (g/day) | (1.02–1.96) | (1.10–2.95) | (0.97–1.98) | 1.09–1.95) | (1.06–1.98) | (1.09–1.92) | (1.06–1.99) | (1.08–1.94) | (1.06–21.97) | (1.08–1.93) |
| p-value | 0.84 | 0.6 | 0.46 | 0.53 | 0.93 | |||||
| Resistant starch | 2.58 | 2.84 | 2.46 | 2.84 | 2.93 | 2.64 | 2.99 | 2.67 | 2.97 | 2.71 |
| (g/day) | (2.04–3.44) | (2.16–3.53) | (2.02–3.05) | (2.13–3.54) | (2.34–3.59) | (2.03–3.41) | (2.34–3.56) | (2.06–3.47) | (2.26–3.54) | (2.10–3.50) |
| p-value | 0.18 | 0.02 | <0.01 | 0.01 | 0.11 | |||||
| Total fiber, grains (g/day) | 9.25 | 9.36 | 7.64 | 9.33 | 9.43 | 9.09 | 9.58 | 8.99 | 9.55 | 9.14 |
| (6.52–11.09) | (7.06–11.85) | (5.76–12.23) | (7.0–11.8) | (7.4–11.8) | (6.73–12.05) | (7.52–12.04) | (6.69–11.62) | (7.47–11.92) | (6.73–11.70) | |
| p-value | 0.21 | 0.12 | 0.2 | 0.02 | 0.12 | |||||
| Total fiber, gluten | 7.31 | 7.81 | 6.41 | 7.73 | 8.11 | 7.2 | 8.1 | 7.42 | 7.91 | 7.41 |
| rich foods (g/day) | (5.10–10.31) | (5.50–10.40) | (4.40–10.90) | (5.51–10.32) | (5.71–10.50) | (5.10–10.21) | (5.91–10.90) | (5.10–10.21) | (5.61–10.80) | (5.10–10.31) |
| p-value | 0.35 | 0.14 | 0.02 | 0.01 | 0.09 | |||||
| Total fiber, green | 1.18 | 1.08 | 1.13 | 1.11 | 1.11 | 1.11 | 1.19 | 1.09 | 1.17 | 1.09 |
| vegetables (g/day) | (0.8–1.8) | (0.70–1.58) | (0.84–1.76) | (0.70–1.62) | (0.75–1.70) | (0.65–1.55) | (0.77–1.77) | (0.68–1.55) | (0.79–1.62) | (0.68–1.63) |
| p-value | 0.31 | 0.57 | 0.25 | 0.04 | 0.19 | |||||
| Total fiber, fruit | 3.14 | 3.69 | 3.33 | 3.35 | 3.61 | 3.51 | 3.61 | 3.52 | 3.86 | 3.47 |
| (g/day) | (1.9–4.8) | (2.37–5.44) | (1.94–4.91) | (2.26–5.23) | (2.20–5.22) | (2.25–5.14) | (2.16–5.38) | (2.28–5.20) | (2.18–5.56) | (2.44–4.78) |
| p-value | <0.01 | 0.27 | 0.51 | 0.76 | 0.26 | |||||
| Allergen Sensitization | IgE-Mediated Food Allergy | |||||
|---|---|---|---|---|---|---|
| Maternal Dietary Intakes | Yes 165/629 (26%) | No 464/629 (74%) | p Value | Yes 91/632 (14%) | No 541/632 (86%) | p Value |
| Energy | 7959 | 8094 | 0.94 | 8107 | 7975 | 0.75 |
| (6880–9713) | (6469–10,079) | (6854–9828) | (6528–9864) | |||
| Carbohydrate | 212.96 | 204.50 | 0.16 | 217.37 | 205.00 | 0.08 |
| (167.8–272.0) | (150.94–268.05) | (179.81–272.73) | (153.59–268.90) | |||
| Total fiber | 24.10 | 23.49 | 0.60 | 23.62 | 23.90 | 0.47 |
| (19.61–28.51) | (18.40–29.31) | (19.43–27.41) | (18.61–29.54) | |||
| Soluble fiber | 5.71 | 5.55 | 0.55 | 5.69 | 5.57 | 0.83 |
| (4.42–7.00) | (4.41–6.94) | (4.41–6.71) | (4.41–6.96) | |||
| Insoluble fiber | 7.06 | 6.59 | 0.30 | 7.08 | 6.60 | 0.18 |
| (5.19–8.66) | (4.99–8.58) | (5.61–8.89) | (5.60–8.61) | |||
| Prebiotic fiber | 1.41 | 1.50 | 0.63 | 1.40 | 1.50 | 0.31 |
| (1.06–1.93) | (1.07–1.96) | (1.01–1.86) | (1.09–1.96) | |||
| Resistant | 2.84 | 2.70 | 0.40 | 2.90 | 2.70 | 0.28 |
| starch | (2.22–3.51) | (2.11–3.54) | (2.31–3.50) | (2.10–3.52) | ||
| Total fiber, grains | 9.44 | 9.20 | 0.78 | 9.70 | 9.20 | 0.38 |
| (6.96–12.08) | (7.00–11.63) | (7.23–12.26) | (6.91–11.70) | |||
| Total fiber, gluten rich | 7.40 | 7.70 | 0.73 | 7.40 | 7.70 | 0.46 |
| (5.71–10.92) | 5.42–10.21 | (5.91–11.20) | (5.41–10.21) | |||
| Total fiber, vegetables | 3.56 | 3.50 | 0.84 | 3.59 | 3.46 | 0.90 |
| (2.44–4.71) | (2.48–4.77) | (2.44–4.51) | (2.47–4.84) | |||
| Total fiber, green vegetables | 1.17 | 1.10 | 0.43 | 1.30 | 1.10 | 0.23 |
| (0.74–1.63) | (0.70–1.62) | (0.75–1.67) | (0.72–1.62) | |||
| Total fiber, fruit | 3.50 | 3.50 | 0.85 | 3.30 | 3.58 | 0.12 |
| (2.23–5.25 | (2.22–5162) | (1.77–4.75) | (2.29–5.25) | |||
| Parent Reported Wheeze | Doctor Diagnosed Wheeze | Parent Reported Eczema | Doctor Diagnosed Eczema | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) § | OR (95% CI) | aOR (95% CI) § | OR (95% CI) | aOR (95% CI) § | OR (95% CI) | aOR (95% CI) § | |
| Carbohydrate | 0.99 (0.99, 1.00) | 0.99 (0.99, 1.04) | 1.00 (1.00, 1.00) | 1.00 (0.99, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
| p value | 0.39 | 0.32 | 0.32 | 0.11 | 0.10 | 0.14 | 0.02 | 0.05 |
| Total dietary fiber | 0.98 (0.96, 1.00) | 0.99 (0.99, 1.00) | 0.96 (0.93, 1.00) | 0.98 (0.94, 1.01) | 1.02 (1.01, 1.04) | 1.03 (1.01, 1.05) | 1.02 (0.99, 1.04) | 1.01 (0.99, 1.04) |
| p value | 0.08 | 0.31 | 0.1 | 0.2 | 0.04 | 0.04 | 0.08 | 0.18 |
| Soluble fiber | 0.89 (0.82, 0.98) | 0.91 (0.83, 1.00) | 0.85 (0.73, 0.99) | 0.85 (0.73, 1.00) | 0.99 (0.93, 1.07) | 0.98 (0.91, 1.07) | 1.03 (0.95, 1.11) | 1.01 (0.93, 1.10) |
| p value | 0.02 | 0.06 | 0.04 | 0.05 | 0.91 | 0.77 | 0.48 | 0.81 |
| Insoluble fiber | 0.96 (0.90, 1.02) | 0.97 (0.92, 1.03) | 1.00 (0.92, 1.09) | 0.99 (0.90, 1.09) | 0.95 (0.91, 0.99) | 0.93 (0.89, 0.98) | 0.99 (0.94, 1.04) | 0.97 (0.92, 1.02) |
| p value | 0.17 | 0.35 | 0.85 | 0.94 | 0.04 | 0.01 | 0.71 | 0.25 |
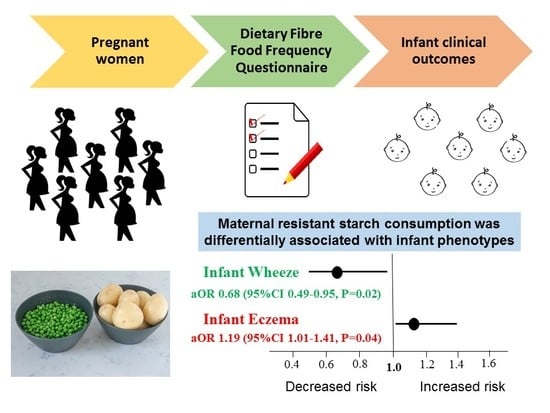
| Resistant starch | 0.93 (0.79, 1.10) | 0.96 (0.81, 1.15) | 0.68 (0.50, 0.94) | 0.68 (0.49, 0.95) | 1.25 (1.08, 1.45) | 1.27 (1.09, 1.49) | 1.19 (1.02, 1.38) | 1.19 (1.01,1.41) |
| p value | 0.39 | 0.67 | 0.02 | 0.02 | <0.01 | <0.01 | 0.02 | 0.04 |
| Prebiotic fiber | 0.94 (0.72, 1.23) | 1.00 (0.75, 1.34) | 0.88 (0.56, 1.38) | 0.95 (0.60, 1.48) | 1.05 (0.83, 1.33) | 1.05 (0.82, 1.36) | 1.04 (0.82, 1.33) | 1.01 (0.78, 1.32) |
| p value | 0.65 | 0.97 | 0.58 | 0.82 | 0.63 | 0.68 | 0.74 | 0.93 |
| Fiber, grains | 0.97 (0.93, 1.02) | 0.98 (0.93, 1.02) | 0.94 (0.87, 1.01) | 0.93 (0.86, 1.02) | 1.01 (0.98, 1.05) | 1.01 (0.97, 1.05) | 1.03 (0.99, 1.08) | 1.02 (0.98, 1.07) |
| p value | 0.18 | 0.36 | 0.12 | 0.09 | 0.47 | 0.69 | 0.07 | 0.25 |
| Fiber, gluten foods | 0.97 (0.94, 1.02) | 0.98 (0.94,1.04) | 0.95 (0.89, 1.03) | 0.95 (0.88, 1.03) | 1.03 (0.99, 1.07) | 1.03 (0.99, 1.07) | 1.05 (1.01, 1.09) | 1.03 (0.99, 1.08) |
| p value | 0.34 | 0.63 | 0.24 | 0.25 | 0.09 | 0.16 | 0.03 | 0.1 |
| Fiber, green veg | 1.09 (0.87, 1.36) | 1.08 (0.85, 1.36) | 1.19 (0.84, 1.67) | 1.09 (0.76, 1.55) | 1.14 (0.93, 1.39) | 1.18 (0.95, 1.46) | 1.27 (1.03, 1.56) | 1.32 (1.06, 1.64) |
| p value | 0.45 | 0.53 | 0.33 | 0.61 | 0.22 | 0.12 | 0.02 | 0.01 |
| Fiber, fruits | 0.89 (0.82, 0.97) | 0.90 (0.83, 0.98) | 0.93 (0.79, 1.11) | 0.94 (0.83, 1.09) | 1.00 (0.94, 1.07) | 0.99 (0.93, 1.06) | 0.99 (0.93, 1.07) | 0.98 (0.92, 1.05) |
| p value | <0.01 | 0.02 | 0.27 | 0.39 | 0.96 | 0.79 | 0.98 | 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pretorius, R.A.; Bodinier, M.; Prescott, S.L.; Palmer, D.J. Maternal Fiber Dietary Intakes during Pregnancy and Infant Allergic Disease. Nutrients 2019, 11, 1767. https://doi.org/10.3390/nu11081767
Pretorius RA, Bodinier M, Prescott SL, Palmer DJ. Maternal Fiber Dietary Intakes during Pregnancy and Infant Allergic Disease. Nutrients. 2019; 11(8):1767. https://doi.org/10.3390/nu11081767
Chicago/Turabian StylePretorius, Rachelle A., Marie Bodinier, Susan L. Prescott, and Debra J. Palmer. 2019. "Maternal Fiber Dietary Intakes during Pregnancy and Infant Allergic Disease" Nutrients 11, no. 8: 1767. https://doi.org/10.3390/nu11081767
APA StylePretorius, R. A., Bodinier, M., Prescott, S. L., & Palmer, D. J. (2019). Maternal Fiber Dietary Intakes during Pregnancy and Infant Allergic Disease. Nutrients, 11(8), 1767. https://doi.org/10.3390/nu11081767