‘Low-Salt’ Bread as an Important Component of a Pragmatic Reduced-Salt Diet for Lowering Blood Pressure in Adults with Elevated Blood Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethical Considerations
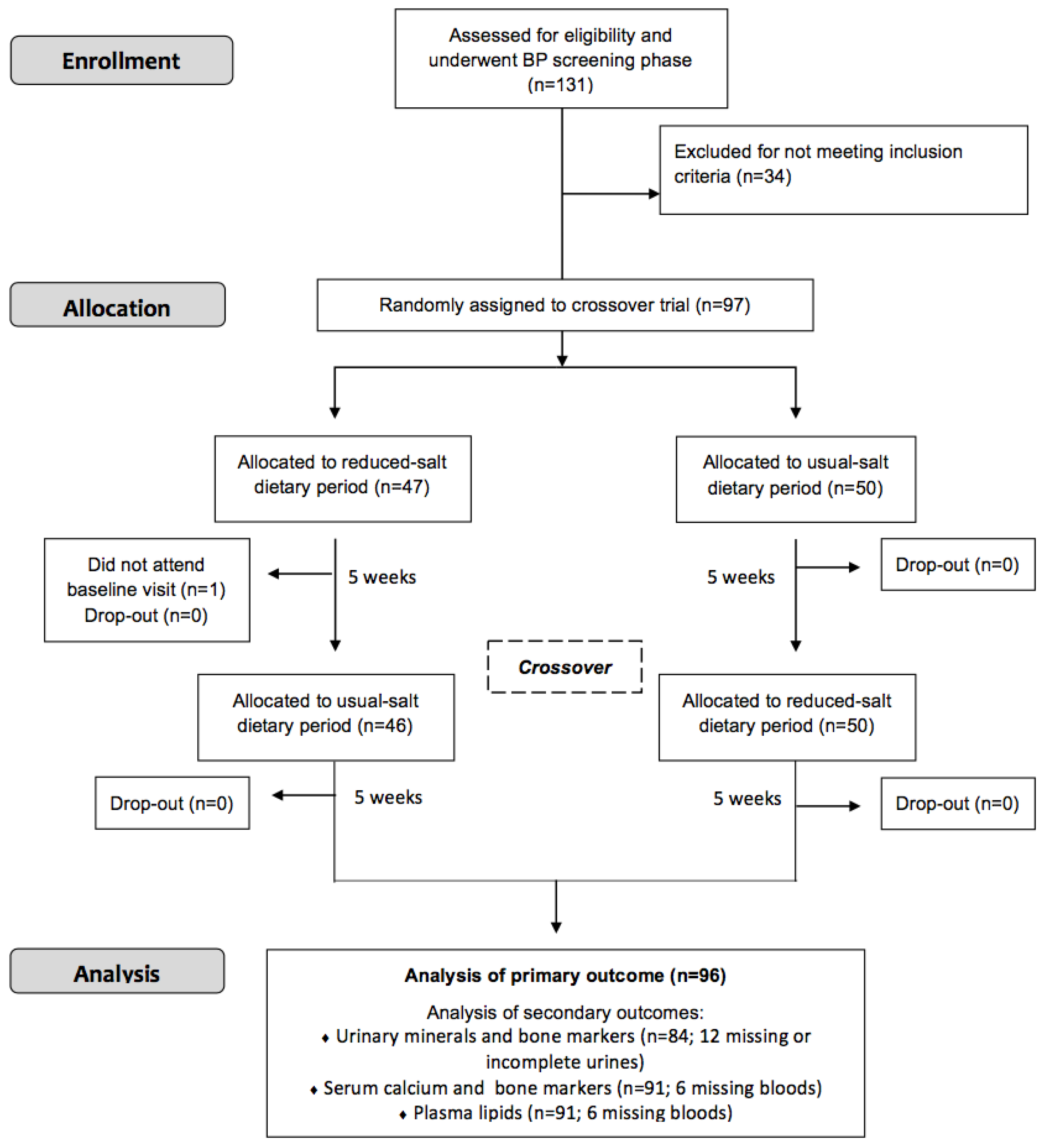
2.3. Design and Conduct of Study
2.4. Production of Low-Salt and Usual-Salt Bread and Luncheon Meats with No Added Salt
2.5. Blood Pressure Measurements
2.6. Preparation of Biological Samples
2.7. Biochemical Analysis
2.7.1. Urinary Creatinine
2.7.2. Urinary Calcium, Sodium, and Potassium
2.7.3. Urinary N-Telopeptides of Type I Collagen
2.7.4. Serum C-Telopeptide of Type I Collagen
2.7.5. Serum Osteocalcin and Bone-Specific Alkaline Phosphatase
2.7.6. Serum 25-Hydroxyvitamin D
2.7.7. Serum Intact Parathyroid Hormone
2.7.8. Plasma Lipids
2.8. Sample Size Calculation
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Causes of Death in 2008. Available online: www.who.int/gho/mortality_burden_disease/causes_death_2008/en/index.html (accessed on 4 February 2013).
- Mendis, S.; Puska, P.; Norrving, B. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization: Geneva, Switzerland, 2011; Available online: http://whqlibdoc.who.int/publications/2011/9789241564373_eng.pdf (accessed on 4 February 2013).
- Lawes, C.M.; Vander Hoorn, S.; Rodgers, A. Global burden of blood-pressure-related disease, 2001. Lancet 2008, 371, 1513–1518. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Reprint of: Prevention and control of hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 2996–3011. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization and World Economic Forum. From burden to “best buys” reducing the economic impact of non-communicable diseases in low- and middle-income countries. World Economic Forum. 2011. Available online: http://www.who.int/nmh/ publications/best_buys_summary/en/ (accessed on 1 November 2013).
- Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Available online: https://www.who.int/nmh/events/ncd_action_plan/en/ (accessed on 21 June 2019).
- World Health Organization. Technical Consultation. In Proceedings of the Salt Reduction and Iodine Fortification Strategies in Public Health, Sydney, Australia, 25–27 March 2013. [Google Scholar]
- Food Safety Authority of Ireland. ‘The FSAI Salt Reduction Programme—Ten years of progress’ by Karl McDonald. Available online: https://www.fsai.ie/uploadedFiles/Science_and_Health/Salt_and_Health/SRP_2013_Karl_McDonald_FSAI.pdf (accessed on 4 June 2019).
- Irish Universities Nutrition Alliance North/South. Ireland Food Consumption Survey Database. Dublin (Ireland) Food Safety Promotion Board 2001. Available online: http://www.iuna.net/docs/NSIFCSummary.pdf (accessed on 15 July 2016).
- Food Safety Authority of Ireland. ‘Salt Intakes in the Irish Population: Estimates and Trends’ by Janette Walton. Available online: https://www.fsai.ie/uploadedFiles/Science_and_Health/Salt_and_Health/SRP_2013_Janette%20Walton_IUNA.pdf (accessed on 4 June 2019).
- The Regulation (EC) No. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:404:0009:0025:EN:PDF (accessed on 4 June 2019).
- Lynch, E.J.; Dal, B.F.; Sheehan, E.M.; Cashman, K.D.; Arendt, E.K. Fundamental studies on the reduction of salt on dough and bread characteristics. Food Res. Int. 2009, 42, 885–891. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lietz, G.; Avenell, A.; Robins, S.P. Short-term effects of dietary sodium intake on bone metabolism in postmenopausal women measured using urinary deoxypyridinoline excretion. Br. J. Nutr. 1997, 78, 73–82. [Google Scholar] [CrossRef][Green Version]
- Lin, P.H.; Ginty, F.; Appel, L.J.; Aickin, M.; Bohannon, A.; Garnero, P.; Barclay, D.; Svetkey, L.P. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J. Nutr. 2003, 133, 3130–3136. [Google Scholar] [CrossRef]
- Lucey, A.J.; Paschos, G.K.; Cashman, K.D.; Martínéz, J.A.; Thorsdottir, I.; Kiely, M. Influence of moderate energy restriction and seafood consumption on bone turnover in overweight young adults. Am. J. Clin. Nutr. 2008, 87, 1045–1052. [Google Scholar] [CrossRef][Green Version]
- Korhonen, M.H.; Järvinen, R.M.; Sarkkinen, E.S.; Uusitupa, M.I. Effects of a salt-restricted diet on the intake of other nutrients. Am. J. Clin. Nutr. 2000, 72, 414–420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belz, M.C.; Mairinger, R.; Zannini, E.; Ryan, L.A.; Cashman, K.D.; Arendt, E.K. The effect of sourdough and calcium propionate on the microbial shelf-life of salt reduced bread. Appl. Microbiol. Biotechnol. 2012, 96, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2014, 23, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Land, M.A.; Webster, J.; Christoforou, A.; Praveen, D.; Jeffery, P.; Chalmers, J.; Smith, W.; Woodward, M.; Barzi, F.; Nowson, C.; et al. Salt intake assessed by 24 h urinary sodium excretion in a random and opportunistic sample in Australia. BMJ Open 2014, 16, e003720. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Hill, T.R.; Cotter, A.A.; Boreham, C.A.; Dubitzky, W.; Murray, L.; Strain, J.; Flynn, A.; Robson, P.J.; Wallace, J.M.; et al. Low vitamin D status adversely affects bone health parameters in adolescents. Am. J. Clin. Nutr. 2008, 87, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- He, F.J.; MacGregor, G.A. Effect of modest salt reduction on blood pressure: A meta-analysis of randomized trials. Implications for public health. J. Hum. Hypertens. 2002, 16, 761–770. [Google Scholar] [CrossRef]
- Jones, B.; Kenward, M.G. Design and Analysis of Cross-Over Trials; Cox, D., Hinkley, D., Rubin, D., Silverman, B., Eds.; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Nowson, C.A.; Morgan, T.O.; Gibbons, C. Decreasing dietary sodium while following a self-selected potassium-rich diet reduces blood pressure. J. Nutr. 2003, 133, 4118–4123. [Google Scholar] [CrossRef]
- Pan American Health Organization. Preventing Cardiovascular Disease in the Americas by Reducing Dietary Salt Intake Population-wide: A Pan American Health Organization Initiative. Available online: www.interamericanheart.org/files/saltpolicystatementengweb.pdf (accessed on 4 February 2013).
- Vasan, R.S.; Larson, M.G.; Leip, E.P.; Evans, J.C.; O’Donnell, C.J.; Kannel, W.B.; Levy, D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N. Engl. J. Med. 2001, 345, 1291–1297. [Google Scholar] [CrossRef]
- Stamler, J.; Rose, G.; Stamler, R.; Elliott, P.; Dyer, A.; Marmot, M. INTERSALT study findings. Public health and medical care implications. Hypertension 1989, 14, 570–577. [Google Scholar] [CrossRef]
- Food Safety Authority of Ireland. Salt and Health: Review of the Scientific Evidence and Recommendations for Public Policy in Ireland (Revision 1). Available online: https://www.fsai.ie/science_and_health/salt_and_health/the_science_of_salt_and_health.html (accessed on 6 June 2019).
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Svetkey, L.P.; Sacks, F.M.; Obarzanek, E.; Vollmer, W.M.; Appel, L.J.; Lin, P.H.; Karanja, N.M.; Harsha, D.W.; Bray, G.A.; Aickin, M.; et al. The DASH Diet, Sodium Intake and Blood Pressure Trial (DASH-sodium): Rationale and design. J. Am. Diet. Assoc. 1999, 99, 96–104. [Google Scholar] [CrossRef]
- Nowson, C.A.; Worsley, A.; Margerison, C.; Jorna, M.K.; Frame, A.G.; Torres, S.J.; Godfrey, S.J. Blood pressure response to dietary modifications in free-living individuals. J. Nutr. 2004, 134, 2322–2329. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; MacGregor, G.A. Does potassium supplementation lower blood pressure? A meta-analysis of published trials. J. Hypertens. 1991, 9, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, M.H.; Litmanen, H.; Rauramaa, R.; Väisänen, S.B.; Niskanen, L.; Uusitupa, M. Adherence to the salt restriction diet among people with mildly elevated blood pressure. Eur. J. Clin. Nutr. 1999, 53, 880–885. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. CFR—Code of Federal Regulations Title 21, Part 101—Food Labelling; Sec. 101.61 Nutrient Content Claims for the Sodium Content of Foods. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=101.61 (accessed on 6 June 2019).
- Gormley, T.R.; Morrissey, A. Evaluation of reduced salt breads. Farm Food 1993, 3, 12. [Google Scholar]
- Belz, M.C.E.; Axel, C.; Arendt, E.K.; Lynch, K.M.; Brosnan, B.; Sheehan, E.M.; Coffey, A.; Zannini, E. Improvement of taste and shelf life of yeasted low-salt bread containing functional sourdoughs using Lactobacillus amylovorus DSM 19280 and Weisella cibaria MG1. Int. J. Food Microbiol. 2018, 302, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Food Safety Authority of Ireland. Monitoring of Sodium and Potassium in Processed Foods. Period: September 2003 to December 2018. Available online: https://www.fsai.ie/uploadedFiles/Science_and_Health/Salt_and_Health/Salt_Surveys_2003_onwards.pdf (accessed on 6 June 2019).
- Cashman, K.D.; Flynn, A. Sodium effects on bone and calcium metabolism. In Nutritional Aspects of Bone Health; Lanham-New, S.A., Bonjour, J.-P., Eds.; The Royal Society of Chemistry: London, UK, 2003; pp. 267–289. [Google Scholar]
- Harsha, D.W.; Sacks, F.M.; Obarzanek, E.; Svetkey, L.P.; Lin, P.H.; Bray, G.A.; Aickin, M.; Conlin, P.R.; Miller, E.R., 3rd; Appel, L.J. Effect of dietary sodium intake on blood lipids: Results from the DASH-sodium trial. Hypertension 2004, 43, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.R.C.; He, F.J.; Tan, M.; Cappuccio, F.P.; Neal, B.; Woodward, M.; Cogswell, M.E.; McLean, R.; Arcand, J.; MacGregor, G.; et al. The International Consortium for Quality Research on Dietary Sodium/Salt (TRUE) position statement on the use of 24-h, spot, and short duration (<24 h) timed urine collections to assess dietary sodium intake. J. Clin. Hypertens. 2019, 21, 700–709. [Google Scholar]
- Ljungman, S.; Aurell, M.; Hartford, M.; Wikstrand, J.; Wilhelmsen, L.; Berglund, G. Sodium excretion and blood pressure. Hypertension 1981, 3, 318–326. [Google Scholar] [CrossRef]
- McCoy, C.E. Understanding the Intention-to-treat Principle in Randomized Controlled Trials. West. J. Emerg Med. 2017, 18, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Belz, M.C.; Ryan, L.A.; Arendt, E.K. The impact of salt reduction in bread: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 514–524. [Google Scholar] [CrossRef] [PubMed]
| Mean (Number) | SD | |
|---|---|---|
| Age (years) | 47.8 | 9.3 |
| Men:women | (55:41) | - |
| Height (m) | 1.71 | 0.10 |
| Weight (kg) | 81.8 | 14.6 |
| BMI (kg/m2) | 27.2 | 6.4 |
| Systolic blood pressure (mmHg) | 138.5 | 10.4 |
| Diastolic blood pressure (mmHg) | 86.5 | 8.8 |
| Urinary sodium (mmol/day) | 108.3 | 45.7 |
| Current smokers | (11) | - |
| Dietary Period | Usual-Salt | Reduced-Salt | p-Value 1 | ||
|---|---|---|---|---|---|
| Blood pressure: | |||||
| Systolic (mmHg) | 134.3 | 12.1 | 131.0 | 11.0 | <0.0001 |
| Diastolic (mmHg) | 84.7 | 8.5 | 84.6 | 8.2 | 0.815 |
| Urine 2: | |||||
| Sodium (mmol/day) | 106.0 | 53.6 | 77.6 | 35.6 | <0.0001 |
| Potassium (mmol/day) | 97.6 | 51.5 | 95.9 | 51.5 | 0.771 |
| Calcium (mmol/day) | 4.36 | 2.35 | 4.10 | 2.52 | 0.183 |
| NTx (nmol BCE/mmol creatinine) | 55.0 | 36.7 | 54.6 | 42.9 | 0.654 |
| Serum 3: | |||||
| 25(OH)D (nmol/L) | 53.6 | 16.7 | 52.6 | 14.7 | 0.414 |
| BAP (U/L) | 27.2 | 9.3 | 27.7 | 9.5 | 0.137 |
| Osteocalcin (g/L) | 12.5 | 4.3 | 13.0 | 5.6 | 0.208 |
| CTx (g/L) | 0.69 | 0.31 | 0.69 | 0.29 | 0.964 |
| PTH (pg/mL) | 64.2 | 31.0 | 62.1 | 30.9 | 0.271 |
| Dietary Period | Usual-Salt | Reduced-Salt | p-Value 1 | ||
|---|---|---|---|---|---|
| Plasma 2: | |||||
| Total cholesterol (mg/dL) | 192.9 | 33.7 | 195.9 | 33.9 | 0.262 |
| LDL cholesterol (mg/dL) | 119.3 | 29.6 | 122.0 | 30.3 | 0.266 |
| HDL cholesterol (mg/dL) | 54.4 | 14.9 | 53.9 | 14.7 | 0.451 |
| TAG (mg/dL) | 96.0 | 44.9 | 100.9 | 46.9 | 0.184 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cashman, K.D.; Kenny, S.; Kerry, J.P.; Leenhardt, F.; Arendt, E.K. ‘Low-Salt’ Bread as an Important Component of a Pragmatic Reduced-Salt Diet for Lowering Blood Pressure in Adults with Elevated Blood Pressure. Nutrients 2019, 11, 1725. https://doi.org/10.3390/nu11081725
Cashman KD, Kenny S, Kerry JP, Leenhardt F, Arendt EK. ‘Low-Salt’ Bread as an Important Component of a Pragmatic Reduced-Salt Diet for Lowering Blood Pressure in Adults with Elevated Blood Pressure. Nutrients. 2019; 11(8):1725. https://doi.org/10.3390/nu11081725
Chicago/Turabian StyleCashman, Kevin D., Sorcha Kenny, Joseph P. Kerry, Fanny Leenhardt, and Elke K. Arendt. 2019. "‘Low-Salt’ Bread as an Important Component of a Pragmatic Reduced-Salt Diet for Lowering Blood Pressure in Adults with Elevated Blood Pressure" Nutrients 11, no. 8: 1725. https://doi.org/10.3390/nu11081725
APA StyleCashman, K. D., Kenny, S., Kerry, J. P., Leenhardt, F., & Arendt, E. K. (2019). ‘Low-Salt’ Bread as an Important Component of a Pragmatic Reduced-Salt Diet for Lowering Blood Pressure in Adults with Elevated Blood Pressure. Nutrients, 11(8), 1725. https://doi.org/10.3390/nu11081725





