A Strategy to Deliver Precise Oral Doses of the Glucosinolates or Isothiocyanates from Moringa oleifera Leaves for Use in Clinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources and Preparation of Moringa oleifera Leaf Powder
2.2. Extraction of Phytochemicals (Tea Preparation)
2.3. Determination of Glucosinolate (Glucomoringin) Content
2.4. Determination of Isothiocyanate (Moringin) Content by Cyclocondensation
2.5. Myrosinase Activity and Stability
2.6. Soluble Protein Content
2.7. Anti-Inflammatory Activity Assay
2.8. Evaluation of Microbiological Load in Moringa Preparations
2.9. Reagents and Equipment
2.10. Statistical Analysis
3. Results
3.1. Preliminary Analyses of Moringa Leaf Powders for Use in Water Extracts (Teas)
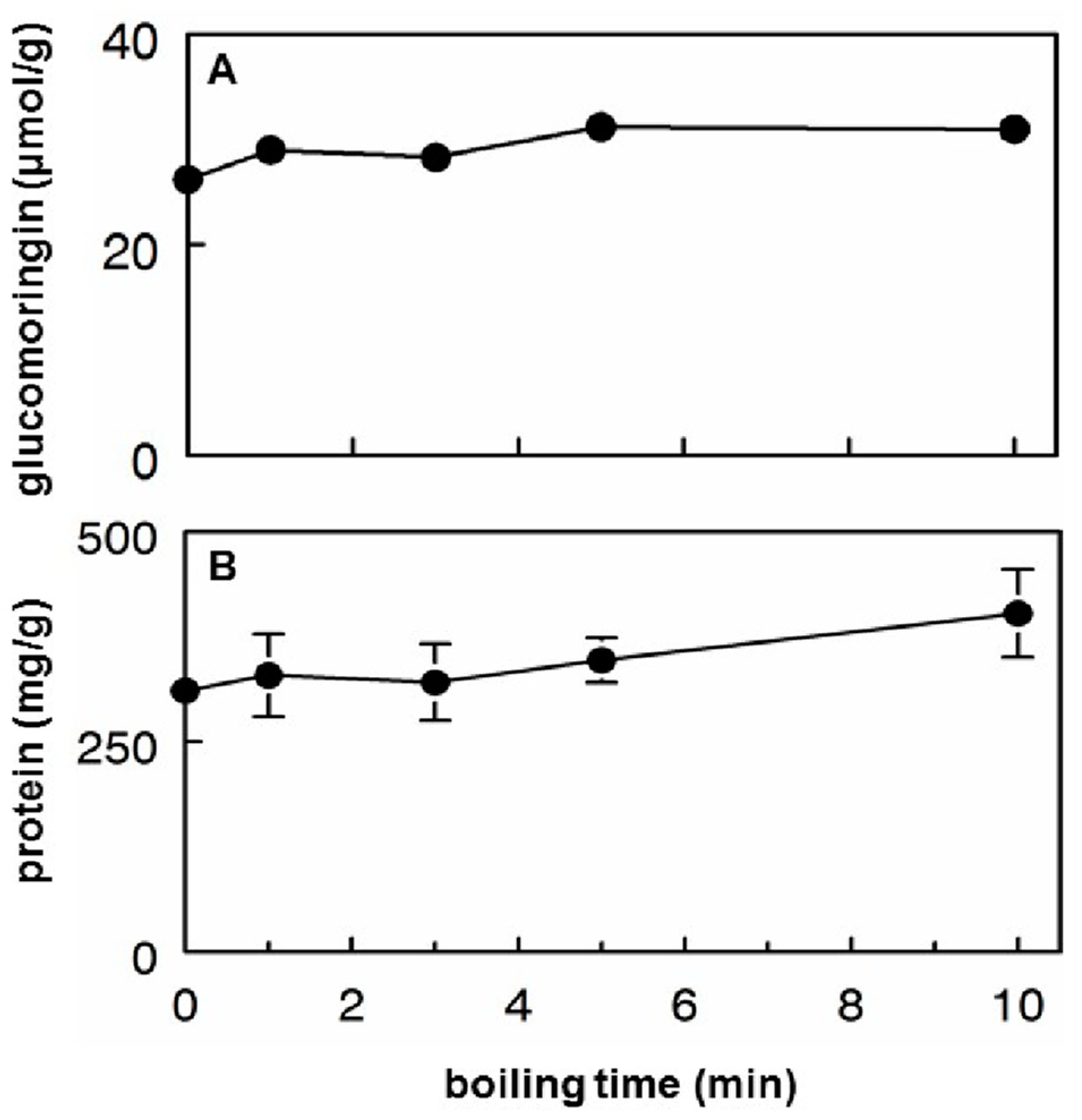
3.2. Glucomoringin and Protein Extraction into Hot Tea
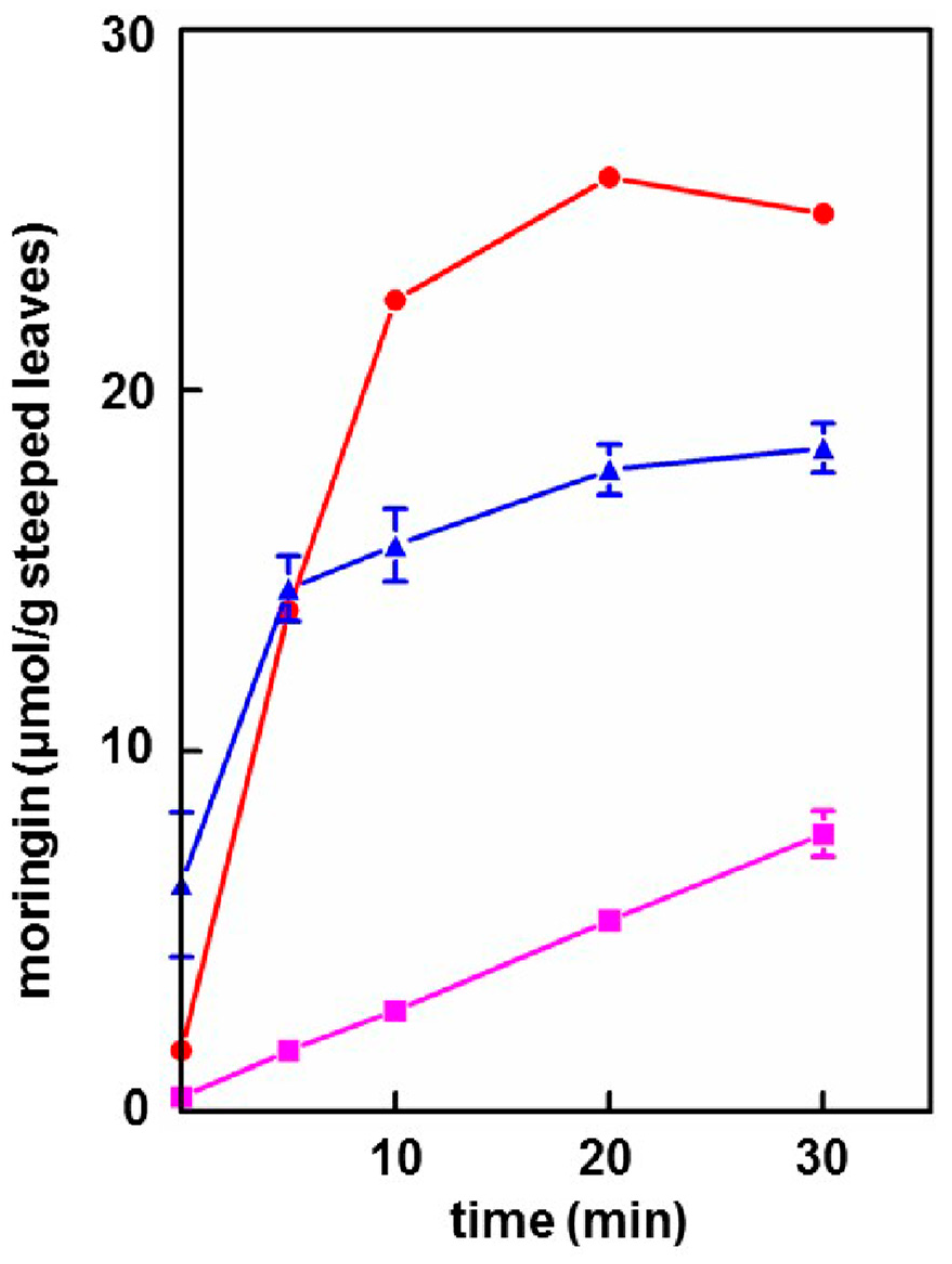
3.3. Extraction of Moringin into Cold Tea
3.4. Microbiological Analysis of Cold Tea Following Non-refrigerated Storage
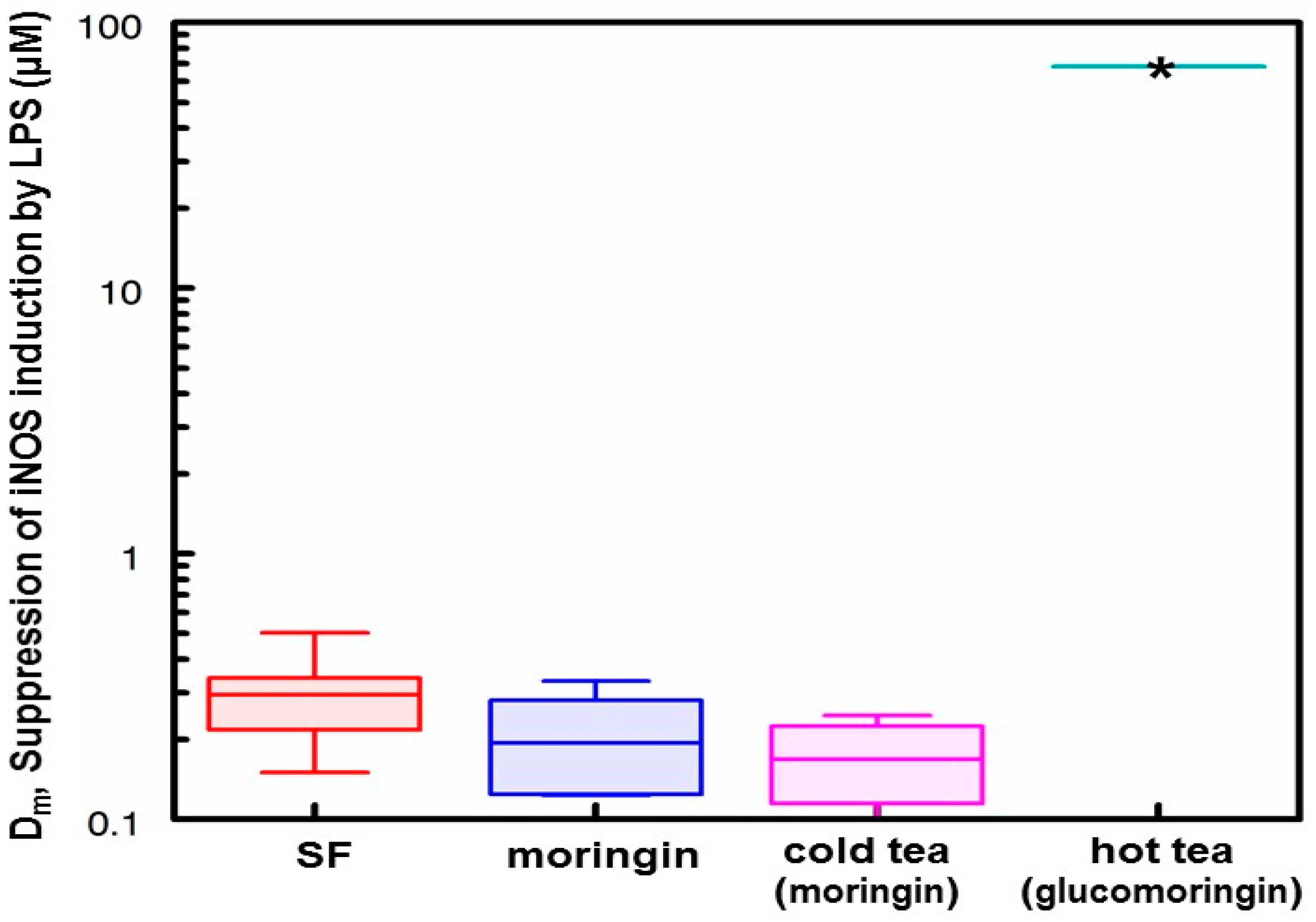
3.5. Anti-Inflammatory Activity of Hot and Cold Teas
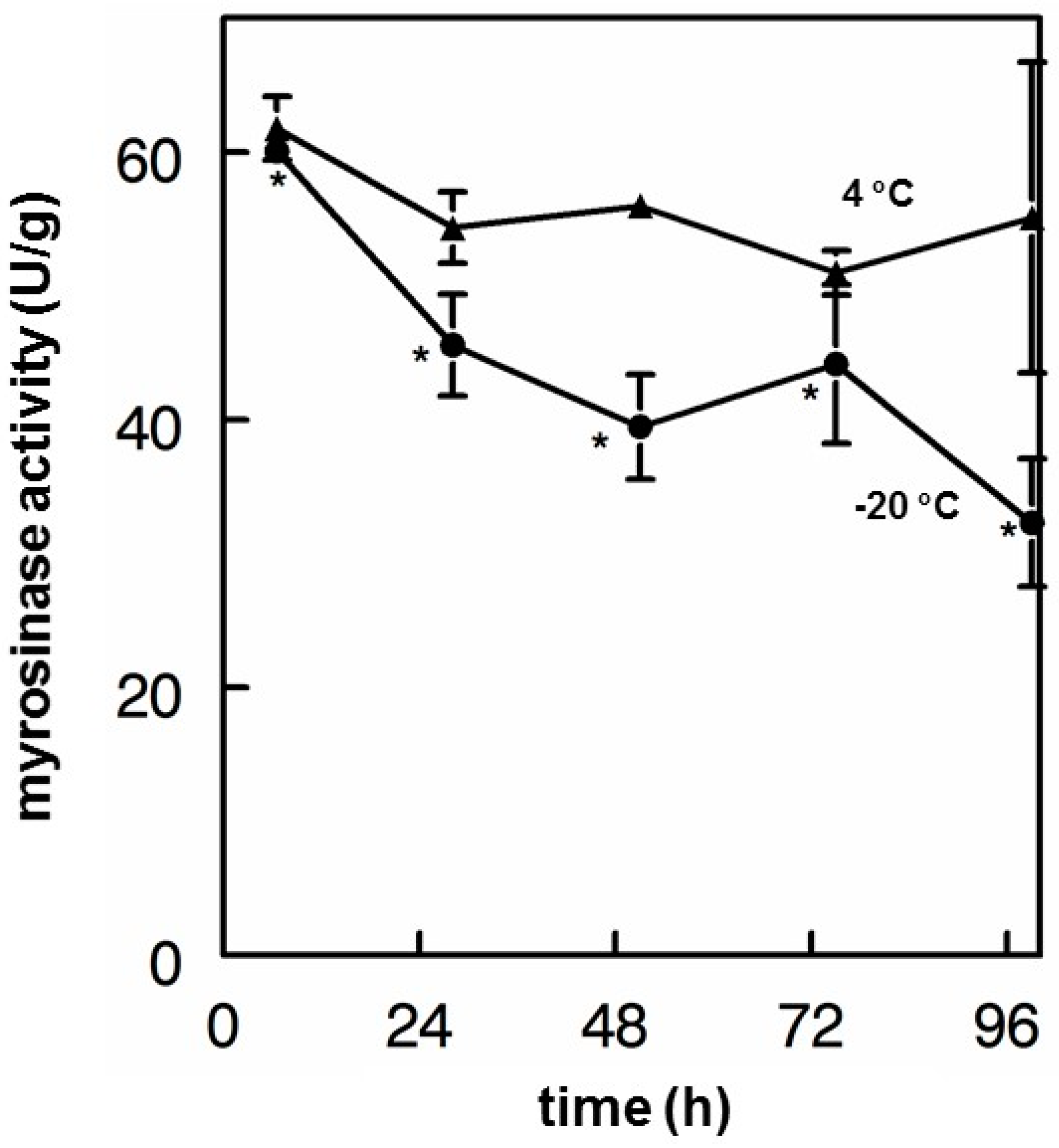
3.6. Myrosinase Stability
4. Discussion
- (A)
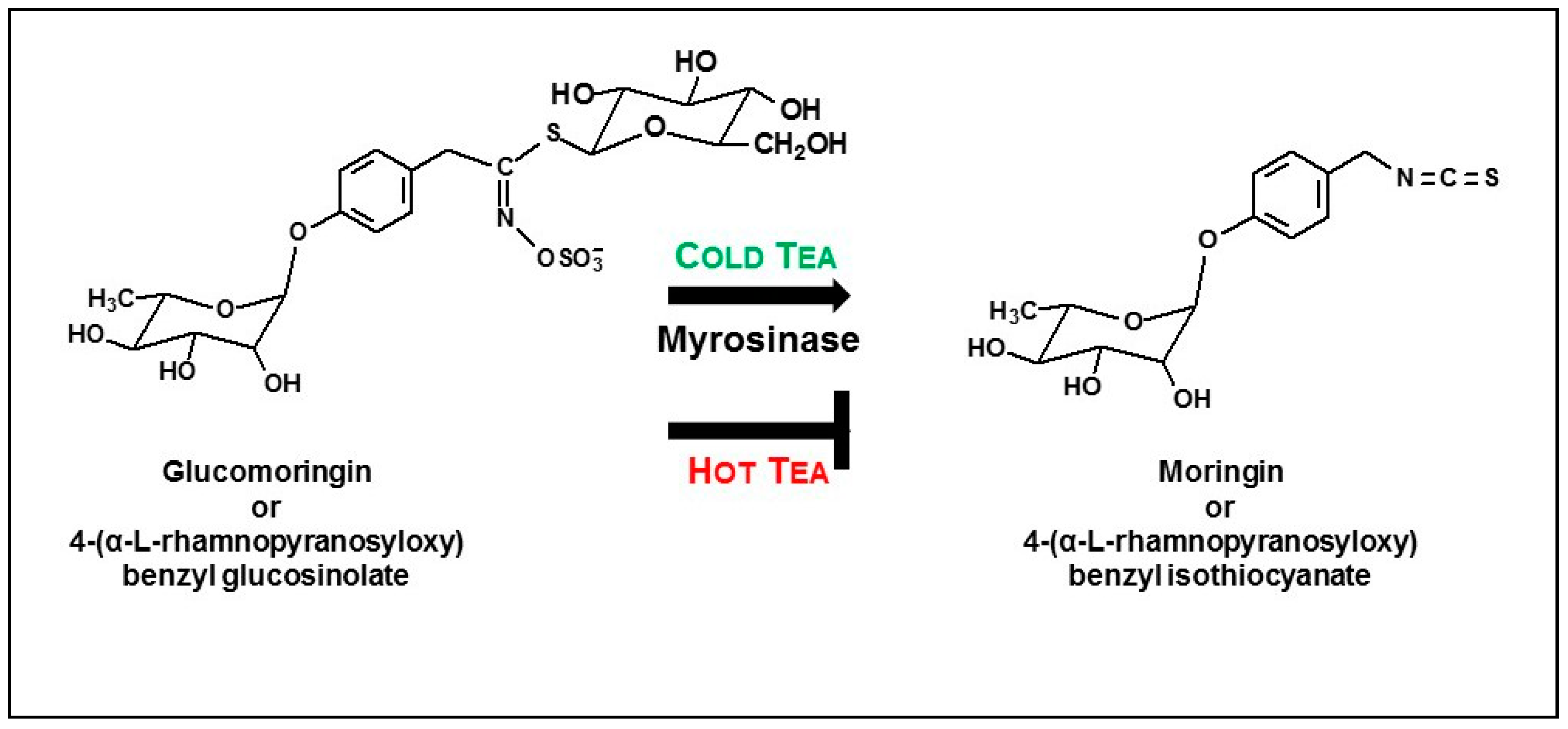
- About half of the available glucomoringin (the highly water soluble but biologically inactive precursor to the chemoprotective ITC) could be rapidly extracted into a boiling water tea made from the leaves. This yield was almost complete in that a tea sample removed immediately upon placing powder into boiling water contained essentially all the glucomoringin that could be extracted even with much longer boiling times (up to 30 min; data not shown). The comparator in all cases was a quadruple organic solvent extraction (water, acetonitrile, dimethyl sulfoxide and dimethyl fumarate, in equal portions) in which the powder was thoroughly extracted using a mechanical tissue homogenizer.
- (B)
- Particle size did not matter, so that using a finely ground and sieved powder yielded no more glucomoringin than using coarsely chopped leaves. This of course has implications for making tea infusions from dried (whole or coarsely chopped) moringa leaflets because it is much easier to filter, and requires less processing. That the fineness of milling is not a critical factor influencing glucomoringin yield means that relatively comparable results can be achieved across studies in different parts of the world.
- (C)
- Provided that the powder contained active myrosinase, a cold water (23 °C) tea in which the powder incubated, or steeped, to produce an infusion, yielded slightly less of the cognate ITC (on a molar basis) than the GS extracted into a comparable boiling water tea. Yield from leaf powders with highly active myrosinase and high glucomoringin content plateaued within about 10 min, whereas yield from powder with less myrosinase kept increasing even after 30 min. The fact that moringin yield from cold teas made from PA and PB plateau within the 30 min incubation time, indicated completed extraction and hydrolysis, whereas the linear response at 30 min from PC indicated that it may benefit from a longer incubation time.
- (D)
- Bacterial titer in hot teas was not a concern with imminent consumption, whereas titer (aerobic plate count) in cold teas that had been sitting at room temperature for 48 h was still perfectly acceptable. (A limit of 105 CFU/mL is recommended by the United States Pharmacopoeia Convention for dried or powdered botanicals and a limit of 107 CFU/g is recommended by the International Standard/American National Standard for Dietary Supplements (NSF/ANSI), [29]). These low bacterial counts were most likely due to the bacteriostatic activity of the moringin that was produced in the tea [36,37].
- (E)
- Based on the number of μmol of moringin or glucomoringin in the teas, moringin-containing tea was as potent as pure moringin in inhibiting LPS-stimulated NO production, which is an anti-inflammatory response indicator. Pure moringin was even more potent, on a molar basis, than sulforaphane from broccoli sprouts. Conversely, the glucomoringin-containing boiled tea had no inherent anti-inflammatory activity, though it is presumed that upon ingestion and digestion (the gut microflora contains some myrosinase activity), some moringin would be produced and absorbed.
- (F)
- Taste of both hot and cold teas was judged to be highly acceptable, by casual survey of friends and family who tasted them. Further larger studies are warranted to confirm this finding in the general population and the ASD population. Moringa tea is widely sold commercially, showing that it already has some acceptance worldwide. We have thus abandoned earlier effort to develop taste masking strategies for delivery of dried leaf powder to children with ASD. This was something we had regarded as a necessary prerequisite for delivery in a clinical study to children who characteristically have very strong taste aversions or preferences, and who by-and-large do not, will not, or cannot take the pills (capsules or tablets) that would be required to deliver a sufficient quantity of leaf powder to provide enough glucormoringin (e.g., 200 μmol or more) for the trials that we envisioned. The favorable taste profile combined with the extraordinarily low bacterial plate count of these teas may be useful for people interested in the preparation of cold (ITC-rich) moringa leaf powder teas that we feel will have the greatest chemoprotection potential.
- (G)
- Finally, myrosinase isolated and purified from moringa and cruciferous vegetables has historically been viewed as being highly unstable. We found this not to be the case, showing that enzyme in saline solution was quite stable for years, and that these solutions could be repeatedly frozen and thawed but could also be refrigerated for multiple days without substantial attenuation of activity. Moreover, we have determined that dry moringa leaf powder (e.g., “PC”) could be stored at 4 °C for 13 years and retain enough myrosinase activity to make a potent cold tea, hydrolyzing glucomoringin to moringin.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olson, M.E.; Flora of North America Editorial Committee. Moringaceae Martinov. Drumstick Tree Family. Flora N. Am. 1993, 7, 163–169. [Google Scholar]
- Fahey, J.W. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 2005, 1, 1–15. [Google Scholar]
- Olson, M.E.; Sankaran, R.P.; Fahey, J.W.; Grusak, M.A.; Odee, D.; Nouman, W. Leaf protein and mineral concentrations across the “miracle tree” genus Moringa. PLoS ONE 2016, 11, e0159782. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W. Moringa oleifera: A review of the medical potential. Acta Horticult. 2017. [Google Scholar] [CrossRef]
- Thurber, M.; Fahey, J.W. Adoption of Moringa oleifera to combat under-nutrition viewed through the lens of the ‘Diffusion of Innovations’ theory. Ecol. Food Nutr. 2009, 48, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W. Viewpoint: Superfood or Super-Hype? Johns Hopkins Health Rev. 2017, 4, 66–67. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51, [corrigendum: Phytochemistry 59, 237]. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Mellon, F.A.; Foidl, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (horseradish tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Yuan, J.-M.; Fahey, J.W.; Kensler, T.W. Isothiocyanates: Translating the power of plants to people. Mol. Nutr. Food Res. 2018, 62, 1700965. [Google Scholar] [CrossRef]
- Johnson, T.L.; Dinkova-Kostova, A.T.; Fahey, J.W. Glucosinolates from the Brassica Vegetables and their Health Effects; Caballero, B., Finglas, P., Toldrá, F., Eds.; Elsevier, Inc.: Philadelphia, PA, USA, 2018; pp. 248–255. [Google Scholar]
- Panjwani, A.A.; Liu, H.; Fahey, J.W. Crucifers and related vegetables and supplements for neurologic disorders: What is the evidence? Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.J.; Brugha, T.S.; Erskine, H.E.; Scheurer, R.W.; Vos, T.; Scott, T.G. The epidemiology and global burden of autism spectrum disorders. Psychol. Med. 2015, 45, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Kensler, T.W. Frugal medicine: Health-span extension through green chemoprevention. AMA J. Ethics 2013, 15, 311–318. [Google Scholar]
- Fahey, J.W.; Talalay, P.; Kensler, T.W. Notes from the field: “Green” chemoprevention as frugal medicine. Cancer Prev. Res. 2012, 5, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Lalas, S.; Athanasiadis, V.; Karageorgon, I.; Batron, G.; Makais, N.; Makris, D.P. Nutritional characterization of leaves and herbal teas of Moringa oleifera cultivated in Greece. J. Herbs Spices Med. Plants 2017, 23, 320–333. [Google Scholar] [CrossRef]
- Wade, K.L.; Garrard, I.J.; Fahey, J.W. Improved hydrophilic interaction chromatography method for the identification and quantification of glucosinolates. J. Chrom. A 2007, 1154, 469–472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Cho, C.-G.; Posner, G.H.; Talalay, P. Spectroscopic quantitation of organic isothiocyanates by cyclocondensation with vicinal dithiols. Anal. Biochem. 1992, 205, 100–107. [Google Scholar] [CrossRef]
- Ye, L.; Dinkova-Kostova, A.T.; Wade, K.L.; Zhang, Y.; Shapiro, T.A.; Talalay, P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: Pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin. Chim. Acta 2002, 316, 43–53. [Google Scholar] [CrossRef]
- Palmieri, S.; Leoni, O.; Iori, R. A steady-state kinetics study of myrosinase with direct ultraviolet spectrophotometric assay. Anal. Biochem. 1982, 123, 320–324. [Google Scholar] [CrossRef]
- Shikita, M.; Fahey, J.W.; Golden, T.R.; Holtzclaw, W.D.; Talalay, P. An unusual case of ‘uncompetitive activation’ by ascorbic acid: Purification and kinetic properties of a myrosinase from Raphanus sativus seedlings. Biochem. J. 1999, 341, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Chodur, G.M.; Olson, M.E.; Wade, K.L.; Stephenson, K.K.; Nouman, W.; Garima; Fahey, J.W. Wild type and domesticated Moringa oleifera differ markedly in taste, glucosinolate composition, and antioxidant potential, but not myrosinase activity or protein content. Sci. Rep. 2018, 8, 7995. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Liu, H.; Dinkova-Kostova, A.T.; Talalay, P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc. Nat. Acad. Sci. USA 2008, 105, 15926–15931. [Google Scholar] [CrossRef] [PubMed]
- Suh, N.; Honda, T.; Finlay, H.J.; Barchowsky, A.; Williams, C.; Benoit, N.E.; Xie, Q.-W.; Nathan, C.; Gribble, G.W.; Sporn, M.B. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998, 58, 717–723. [Google Scholar] [PubMed]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Chou, T.-C.; Martin, N. CompuSyn for Drug Combinations. A Computer Software for Quantitation of Synergism and Antagonism, and the Determination of IC50, ED50 and LD50 Values, (ComboSyn, Paramus, NJ). [PC software and user’s guide]. 2005.
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- American Herbal Products Association. Recommended Microbial Limits for Botanical Ingredients. 2014. Available online: http://www.ahpa.org/Portals/0/PDFs/Policies/14_0206_AHPA_micro_limits_comparisons.pdf (accessed on 15 May 2019).
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef]
- Fahey, J.W.; Olson, M.E.; Stephenson, K.K.; Wade, K.L.; Chodur, G.M.; Odee, D.; Noumann, W.; Massiah, M.; Alt, J.; Egner, P.A.; et al. The diversity of chemoprotective glucosinolates in moringaceae (Moringa spp.). Sci. Rep. 2018, 8, 7994. [Google Scholar] [CrossRef]
- Chen, J.-G.; Johnson, J.; Egner, P.A.; Ng, D.K.; Zhu, J.; Wang, J.-B.; Xue, X.-F.; Sun, Y.; Zhang, Y.-H.; Lu, L.-L.; et al. Dose-dependent detoxication of the airborne pollutant benzene by broccoli sprout beverage in randomized trial in Qidong, China. Am. J. Clin. Nutr. 2019, nqz122. [Google Scholar] [CrossRef]
- Egner, P.A.; Chen, J.-G.; Zarth, A.T.; Ng, D.K.; Wang, J.-B.; Kensler, K.H.; Jacobson, L.P.; Muñoz, A.; Johnson, J.L.; Groopman, J.D.; et al. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: Results of a randomized clinical trial in China. Cancer Prev. Res. 2014, 7, 813–823. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Stephenson, K.K.; Wade, K.L.; Liu, H.; Fahey, J.W. Structure-activity analysis of flavonoids: Direct and indirect antioxidant, and anti-inflammatory potencies and toxicities. Nutr. Cancer 2013, 65, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Wade, K.L.; Ito, Y.; Ramarathnam, A.; Holtzclaw, W.D.; Fahey, J.W. Novel purification of active myrosinase from plants by aqueous two-phase counter-current chromatography. Phytochem. Anal. 2015, 26, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Haristoy, X.; Fahey, J.W.; Scholtus, I.; Lozniewski, A. Evaluation of antimicrobial effect of several isothiocyanates on Helicobacter pylori. Planta Med. 2005, 71, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef] [PubMed]
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin 534 resistance, and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 2015, 59, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
| Moringa Leaf Powder | Bacterial Titer c (CFU/g) | Myrosinase Activity d (U/g) | Glucomoringin Content d,e (µmol/g)* |
|---|---|---|---|
| PA a | 2000 | 11.56 ± 0.27 | 77.03 ± 6.52 |
| PB b | 24,000 | 8.71 ± 0.09 | 49.65 ± 4.58 |
| PC b | 2000 | 6.25 ± 1.61 | 40.25 ± 2.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Shi, Y.; Liu, H.; Panjwani, A.A.; Warrick, C.R.; Olson, M.E. A Strategy to Deliver Precise Oral Doses of the Glucosinolates or Isothiocyanates from Moringa oleifera Leaves for Use in Clinical Studies. Nutrients 2019, 11, 1547. https://doi.org/10.3390/nu11071547
Fahey JW, Wade KL, Stephenson KK, Shi Y, Liu H, Panjwani AA, Warrick CR, Olson ME. A Strategy to Deliver Precise Oral Doses of the Glucosinolates or Isothiocyanates from Moringa oleifera Leaves for Use in Clinical Studies. Nutrients. 2019; 11(7):1547. https://doi.org/10.3390/nu11071547
Chicago/Turabian StyleFahey, Jed W., Kristina L. Wade, Katherine K. Stephenson, Yuzhu Shi, Hua Liu, Anita A. Panjwani, Collin R. Warrick, and Mark E. Olson. 2019. "A Strategy to Deliver Precise Oral Doses of the Glucosinolates or Isothiocyanates from Moringa oleifera Leaves for Use in Clinical Studies" Nutrients 11, no. 7: 1547. https://doi.org/10.3390/nu11071547
APA StyleFahey, J. W., Wade, K. L., Stephenson, K. K., Shi, Y., Liu, H., Panjwani, A. A., Warrick, C. R., & Olson, M. E. (2019). A Strategy to Deliver Precise Oral Doses of the Glucosinolates or Isothiocyanates from Moringa oleifera Leaves for Use in Clinical Studies. Nutrients, 11(7), 1547. https://doi.org/10.3390/nu11071547






